- 1Molecular Biology Research Laboratory, Department of Otorhinolaryngology, Charité - Universitätsmedizin Berlin, Campus Charité Mitte, Berlin, Germany
- 2Tinnitus Center, Charité - Universitätsmedizin Berlin, Campus Charité Mitte, Berlin, Germany
- 3Department of Otorhinolaryngology, Charité - Universitätsmedizin Berlin, Campus Virchow Klinikum, Berlin, Germany
The aim of this review is to focus the attention of clinicians and basic researchers on the association between psycho-social stress and tinnitus. Although tinnitus is an auditory symptom, its onset and progression often associates with emotional strain. Recent epidemiological studies have provided evidence for a direct relationship between the emotional status of subjects and tinnitus. In addition, studies of function, morphology, and gene and protein expression in the auditory system of animals exposed to stress support the notion that the emotional status can influence the auditory system. The data provided by clinical and basic research with use of animal stress models offers valuable clues for an improvement in diagnosis and more effective treatment of tinnitus.
Introduction
The term “stress” was originally used in physics to define a pressure causing deformation of a physical body. In biology and medicine, the term “stress” is used to describe a reaction of an organism to a stressor. Stressors can be of physical or psycho-social nature (Figure 1). Generally speaking, stress is a positive reaction because it increases the chance of survival by initiating adaptation and coping with new situation (Lupien et al., 2009). Changes provoked by stress can be presented as a chain of reactions involving alarm stage, and—if the stressor is not removed—resistance, and exhaustion (Tsigos and Chrousos, 2002). Another, allostatic model of stress-induced reactions is introduced below.
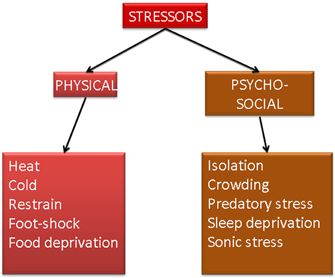
Figure 1. Schematic representation of two main types of stress and different stress models used in basic research.
Stress stimulates neuroendocrine axes such as hypothalamus-pituitary-thyroid axis (Mebis and van den Berghe, 2009), hypothalamus-pituitary-gonadal axis (Whirledge and Cidlowski, 2010) and hypothalamus-pituitary-adrenal axis (HPA axis) (Lupien et al., 2007). Further, stress can also activate sympathetic nervous system (Ulrich-Lai and Herman, 2009). Adaptation of neuronal system to stress-induced condition is reflected by neuronal plasticity. Neuronal plasticity is not only essential for learning and memory formation but also for the induction of mood illnesses (Berlucchi and Buchtel, 2009; Calabrese et al., 2009). The process of coping with conditions altered by stress is called allostasis and means a change from the usual, homeostatic status into a status, in which the organism can adapt to changes (McEwen and Gianaros, 2011). Abuse or chronic deregulation of allostatic processes (such as prolonged or repeated stress) may lead to so-called allostatic load, which is a negative physiological and behavioral effect of stress (Figure 2) (McEwen and Wingfield, 2003). Allostatic load can affect various tissues and organs and include neuronal atrophy, impaired immunity, atherosclerosis, obesity, bone demineralization and mood disorders (McEwen, 2003).
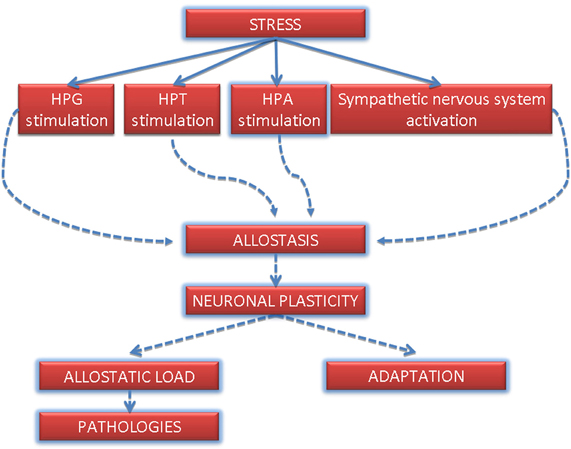
Figure 2. Most representative stress-induced pathways and hypothetical involvement in the induction of allostatic load. HPT, hypothalamus-pituitary-thyroid axis; HPG, hypothalamus-pituitary-gonadal axis; HPA, hypothalamus-pituitary-adrenal axis.
Tinnitus is a subjective perception of sound without external acoustic signal caused by inappropriate activation of auditory cortex. This activation has been documented in tinnitus patients by using either positron emission tomography (PET) or functional magnetic resonance imagining (fMRI) (Lanting et al., 2009). The results of animal and human studies have helped to determine various possible causes of inappropriate activation of auditory cortex leading to neuronal plasticity. These causes include changes in spontaneous firing rate, increased gamma band reflecting synchronous firing of auditory cortex and tonotopic reorganization (Nava and Roder, 2011).
Tinnitus can be induced by a variety of pathological conditions via modification of the middle or inner ear functions (e.g., otosclerosis, chronic otitis media, labirintitis, ototoxicity, noise, genetic defects), or by affecting directly or indirectly neurons in the auditory pathway (e.g., multiple sclerosis, acoustic neuroma/vestibular schwannomas, meningiomas, stroke, hemorrhage, head trauma). Accumulating evidence suggests that changes induced by these diverse conditions may result in similar phenotype, which is inappropriate activation of the auditory cortex.
In this review, we will describe some of HPA axis-mediated effects induced by stress and will discuss their possible influence on the auditory system with special focus on tinnitus. In addition, we will attempt to transfer some of the in vitro knowledge into a clinical practice.
Stress: Mechanisms (HPA Axis), Models, and Auditory System
The Hypothalamic-Pituitary-Adrenal Axis (HPAa)
Stress induces secretion of corticotropin-releasing hormone (CRH) from hypothalamus. CRH stimulates in turn the secretion of adrenocorticothropin (ACTH) from pituitary gland. Finally, release of ACTH to blood causes secretion of stress hormones from the adrenal glands (Lupien et al., 2007). Stress hormones comprise glucocorticoids (corticosterone, cortisol) and mineralocorticoids (aldosterone) (de Kloet et al., 2005). Cortisol (corticosterone in rodents)—is released not only upon exposure to stress but also in a circadian rhythm (Weitzman et al., 1971) and regulates in genomic and non-genomic way a variety of processes, from inflammation to behavioral changes (Amsterdam and Sasson, 2002; Amsterdam et al., 2002; Kudielka et al., 2004; de Kloet et al., 2005). Corticosteroids act via respective receptors: mineralocorticoid receptor (aldosterone receptor, MR) and glucocorticoid receptor (GR) and elicit two types of reactions: genomic and non-genomic (Figure 3). The genomic reactions occur due to the fact that both GRs and mineralcorticoid receptors are cytoplasmic, ligand-activated transcription factors (Funder, 1997). Binding of steroids to GRs or mineralcorticoid receptors induces translocation of the ligand-receptor complex to the nucleus, where the transcription of selected genes is either induced or suppressed (Datson et al., 2008). The genomic response is relatively slow and on average takes few hours. Non-genomic responses induced by corticosteroids and mineralocorticoids are extremely rapid (seconds to minutes) and are not mediated by a cytoplasmic but by a minor, membrane-bound form of GRs or mineralcorticoid receptors (Groeneweg et al., 2012). Both receptors were shown to be localized to the lipid rafts—more precisely to caveolae on a cell surface. Caveolae are rich in signaling proteins such as G-proteins and kinases, thus, they support formation of various signalosomes. The precise signaling pathways induced by corticosteroids and mineralocorticoids remain to be confirmed but it is already apparent that the kinase pathways are predominant (Groeneweg et al., 2012).
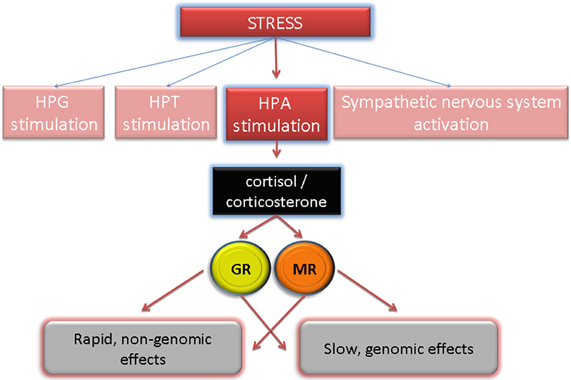
Figure 3. HPA-induced stress hormone release and their signaling via respective receptors induce genomic (slow) and non-genomic (rapid) changes. GR, glucocorticoid receptor; MR, mineralcorticoid receptor.
Hypothalamic-Pituitary-Adrenal Axis and Auditory System
HPA-induced steroids signal trough their respective receptors: glucocorticoid and mineralcorticoid receptors. In contrast to ubiquitously expressed GRs, expression of mineralcorticoid receptors is restricted to selected tissues including brain, eye, intestine, kidney, mammary gland, pancreas, pituitary gland, and the inner ear. In fact, analyses of expressed sequence tag profile in mice demonstrated the highest expression level of mineralcorticoid receptor mRNA in the inner ear, as compared to other tissues (NCBI accession number: Mm.324393). Expression of mineralcorticoid receptors is not incidental—it denotes aldosterone-sensitive tissues, in which mineralcorticoid receptors regulate the ionic and water transports (mainly the epithelial sodium channel, Na+/K+ pump, serum, and glucocorticoid-induced kinase or SGK1) resulting in the re-absorption of sodium and an excretion of potassium (Thomas and Harvey, 2011).
Presence and localization of mineralcorticoid and glucocorticoid receptors was studied in the rat cochlea and their expression was positively confirmed (Zuo et al., 1995; Yao and Rarey, 1996). Not surprisingly, mineralcorticoid receptor was found to be predominantly localized in stria vascularis and in the spiral ganglion neurons (Furuta et al., 1994). Hyperactivation of mineralcorticoid receptors in the inner ear could lead to improper potassium-sodium balance in the scala tympani and was, in fact, suspected to play a crucial role in the vertigo and tinnitus/deafness attacks in the Ménière's disease. In a clinical study, where the concentration of aldosterone was measured in plasma of Ménière's patients obtained between the attacks, no abnormal fluctuation was found, suggesting that this hormone does not contribute to the sickness symptomes (Mateijsen et al., 2001). However, the question if aldosterone concentration is being altered during active periods of Ménière's disease remains open. On the other hand, Ménière's patients have elevated concentration of cortisol in blood (van Cruijsen et al., 2005), which could contribute to Ménière's symptoms but could also be a secondary marker of stress perceived by the patients.
Recently, local HPA-equivalent signaling system was discovered in the cochlea of mice (Graham et al., 2010; Graham and Vetter, 2011). This local HPA system is independent of the systemic HPA signaling. It consists of locally produced corticotropin-releasing factor (CRF), CRF1-receptor and ACTH. Interestingly, deletion of CRF1-receptor gene resulted in auditory impairment of knock-out animals (Graham and Vetter, 2011). This impairment was accompanied by reduced expression of glutamine synthetase together with abnormal innervation features and was attributed to the developmental role that CRFR1 potentially plays in the inner ear. Future experiments should determine the connection between cochlear and systemic HPA systems.
Stress and the HPA Axis-Induced Neuronal Plasticity
Both acute and chronic stress were demonstrated to influence the glutamate neurotransmission and in this way contribute to the neuronal plasticity (Krugers et al., 2010; Popoli et al., 2011). Induction of neuronal plasticity was shown to be possible by generation of changes on a pre-synaptic (synthesis, transport, release) and/or post-synaptic level (glutamate recycling, binding, and signaling via glutamate receptors).
Glutamate is an abandoned neurotransmitter in CNS and is involved in the process of memory, learning, and also in the auditory processing. Special feature of glutamate circuits is their involvement in the process of plasticity, for the reason that glutamate and glutamate receptors NMDAR (N-methyl-D-aspartate receptor) and AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) regulate the strength and function of neuronal synapses. To date, mechanisms determined as responsible for the synaptic plasticity are glutamate receptors (NMDAR)—related long-term potentiation (LTP) and long-term depression (LTD). In addition, changes in AMPAR composition and density on the synapses were shown to be essential for the plasticity process.
Pre-synaptic neuronal plasticity can be mediated by changes in glutamate transport. Predominant type of glutamate transporter present in the organ of Corti (in the supporting cells) is GLAST/EAAT1 (Ruel et al., 2007). Upregulation of GLAST/EAAT1 was demonstrated in astrocytes of animals subjected to chronic physical stress (Madrigal et al., 2003). However, the influence of stress or glucocorticoids on cochlear GLAST/EAAT1 is still unknown.
Post-synaptic neuronal plasticity can be induced by changes in the expression and trafficking of glutamate receptors AMPAR. AMPAR are multimers composed of various subunits, quantity, and ratio of which influences the synaptic strength. The mechanism that mediates HPA-induced changes in AMPA receptors trafficking is attributed to the genomic and non-genomic effects of glucocorticoids (Figure 4). In the prefrontal cortex, stress has been demonstrated to activate in a non-genomic way the glucocorticoid-inducible kinase SGK (Popoli et al., 2011). SGK1 is also expressed in stria vascularis, spiral ligament, spiral limbus, organ of Corti, Reissner's membrane and in the spiral ganglion of rats (Zhong and Liu, 2009) but role of SGK1 in the inner ear has not yet been experimentally addressed. The rapid, non-genomic effects of stress are attributed to the presence of mineralcorticoid receptor (Karst et al., 2005; Groeneweg et al., 2012), which is also expressed in the cochlea (Furuta et al., 1994; Yao and Rarey, 1996).
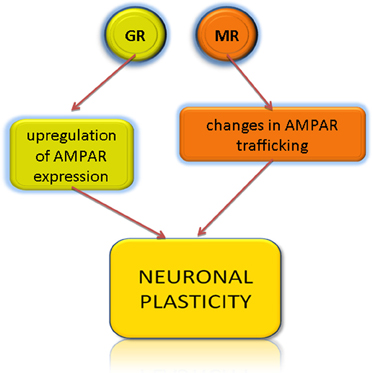
Figure 4. Glucocorticoids induce neuronal plasticity via respective receptors. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GR, glucocorticoid receptor; MR, mineralcorticoid receptor.
An example of slow, genomic effects of glucocorticoids is the cortisol/corticosterone-induced increase in transcription and translation of GluA2 AMPA receptor subunit (Krugers et al., 2010). In the auditory system, the expression of GluA2 was demonstrated in the cochlear nucleus and in the medial nucleus of the trapezoid body (Hermida et al., 2010; Wang et al., 2011) but the stress-induced changes in its expression were not yet studied.
Taken together, peripheral and central auditory systems express molecules, which are modulated by stress in the limbic, memory, and learning centers of CNS. This modulation is responsible for neuronal plasticity in above areas. If stress and activated HPA axis could induce plastic changes in the auditory pathway via modification of glutamate neurotransmission, remains to be established.
Stress Models
Extended presence of stressor such as inability to escape from a stressing situation (chronic stress) or a high impact stressor (acute stress) may induce allostatic load reflected by pathological reactions or conditions. However, the outcome of allostatic load not only depends on duration and category of stress but also on age, gender and the genetic makeup of stressed organism (Joels and Baram, 2009).
To date, research has concentrated mainly on the stress-induced changes in learning, memory, cognition, and on morphological and molecular modifications in the respective brain structures (Lupien et al., 2007; Joels and Baram, 2009). Majority of stress research requiring information regarding histology, molecular or cell biology has been preformed with use of different animal stress models. The goal of animal stress models is to mimic and study stress experienced by people under certain conditions. Therefore, various physical or psycho-social stressors are used (Figure 1). To the physical stress models belongs between many others the immersion in cold water, restrain, cold-water restrain, electric foot shock, and food deprivation. Psycho-social stress models use as stressors neonatal isolation (isolation of offspring from mother), isolation, crowding (too many animals per cage), predatory (exposure of mice or rats to cat or any substance having its smell), sleep deprivation and sonic stress (harmful or non-harmful to the auditory system) (Quarcoo et al., 2009; Jaggi et al., 2011; Vicario et al., 2012).
Psycho-social stress is quite different in nature than physical stress. In fact, psycho-social stress was shown to induce changes in some areas of brain that were not affected by physical stress (Nakagawa et al., 1981; Iimori et al., 1982). In addition to the type of stress, important is also stress duration. In acute settings, the stressor is used for a short time. In chronic settings, the stressor is applied from 24 h to up to 40 days. Lastly, there are models that mix different types of acute and chronic stress in an unpredictable way (Jaggi et al., 2011).
Taken together, caution needs to be taken when designing, interpreting, and comparing experiments that use various animal stress models.
Stress Models in Auditory Research
In the auditory research, influence of stress on the auditory processing was often studied using physical acute stress. Severe pain (tracheotomy and bladder catheterization performed without general anesthetics) used in guinea pigs as stressors was demonstrated to induce auditory threshold shift, which was later explained by a cochlear hypoxia (Muchnik et al., 1980; Hildesheimer et al., 1985). Sprague-Dawley rats subjected to restraint stress for 10 days, 2 h per day (physical stressor; chronic stress) have developed auditory impairment and significant atrophy of inferior colliculus (Dagnino-Subiabre et al., 2005) and of medial geniculate nucleus (Bose et al., 2010). The mechanisms mediating atrophic degeneration in the auditory pathway have not been fully clarified, but it is apparent that degeneration is auditory tissues-specific, since the visual system (e.g., the superior colliculus adjacent to the inferior colliculus) is not affected by stress. From the perspective of allostatic model, one could postulate that chronic physical stress may induce the allostatic load in auditory pathway.
Stress (e.g., heat or restraint) was found not only to damage but also to protect the hearing (Yoshida et al., 1999; Wang and Liberman, 2002). Physical type of stressor (restraint) in the acute settings (4 h of duration) increased the concentration of corticosterone in blood and as a consequence, protected the animals form the noise-induced trauma. Similar protective effects had the administration of glucocorticoid-based drugs prior to acoustic trauma (reviewed in Meltser and Canlon, 2011). From the perspective of allostatic model, one could hypothesize that the acute physical stress is unable to induce allostatic load in auditory pathway. On the contrary, activation of HPA axis resulting in the production of corticosteroids protects the auditory system against the noise trauma. This endogenous corticosteroid protection is comparable to the application of synthetic corticosteroids, used in therapy of acute hearing loss (Meltser and Canlon, 2011).
Under special circumstances, the administration of corticosteroids can also have adverse effects on the auditory system. Prenatal, long-term administration of glucocorticoids, increased the susceptibility of the of the Sprague-Dawley offspring rats to noise trauma (Canlon et al., 2003). However, these findings could not be reproduced by other group in Wistar rats, possibly reflecting inter-strain genetic differences in stress and corticosteroid susceptibility (Hougaard et al., 2007).
In addition to physical stress, psycho-social stress model was also used in the auditory research. Wistar rats subjected to 24 h of stress (non-harmful sonic stress: sound pressure level 61–65 dB, sound frequency 300 Hz, 1 s sound in intervals of 15 s) have developed temporary but significant reduction of the ABR thresholds in all frequencies tested and of the DPOAE thresholds in low frequencies, consistent with auditory hypersensitivity (Mazurek et al., 2010). This implies that chronic, psycho-social stress may influence the function of auditory pathway. Long-term consequences of such influence remain to be determined.
Stress and Tinnitus
There is a bulk of evidence supporting the view that tinnitus induces stress in patients. However, little is known about the other side of this interaction—that is about stress inducing tinnitus. It has been a frequent observation made by otologists and audiologists that many tinnitus patients complain of psycho-social distress prior to or during the onset and progression of tinnitus. One of the earliest published observations connecting the onset of tinnitus with psycho-social distress was made by John Harrison Curtis, the surgeon of the Royal Dispensary for Diseases of the Ear in London (the first hospital in England offering specialized care for ear diseases, est. 1817). Dr. Curtis has noticed that in two of five cases, affected patients attributed the beginning of tinnitus to a psycho-social strain caused by death in immediate family (Curtis, 1841).
Hundred and seventy years later, two large-scale studies provided epidemiological information about the association of psycho-social stress with tinnitus (Baigi et al., 2011; Hasson et al., 2011). The first study demonstrated that the probability of developing tinnitus is approximately the same for highly stressed persons as it is for persons exposed to occupational noise (Baigi et al., 2011). Importantly, the authors also have noticed that psycho-social stress contributes to worsening of tinnitus symptoms. Interestingly, exposure to high level of stress and occupational noise doubles the probability of developing tinnitus. In the second study, a self-completion questionnaire was used to inquire about work- and health-related stressors and hearing problems, such as tinnitus. About one-third of working population reported hearing problems or tinnitus or both. In addition, prevalence of sleeping problems was significantly higher in subjects with tinnitus, than in the tinnitus-free subjects. Importantly, the authors found linear association between tinnitus and the magnitude and duration of stress, such as for instance occupational stress (Hasson et al., 2011). Both studies were performed with more than 10,000 subjects each, thus, providing statistical strength. Recently, we also have shown that the patients with disturbing chronic tinnitus have higher scores than patients with non-disturbing tinnitus in the subscales “worries” and “tension” measured by stress-oriented Perceived Stress Questionnaire (Seydel et al., 2010).
Interesting for the issue tinnitus and stress is the HPA axis, which seems to be disturbed in tinnitus patients. Hebert and Lupien have observed that the basal levels of salivary cortisol are chronically elevated in tinnitus patients, who had disturbing tinnitus on average for 5.5 years (Hebert et al., 2004). In addition, patients who had disturbing tinnitus for a longer time (on average 14.7 years) were shown to develop improper HPA responses to an experimental, psycho-social stress (Hebert and Lupien, 2007). HPA axis in tinnitus patients under stress seems to be activated later and to a lesser extend than in the healthy controls, consistent with glucocorticoid inefficiency (Yehuda et al., 1996). Such inefficiency is also found in some stress-related disorders: chronic fatigue syndrome, posttraumatic stress disorder and in burnout syndrome (Kudielka and Wust, 2010; Juster et al., 2011). Interestingly, high comorbidity of tinnitus and posttraumatic stress disorder was observed (Hinton et al., 2006; Fagelson, 2007). It is, however, possible that the observed changes in HPA axis of tinnitus patients may be a result and not a cause of tinnitus.
The link between tinnitus and negative emotional arousal was proposed many years ago by Jastreboff and Hazell to explain why is tinnitus perceived as unpleasant or even dangerous sound (Jastreboff and Hazell, 1993). Recent review of Kraus and Canlon delineates known to date anatomical connectivities between the auditory and limbic systems (reviewed in detail by Kraus and Canlon, 2012). In addition, this important work collected evidence supporting the notion of noise generating synaptic plasticity in the limbic structures. Induction of limbic plasticity is possible due to multi-level projections of the auditory system to the limbic structures. However, known projections of limbic system to the auditory system are rather limited (e.g., projections of amydala to inferior colliculus). If these projections are activated by stress and if this activation could induce plastic changes in the auditory system, remains to be determined.
Scientists in the field currently agree that tinnitus may be triggered by an injury to the inner ear causing decreased activity of the auditory nerve and lastly in plastic changes in central auditory system (Kaltenbach, 2011). Resulting plastic changes in central auditory activity are coupled with altered attention and negative emotions. Can stress induce such critical injury to the inner ear or induce plasticity in higher auditory structures? An important hint was recently delivered by a group studying mild stress model of depression in Sprague-Dawley rat with PET imaging technique. The type of stress was chronic; the stressors were of mixed types and included physical stressors (e.g., sleep deprivation, water deprivation and heat stress) and psycho-social stressors (e.g., crowding, sonic stress). After 4 weeks of random mixed stressing, brain PET analysis revealed the activation of left auditory cortex and deactivation of left inferior colliculus in stressed animals. Changes in the auditory system correlated significantly with the depressive symptoms of experimental animals (Hu et al., 2010). At the same time, no changes were detectible in the visual pathway. Interestingly, activation of left but not right auditory cortex (Brodmann areas 41 and 42) was also reported for tinnitus patients (Arnold et al., 1996; Wang et al., 2001) and could possibly be used as a tinnitus correlate for the experimental animals.
Clinical definition of stress has been improved in the recent years. Importantly, biomarkers and mechanisms are being indentified in a process of stress definition (Piazza et al., 2010). This provides the researchers and clinicians not only with more basic knowledge about stress-induced processes but also with more diagnostic power. Stress is no longer an undefined, sad state of mind but an important, distinct factor in precipitation and amplification of mental and mood disorders (Holsboer and Ising, 2010). Recent research has implicated that living in a city affects stress processing and may be responsible for increased incidence of anxiety disorders in the cities, as opposed to rural communities (Lederbogen et al., 2011). Stress may also precipitate onset of other, non-mental types of diseases, such as asthma or inflammatory bowel disease (Niess et al., 2002; Quarcoo et al., 2009), thus, stressing the role of allostatic load in non-CNS and non-neuronal organs. Including stress as factor of future investigations in the auditory, tinnitus-related research seems to be a logical consequence of above observations.
Furthermore, information collected here, indirectly implies the requirement for psychological assessment during the diagnosis of tinnitus patients, with focus on perceived stress and psychological comorbidity. Such assessment may be performed in form of a self-filled questionnaire or by a clinical psychologist. Psychological intervention with a goal of stress-management strategies appears to be an indispensable element in tinnitus treatment, especially important to use in very early stages of tinnitus, before the chronification of plastic changes has taken place.
Based on the information collected here, following hypothetical models could possibly explain the causative connection of stress and tinnitus:
- First, stress may potentially activate the local HPA axis in the inner ear. The consequences of local overdrive in the HPA system in cochlea are so far unknown.
- Second, stress-activated HPA corticosterone release may affect mineralcorticoid receptor function in cochlea and possibly influence the concentration of potassium secreted by stria vascularis, resulting in tinnitus.
- Third, stress-induced activation of HPA axis and corticosteroid release could provoke pre- or post-synaptic neuronal plasticity of the auditory system (Figure 5).
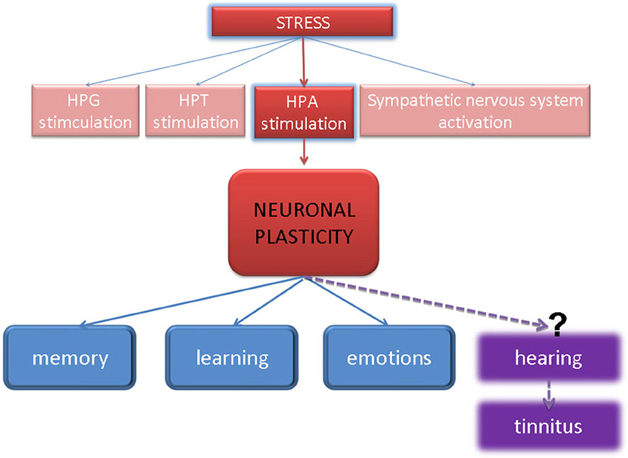
Figure 5. Model of stress-induced neuronal plasticity, which has been accepted in memory, learning, and emotional systems—can it also be truth to explain the induction of auditory pathologies by stress?
Exploring the above models and obtaining a clear-cut animal model, which would combine an appropriate stress type and tinnitus read-out, is a challenging task. In addition, designing and conducting large epidemiological studies, where individuals would be monitored for stress parameters and followed audiometrically for decades, would be an expensive and a time-consuming mission. Nevertheless, step-by step understanding of how and to what degree the psycho-social or physical stress could affect our peripheral and central auditory system should become a goal in the basic and clinical auditory research.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Amsterdam, A., and Sasson, R. (2002). The anti-inflammatory action of glucocorticoids is mediated by cell type specific regulation of apoptosis. Mol. Cell. Endocrinol. 189, 1–9.
Amsterdam, A., Tajima, K., and Sasson, R. (2002). Cell-specific regulation of apoptosis by glucocorticoids: implication to their anti-inflammatory action. Biochem. Pharmacol. 64, 843–850.
Arnold, W., Bartenstein, P., Oestreicher, E., Romer, W., and Schwaiger, M. (1996). Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J. Otorhinolaryngol. Relat. Spec. 58, 195–199.
Baigi, A., Oden, A., Almlid-Larsen, V., Barrenas, M. L., and Holgers, K. M. (2011). Tinnitus in the general population with a focus on noise and stress: a public health study. Ear Hear. 32, 787–789.
Berlucchi, G., and Buchtel, H. A. (2009). Neuronal plasticity: historical roots and evolution of meaning. Exp. Brain Res. 192, 307–319.
Bose, M., Munoz-Llancao, P., Roychowdhury, S., Nichols, J. A., Jakkamsetti, V., Porter, B., Byrapureddy, R., Salgado, H., Kilgard, M. P., Aboitiz, F., Dagnino-Subiabre, A., and Atzori, M. (2010). Effect of the environment on the dendritic morphology of the rat auditory cortex. Synapse 64, 97–110.
Calabrese, F., Molteni, R., Racagni, G., and Riva, M. A. (2009). Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology 34(Suppl. 1), S208–S216.
Canlon, B., Erichsen, S., Nemlander, E., Chen, M., Hossain, A., Celsi, G., and Ceccatelli, S. (2003). Alterations in the intrauterine environment by glucocorticoids modifies the developmental programme of the auditory system. Eur. J. Neurosci. 17, 2035–2041.
Dagnino-Subiabre, A., Terreros, G., Carmona-Fontaine, C., Zepeda, R., Orellana, J. A., Diaz-Veliz, G., Mora, S., and Aboitiz, F. (2005). Chronic stress impairs acoustic conditioning more than visual conditioning in rats: morphological and behavioural evidence. Neuroscience 135, 1067–1074.
Datson, N. A., Morsink, M. C., Meijer, O. C., and de Kloet, E. R. (2008). Central corticosteroid actions: search for gene targets. Eur. J. Pharmacol. 583, 272–289.
de Kloet, E. R., Joels, M., and Holsboer, F. (2005). Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475.
Fagelson, M. A. (2007). The association between tinnitus and posttraumatic stress disorder. Am. J. Audiol. 16, 107–117.
Funder, J. W. (1997). Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Annu. Rev. Med. 48, 231–240.
Furuta, H., Mori, N., Sato, C., Hoshikawa, H., Sakai, S., Iwakura, S., and Doi, K. (1994). Mineralocorticoid type I receptor in the rat cochlea: mRNA identification by polymerase chain reaction (PCR) and in situ hybridization. Hear. Res. 78, 175–180.
Graham, C. E., Basappa, J., and Vetter, D. E. (2010). A corticotropin-releasing factor system expressed in the cochlea modulates hearing sensitivity and protects against noise-induced hearing loss. Neurobiol. Dis. 38, 246–258.
Graham, C. E., and Vetter, D. E. (2011). The mouse cochlea expresses a local hypothalamic-pituitary-adre-nal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity. J. Neurosci. 31, 1267–1278.
Groeneweg, F. L., Karst, H., de Kloet, E. R., and Joels, M. (2012). Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol. Cell. Endocrinol. 350, 299–309.
Hasson, D., Theorell, T., Wallen, M. B., Leineweber, C., and Canlon, B. (2011). Stress and prevalence of hearing problems in the Swedish working population. BMC Public Health 11, 130.
Hebert, S., and Lupien, S. J. (2007). The sound of stress: blunted cortisol reactivity to psychosocial stress in tinnitus sufferers. Neurosci. Lett. 411, 138–142.
Hebert, S., Paiement, P., and Lupien, S. J. (2004). A physiological correlate for the intolerance to both internal and external sounds. Hear. Res. 190, 1–9.
Hermida, D., Mateos, J. M., Elezgarai, I., Puente, N., Bilbao, A., Bueno-Lopez, J. L., Streit, P., and Grandes, P. (2010). Spatial compartmentalization of AMPA glutamate receptor subunits at the calyx of held synapse. J. Comp. Neurol. 518, 163–174.
Hildesheimer, M., Muchnik, C., Rubinstein, M., and Molho, M. (1985). Basic metabolic rate in emotional stress: its potential influence on cochlear function. Laryngoscope 95, 63–66.
Hinton, D. E., Chhean, D., Pich, V., Hofmann, S. G., and Barlow, D. H. (2006). Tinnitus among Cambodian refugees: relationship to PTSD severity. J. Trauma. Stress 19, 541–546.
Holsboer, F., and Ising, M. (2010). Stress hormone regulation: biological role and translation into therapy. Annu. Rev. Psychol. 61, 81–109.
Hougaard, K. S., Barrenas, M. L., Kristiansen, G. B., and Lund, S. P. (2007). No evidence for enhanced noise induced hearing loss after prenatal stress or dexamethasone. Neurotoxicol. Teratol. 29, 613–621.
Hu, H., Su, L., Xu, Y. Q., Zhang, H., and Wang, L. W. (2010). Behavioral and [F-18] fluorodeoxyglucose micro positron emission tomography imaging study in a rat chronic mild stress model of depression. Neuroscience 169, 171–181.
Iimori, K., Tanaka, M., Kohno, Y., Ida, Y., Nakagawa, R., Hoaki, Y., Tsuda, A., and Nagasaki, N. (1982). Psychological stress enhances noradrenaline turnover in specific brain regions in rats. Pharmacol. Biochem. Behav. 16, 637–640.
Jaggi, A. S., Bhatia, N., Kumar, N., Singh, N., Anand, P., and Dhawan, R. (2011). A review on animal models for screening potential anti-stress agents. Neurol. Sci. 32, 993–1005.
Jastreboff, P. J., and Hazell, J. W. (1993). A neurophysiological approach to tinnitus: clinical implications. Br. J. Audiol. 27, 7–17.
Juster, R. P., Sindi, S., Marin, M. F., Perna, A., Hashemi, A., Pruessner, J. C., and Lupien, S. J. (2011). A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology 36, 797–805.
Karst, H., Berger, S., Turiault, M., Tronche, F., Schutz, G., and Joels, M. (2005). Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl. Acad. Sci. U.S.A. 102, 19204–19207.
Kraus, K. S., and Canlon, B. (2012). Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear. Res. doi: 10.1016/j.heares.2012.02.009. [Epub ahead of print].
Krugers, H. J., Hoogenraad, C. C., and Groc, L. (2010). Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat. Rev. Neurosci. 11, 675–681.
Kudielka, B. M., Schommer, N. C., Hellhammer, D. H., and Kirschbaum, C. (2004). Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 29, 983–992.
Kudielka, B. M., and Wust, S. (2010). Human models in acute and chronic stress: assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress 13, 1–14.
Lanting, C. P., de Kleine, E., and van Dijk, P. (2009). Neural activity underlying tinnitus generation: results from PET and fMRI. Hear. Res. 255, 1–13.
Lederbogen, F., Kirsch, P., Haddad, L., Streit, F., Tost, H., Schuch, P., Wust, S., Pruessner, J. C., Rietschel, M., Deuschle, M., and Meyer-Lindenberg, A. (2011). City living and urban upbringing affect neural social stress processing in humans. Nature 474, 498–501.
Lupien, S. J., Maheu, F., Tu, M., Fiocco, A., and Schramek, T. E. (2007). The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 65, 209–237.
Lupien, S. J., McEwen, B. S., Gunnar, M. R., and Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445.
Madrigal, J. L., Caso, J. R., de Cristóbal, J., Cardenas, A., Leza, J. C., Lizasoain, I., Lorenzo, P., and Moro, M. A. (2003). Effect of subacute and chronic immobilisation stress on the outcome of permanent focal cerebral ischaemia in rats. Brain Res. 979, 137–145.
Mateijsen, D. J., Kingma, C. M., de Jong, P. E., Wit, H. P., and Albers, F. W. (2001). Aldosterone assessment in patients with Meniere's disease. ORL J. Otorhinolaryngol. Relat. Spec. 63, 280–286.
Mazurek, B., Haupt, H., Joachim, R., Klapp, B. F., Stover, T., and Szczepek, A. J. (2010). Stress induces transient auditory hypersensitivity in rats. Hear. Res. 259, 55–63.
McEwen, B. S., and Gianaros, P. J. (2011). Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 62, 431–445.
McEwen, B. S., and Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15.
Mebis, L., and van den Berghe, G. (2009). The hypothalamus-pituitary-thyroid axis in critical illness. Neth. J. Med. 67, 332–340.
Meltser, I., and Canlon, B. (2011). Protecting the auditory system with glucocorticoids. Hear. Res. 281, 47–55.
Muchnik, C., Hildesheimer, M., and Rubinstein, M. (1980). Effect of emotional stress on hearing. Arch. Otorhinolaryngol. 228, 295–298.
Nakagawa, R., Tanaka, M., Kohno, Y., Noda, Y., and Nagasaki, N. (1981). Regional responses of rat brain noradrenergic neurones to acute intense stress. Pharmacol. Biochem. Behav. 14, 729–732.
Nava, E., and Roder, B. (2011). Adaptation and maladaptation insights from brain plasticity. Prog. Brain Res. 191, 177–194.
Niess, J. H., Monnikes, H., Dignass, A. U., Klapp, B. F., and Arck, P. C. (2002). Review on the influence of stress on immune mediators, neuropeptides and hormones with relevance for inflammatory bowel disease. Digestion 65, 131–140.
Piazza, J. R., Almeida, D. M., Dmitrieva, N. O., and Klein, L. C. (2010). Frontiers in the use of biomarkers of health in research on stress and aging. J. Gerontol. B Psychol. Sci. Soc. Sci. 65, 513–525.
Popoli, M., Yan, Z., McEwen, B. S., and Sanacora, G. (2011). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37.
Quarcoo, D., Pavlovic, S., and Joachim, R. A. (2009). Stress and airway reactivity in a murine model of allergic airway inflammation. Neuroimmunomodulation 16, 318–324.
Ruel, J., Wang, J., Rebillard, G., Eybalin, M., Lloyd, R., Pujol, R., and Puel, J. L. (2007). Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear. Res. 227, 19–27.
Seydel, C., Haupt, H., Szczepek, A. J., Klapp, B. F., and Mazurek, B. (2010). Long-term improvement in tinnitus after modified tinnitus retraining therapy enhanced by a variety of psychological approaches. Audiol. Neurootol. 15, 69–80.
Thomas, W., and Harvey, B. J. (2011). Mechanisms underlying rapid aldosterone effects in the kidney. Annu. Rev. Physiol. 73, 335–357.
Tsigos, C., and Chrousos, G. P. (2002). Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 53, 865–871.
Ulrich-Lai, Y. M., and Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409.
van Cruijsen, N., Dullaart, R. P., Wit, H. P., and Albers, F. W. (2005). Analysis of cortisol and other stress-related hormones in patients with Meniere's disease. Otol. Neurotol. 26, 1214–1219.
Vicario, M., Alonso, C., Guilarte, M., Serra, J., Martinez, C., Gonzalez-Castro, A. M., Lobo, B., Antolin, M., Andreu, A. L., Garcia-Arumi, E., Casellas, M., Saperas, E., Malagelada, J. R., Azpiroz, F., and Santos, J. (2012). Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology 37, 65–77.
Wang, H., Tian, J., Yin, D., Jiang, S., Yang, W., Han, D., Yao, S., and Shao, M. (2001). Regional glucose metabolic increases in left auditory cortex in tinnitus patients: a preliminary study with positron emission tomography. Chin. Med. J. (Engl.) 114, 848–851.
Wang, H., Yin, G., Rogers, K., Miralles, C., de Blas, A. L., and Rubio, M. E. (2011). Monaural conductive hearing loss alters the expression of the GluA3 AMPA and glycine receptor alpha1 subunits in bushy and fusiform cells of the cochlear nucleus. Neuroscience 199, 438–451.
Wang, Y., and Liberman, M. C. (2002). Restraint stress and protection from acoustic injury in mice. Hear. Res. 165, 96–102.
Weitzman, E. D., Fukushima, D., Nogeire, C., Roffwarg, H., Gallagher, T. F., and Hellman, L. (1971). Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J. Clin. Endocrinol. Metab. 33, 14–22.
Whirledge, S., and Cidlowski, J. A. (2010). Glucocorticoids, stress, and fertility. Minerva Endocrinol. 35, 109–125.
Yao, X., and Rarey, K. E. (1996). Localization of the mineralocorticoid receptor in rat cochlear tissue. Acta Otolaryngol. 116, 493–496.
Yehuda, R., Levengood, R. A., Schmeidler, J., Wilson, S., Guo, L. S., and Gerber, D. (1996). Increased pituitary activation following metyrapone administration in post-traumatic stress disorder. Psychoneuroendocrinology 21, 1–16.
Yoshida, N., Kristiansen, A., and Liberman, M. C. (1999). Heat stress and protection from permanent acoustic injury in mice. J. Neurosci. 19, 10116–10124.
Zhong, S. X., and Liu, Z. H. (2009). Expression patterns of Nedd4 isoforms and SGK1 in the rat cochlea. Acta Otolaryngol. 129, 935–939.
Keywords: tinnitus, HPA axis, emotional stress, neurotransmitter, corticosteroid, gene expression
Citation: Mazurek B, Haupt H, Olze H and Szczepek AJ (2012) Stress and tinnitus—from bedside to bench and back. Front. Syst. Neurosci. 6:47. doi: 10.3389/fnsys.2012.00047
Received: 19 January 2012; Accepted: 26 May 2012;
Published online: 11 June 2012.
Edited by:
Jos J. Eggermont, University of Calagry, CanadaReviewed by:
Sylvie Hébert, Université de Montréal, CanadaDan Hasson, Karolinska Institutet, Sweden
Copyright: © 2012 Mazurek, Haupt, Olze and Szczepek. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Birgit Mazurek, Tinnitus Center, Charité - Universitätsmedizin Berlin, Charitéplatz 1, D-10117 Berlin, Germany. e-mail:YmlyZ2l0Lm1henVyZWtAY2hhcml0ZS5kZQ==
Agnieszka J. Szczepek, Molecular Biology Research Laboratory, Charité - Universitätsmedizin Berlin, Charitéplatz 1, D-10117 Berlin, Germany. e-mail:YWduZXMuc3pjemVwZWtAY2hhcml0ZS5kZQ==

 Heidemarie Haupt1
Heidemarie Haupt1