- 1Department of Pharmacology, Tohoku University Graduate School of Medicine, Sendai, Japan
- 2Cyclotron and Radioisotope Center, Tohoku University, Sendai, Japan
- 3Department of Psychiatry, Tohoku University Graduate School of Medicine, Sendai, Japan
Molecular imaging in neuroscience is a new research field that enables visualization of the impact of molecular events on brain structure and function in humans. While magnetic resonance-based imaging techniques can provide complex information at the level of system, positron emission tomography (PET) enables determination of the distribution and density of receptor and enzyme in the human brain. Previous studies using [11C]raclopride and [11C]FLB457 revealed that the release of neuronal dopamine was increased in human brain by psychostimulants or reward stimuli. Following on from these previous [11C]raclopride studies, we examined whether the levels of neuronal release of histamine might change [11C]doxepin binding to the H1 receptors under the influence of physiological stimuli. The purpose of the present study was to evaluate the test–retest reliability of quantitative measurement of [11C]doxepin binding between morning and afternoon and between resting and attentive waking conditions in healthy human subjects. There was a trend for a decrease in [11C]doxepin binding during attentive calculation tasks compared with that in resting conditions, but the difference (less than 10%) was not significant. Similarly, the binding potential of [11C]doxepin in the cerebral cortex was slightly higher in the morning than that in the afternoon, but it was also insignificant. These data suggest that higher histamine release during wakefulness could not decrease the [11C]doxepin binding in the brain. This study confirmed the reproducibility and reliability of [11C]doxepin in the previous imaging studies to measure the H1 receptor.
Introduction
Histamine is a transmitter in the nervous system and a signaling molecule in the gut, the skin, and the immune system. Histamine neurons in mammalian brain are located exclusively in the tuberomammillary nucleus of the posterior hypothalamus and their axons extend throughout the central nervous system (CNS). Four known histamine receptors and histamine binding to glutamate NMDA receptors serve multiple functions in the brain, particularly control of excitability and plasticity (Haas and Panula, 2003; Haas et al., 2008). The H1 and H2 receptor-mediated actions are mostly excitatory, while H3 receptors act as inhibitory auto- and heteroreceptors. Histamine neurons are proposed to have a dual effect on the CNS, with both stimulatory and suppressive actions (Watanabe and Yanai, 2001). As a stimulator, neuronal histamine is one of the most important systems that stimulate and maintain attentive wakefulness. Brain histamine also functions in bioprotection as a suppressor of various noxious and unfavorable stimuli of convulsion, drug sensitization, denervation supersensitivity, ischemic lesions, and stress susceptibility. We have examined the functions of histamine neurons using various approaches, such as histamine-related gene knockout mice and human positron emission tomography (PET) (Yanai and Tashiro, 2007).
Histamine neurons play an important role in the forebrain waking systems (Lin, 2000; Eriksson et al., 2001; Huang et al., 2001). Their neuronal activity is specific for a high-vigilance waking state and histamine neurons might play a role not in the initiation of wakefulness, but in maintenance of the high level of vigilance necessary for cognitive processes (Takahashi et al., 2008; Sakai et al., 2010). An increase in histaminergic transmission promotes wakefulness, whereas its blockade by sedating antihistamines causes somnolence and impaired performance in humans (Yanai et al., 2011). Several lines of evidence suggest that histamine modulates circadian rhythms (Tuomisto et al., 2001; Abe et al., 2004) and it has even been suggested to play a pivotal role in circadian entrainment (Jacobs et al., 2000). Accordingly, a clear circadian rhythm of histamine release was demonstrated in the anterior hypothalamus by in vivo microdialysis studies (Mochizuki et al., 1992). Histamine release increased before the active phase and was maintained at an elevated level during the active phase.
Stress in our daily lives has been associated with various psychiatric disorders including depression, schizophrenia, cognitive disorders, and psychosomatic diseases such as anorexia nervosa and irritable bowel syndrome. To date, we have conducted several PET studies to elucidate the pathophysiological mechanism behind the above-mentioned disorders, focusing on alteration in neural transmission of the histaminergic neuron systems (Yanai and Tashiro, 2007). For PET studies, [11C]doxepin, a potent antagonist of histamine H1 receptors, has been utilized as an effective PET imaging tracer. Using [11C]doxepin, increasing evidence has been accumulated regarding the role of the histaminergic neuron system in the pathophysiology of stress-related neuropsychiatric disorders. For example, histamine H1 receptor binding was measured using [11C]doxepin in patients with schizophrenia (Iwabuchi et al., 2005), major depression (Kano et al., 2004) and anorexia nervosa (Yoshizawa et al., 2009). Another application of [11C]doxepin-PET is the measurement of histamine H1 receptor occupancy by antihistamines. The inhibition of histaminergic activity at the brain H1 receptor level may have some favorable anxiolytic effects, however, more often this is accompanied by unfavorable effects like increased daytime somnolence, impaired memory and learning, decreased attention, and weight gain. The brain H1 receptor occupancy measured by [11C]doxepin-PET is one of the most reliable and objective methods to estimate the sedating properties of antihistamines (Yanai et al., 2011, 2012). A critical step in validating [11C]doxepin to measure the brain H1 receptor occupancy is to evaluate the reproducibility of its binding potential (Bmax/KD) in the different attentive conditions. Clinically-used PET probes should be examined on the test–retest reproducibility for the in vivo binding (Kim et al., 2006; Yasuno et al., 2007; Edison et al., 2009; Narendran et al., 2011), although [11C]doxepin binding to brain has not been examined on this aspect until now.
Previous studies have shown that imaging with PET radiotracers that are specific for brain receptors can be used to visualize changes in the release of neurotransmitters indirectly. Most successful studies have focused on dopamine, since the dopamine neurons that project to the striatum have been shown to play a critical role in mediating motivational and addictive behaviors. These imaging studies successfully measured increased extracellular dopamine released by psychostimulants and physiological reward stimuli in humans with [11C]raclopride (Koepp et al., 1998; Rinne, 2003; Hommer et al., 2011) and [11C]FLB 457 (Narendran et al., 2011). However, some technical difficulties have been encountered for the imaging of changes in the release of other neurotransmitters. Among these are low sensitivity, changes of neurotransmitter release pulse over time during PET imaging and the issue of affinity states, the contribution of carryover mass in the second PET scan, and internalization of receptors (Boecker et al., 2008). Followed by the previous PET measurement of dopamine release, we examined whether neuronal histamine released as a result of mental stress and the circadian rhythm might change the levels of H1 receptors measured in vivo by PET and [11C]doxepin. Therefore, we undertook the present study for the purpose of evaluating the test–retest reliability of [11C]doxepin binding in the human brain during different attentive conditions and circadian rhythm in healthy human subjects.
Methods
Subjects and Study Design
Japanese male volunteers, who were physically and mentally healthy and had no history of allergy or long-term treatment with H1 antagonists, were recruited to participate in this study. They showed no abnormality in brain magnetic resonance imaging (MRI). All subjects gave written informed consent for all study procedures before participation. Concomitant medications, nicotine, caffeine, grapefruit or grapefruit juice, and alcohol were not allowed during the experimental period. The present study was approved by the Committee on Clinical Investigation at Tohoku University Graduate School of Medicine, Japan, and was performed in accordance with the principles of the Declaration of Helsinki. All experiments were performed at the Cyclotron and Radioisotope Center, Tohoku University.
In the first part of this study involving test–retest measurements, six healthy male volunteers (mean age ± SD: 24.6 ± 2.1 years old) were examined twice with [11C]doxepin-PET during a resting condition in the morning (11:00 a.m.) and afternoon (3:00 p.m.) of the same day in order to evaluate the circadian rhythm of H1 receptor binding. In the second part of this study involving investigation of ligand activation, 10 healthy men (22.3 ± 1.0 years old) were examined during resting and attentive waking conditions. They were scanned twice by PET (SET2400W; Shimadzu Co., Kyoto, Japan) on the same day during attentive calculation tasks involving two-digit addition and during resting conditions with their eyes closed after administration of [11C]doxepin. The task protocols are shown in Figure 1. The order of resting and calculation conditions was randomized. We performed measurements of subjective feelings five times during PET scans before the scan (pre), at interval 1 (int1), interval 2 (int2), interval 3 (int3), and at the end of scan (end). Subjective feelings including alertness, tiredness, and sleepiness were measured during PET scans using Line Analog Rating Scale (LARS). In LARS measurement, subjects mark a series of 100 mm linear analog scales (+50 to −50 mm), indicating their present feeling with regard to a midpoint, which represents their normal state of mind.
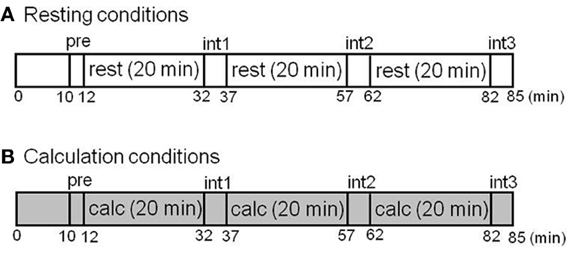
Figure 1. Study protocols of test–retest measurements in the different conditions. (A) Resting condition. (B) Calculation condition. The H1 receptors were examined with [11C]doxepin-PET during the resting and calculation conditions. The subjective feelings during PET scans were also measured during the 90 min before the scan (pre), interval 1 (int1), interval 2 (int2), interval 3 (int3), and at the end of the scan (end).
PET Image Acquisition and Data Analysis
[11C]Doxepin was synthesized by 11C-methylation of desmethyl-doxepin with [11C]methyl triflate as described previously. The radiochemical purity of [11C]doxepin was greater than 99%, and its specific radioactivity at the time of injection was 207.5 ± 61.9 GBq/μmol (5608 ± 1673 mCi/μmol). The single injected dose and cold mass of [11C]doxepin were 119.8 ± 10.5 MBq (3.23 ± 0.283 mCi) and 0.577 ± 0.051 nmol, respectively.
For the measurement of H1 receptors, PET scans were carried out with an SET2400W PET scanner (Shimadzu Co., Kyoto, Japan). PET data were acquired 30 s after the administration of [11C]doxepin with the subject's eyes closed for 90 min.
In order to calculate the binding potential (BP) of H1 receptors, brain PET images of each subject during resting and calculation conditions were subjected to inter-frame motion correction and then co-registered to an identical stereotaxic brain coordinate using a corresponding T1-weighted MRI image. MRI images were obtained with a 1.5-T MR scanner (HiSpeed, ver. 9.1; General Electric Inc., WI, USA). Regions of interest (ROI) were first placed on the following brain regions on the T1 images for which precise anatomical information was available: anterior and posterior cingulate gyrus, inferior prefrontal cortex, superior prefrontal cortex, temporal cortex, and cerebellum. ROI was defined for each cortical region by 3–5 concentric circles with a diameter of 5.0 mm for each hemisphere in 4–5 consecutive brain transaxial slices. An averaged value from all ROIs was used as a representative value of each region. In addition, we produced a time-activity curve (TAC) of each region from ROI data. The TACs were obtained by applying the ROIs to the dynamic PET images. A standardized uptake value (SUV) was calculated for the normalization of ROI-TACs as follows:
Subsequently, Logan graphical analysis with the reference tissue input (LGAR) method was applied to calculate BP using PMOD kinetic modeling tool (PKIN) software (PMOD Technologies Ltd., Zurich, Switzerland) and TAC (Suzuki et al., 2005), and we compared the BP between different conditions (resting vs. calculation; morning vs. afternoon). All data were analyzed by a repeated measure of ANOVA followed by multiple comparisons (Tukey–Kramer test, Scheffe's F test, and Bonferroni–Dunn test), and P < 0.05 was considered statistically significant.
Results
During the performance of the attentive task involving two-digit calculation, the percentage of correct answers was greater than 90%. Subjects felt significantly more tired during the calculation task than in the resting condition (Figure 2A). Subjects in the resting condition tended to have significantly higher sleepiness scores than those in the calculation condition (Figure 2B). These data suggest that there was a significant difference in the attention level between the resting and calculation conditions.
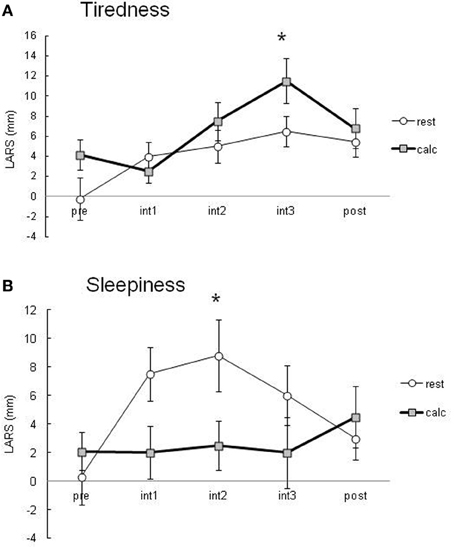
Figure 2. Subjective tiredness (A) and sleepiness (B) evaluated using LARS during PET scans. Data are presented as the means ± SEM of LARS (mm) from 10 healthy subjects in the resting and calculation conditions. All data were analyzed by a repeated measure of ANOVA followed by multiple comparisons and P < 0.05 was considered statistically significant.
Six subjects were tested in the evaluation of the test–retest reliability of [11C]doxepin PET. Given concerns about the possibility that [11C]doxepin binding changes over days and week, the test and re-test trials were performed in the morning (11:00 a.m.) and afternoon (3:00 p.m.) of the same day. As shown in Figure 3A, average SUVs over time from test–retest scans show that the tracer gradually entered the brain and the brain activity remained almost stable. The radioactivity in the anterior cingulate gyrus showed a slightly longer elimination phase in the test trial performed in the morning than that in the afternoon, but the difference was insignificant. The radioactivity in the cerebellum with negligible H1 receptor binding was essentially the same between the test and re-test trials. There was an apparent trend for decreased BP in the afternoon, but the BP values in the brain regions including anterior cingulate gyrus were not significantly higher in the morning test trial (Figure 3B), demonstrating that [11C]doxepin binding in the brain is essentially the same between in the morning and afternoon.
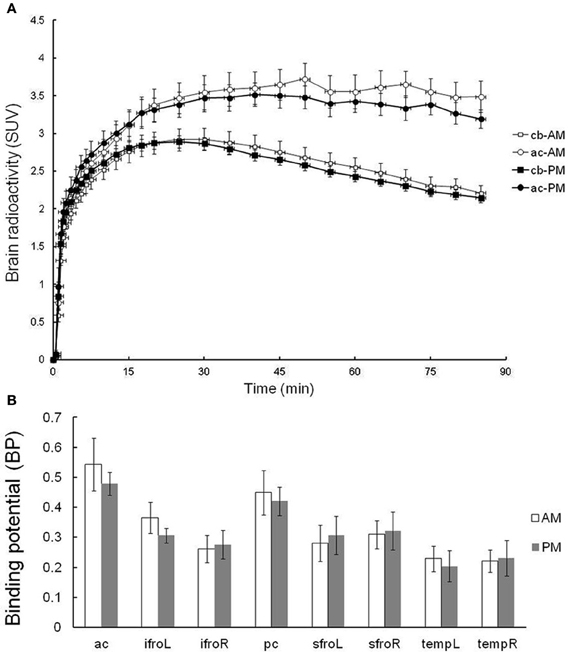
Figure 3. Test–retest trial. (A) TACs in terms of SUVs for the regions of anterior cingulate gyrus (ac) and cerebellum (cb) of test–retest scans. Test–retest scans were performed in the morning and afternoon of the same day, demonstrating a gradual initial increase in SUV followed by a longer elimination phase. Data are expressed as the means ± SEM of six young healthy male volunteers. (B) The binding potential (BP) in the test–retest trial. Abbreviations: ac, anterior cingulate gyrus; ifroL, left inferior prefrontal cortex; ifroR, right inferior prefrontal cortex; pc, posterior cingulate gyrus; sfroL, left superior prefrontal cortex; sfroR, right superior prefrontal cortex; tempL, left temporal cortex; tempR, right temporal cortex.
In order to verify the effects of attentive waking on in vivo PET measurements of H1 receptor binding, [11C]doxepin binding was examined twice in the brains of 10 normal volunteers during resting and attentive waking conditions on the same day, as shown in Figure 4. The order of rest and calculation trials of PET studies was randomized to eliminate the effects of circadian rhythm on H1 receptor binding observed in previous experiments. The time courses in the regions of anterior cingulate gyrus (H1 receptor rich region) and cerebellum (H1 receptor null region) were not significantly different between the resting and attentive calculation conditions (Figure 4A). There was a trend for decreased BP during the calculation task compared with that in the resting condition, but the difference was not significant. These data suggest that [11C]doxepin binding in the brain is not significantly influenced by the performance of a calculation task as an example of an attentive waking condition.
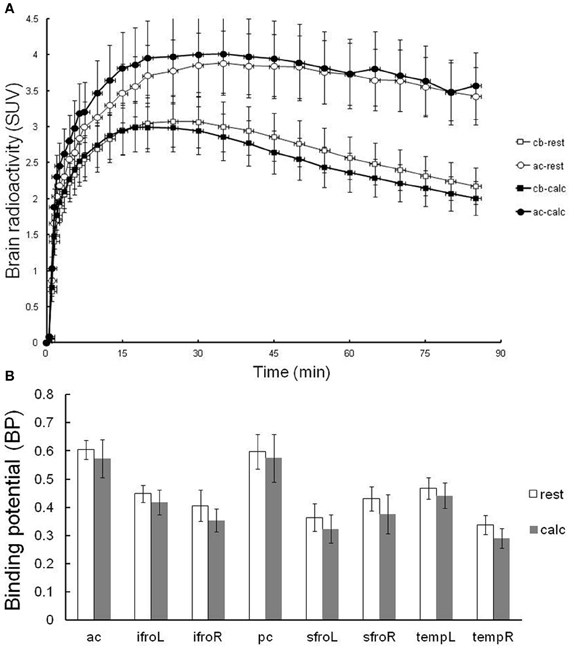
Figure 4. Resting-attentive calculation trial. (A) TACs in terms of SUVs for the regions of anterior cingulate gyrus (ac) and cerebellum (cb) of resting and attentive waking conditions. Data are expressed as the means ± SEM of 10 young healthy male volunteers. (B) The binding potential (BP) in the resting and attentive conditions. Abbreviations: ac, anterior cingulate gyrus; ifroL, left inferior prefrontal cortex; ifroR, right inferior prefrontal cortex; pc, posterior cingulate gyrus; sfroL, left superior prefrontal cortex; sfroR, right superior prefrontal cortex; tempL, left temporal cortex; tempR, right temporal cortex.
Discussion
The main objectives of this study were to analyze the specific brain uptake and kinetics of [11C]doxepin in normal volunteers under different conditions, and to assess the test–retest reliability of quantitative PET measurements between morning and afternoon and between resting and attentive waking conditions. The test–retest reliability for estimated BP was found to be sufficiently high to afford reasonable precision in the tracer binding determinations of H1 receptors. Attentive waking and circadian rhythm might have some influences on the BP of H1 receptors, but only within the range of 10% over all regions. The low variability within 10% in the test–retest studies can be compatible to other PET tracers such as [11C]DASB (serotonin transporter), [18F]SPA-RQ (NK-1 receptor), [11C]PIB (amyloid Aβ), and [11C]FLB457 (D2/3 receptor) (Kim et al., 2006; Yasuno et al., 2007; Edison et al., 2009; Narendran et al., 2011).
Evidence from animal studies has implicated the histaminergic neuron system in the pathophysiology of stress-related disorders. Although several antidepressants and atypical antipsychotics are potent H1R antagonists, the significance of their interaction with H1R in a clinical context of efficacy is still unclear. In previous PET studies, significant reduction in H1 receptor binding was observed in patients with schizophrenia and major depression. It is suggested that prolonged massive histamine release due to repeated stress might lead to the down-regulation and/or internalization of H1R, which may result in decreased binding of [11C]doxepin in stress-related disorders (Endou et al., 2001).
We previously demonstrated that normal female volunteers had significantly higher BP of [11C]doxepin to H1 receptors in the cerebral cortical areas than male volunteers (Yoshizawa et al., 2009). The brain exhibits sexual dimorphism. For example, there are gender differences in the size of the interstitial nucleus of anterior hypothalamus (INAH) (male > female). INAH in homosexual men is only half the size of the nucleus in heterosexual men. The gender difference in human H1 receptors that we observed is reasonable because histamine neurons are exclusively located in the posterior hypothalamus. Sexual dimorphism was also reported for brain histamine in rodents. The density of histamine H1 receptors was higher in female rats than in male rats (Ghi et al., 1999), and hypothalamic histamine release was higher in male rats than in female rats (Ferretti et al., 1998). It is not ruled out that neuronal histamine release and in vivo H1 receptor binding are closely correlated. Therefore, we should carefully consider unknown factors influencing in vivo [11C]doxepin binding in the brain.
One of the most commonly used types of drug for allergies is the antihistamines. There are many available antihistamines with different sedating properties. Therefore, it is important to develop an objective and reliable method for measuring the strength of such sedative side effects, on which we have conducted numerous PET studies (Tagawa et al., 2001; Yanai et al., 2011, 2012). [11C]Doxepin-PET has been shown to be useful for evaluating their sedating side effects and the mechanisms involved. We succeeded in quantifying the strength of the sedating properties of antihistamines in terms of brain histamine H1 receptor occupancy. We previously reported an age-related decline in H1 receptor binding in normal human brain, especially in the prefrontal, temporal, cingulate, and parahippocampal regions (Yanai et al., 1992), which are known to be involved in attention and cognition. Therefore, we chose only young male volunteers for these studies of receptor occupancy. This study confirmed the reliability of values in H1 receptor occupancy by antihistamines because different PET studies were summarized to make the figures of occupancy (Yanai et al., 2011, 2012).
This study demonstrates for the first time that subjects' attentive conditions do not affect the reliability of H1 receptor binding measured by [11C]doxepin-PET. This study does not necessarily rule out the feasibility of measuring neuronal histamine release in the living human brain using PET, although PET tracers with better signal-to-noise properties should be developed in the future. For this purpose, H3 receptor binding would be more appropriate because H3 receptors are easily down-regulated by stress-related histamine release. The previous studies reported that histamine release was significantly increased during stressful conditions, and that the H3 receptor density rapidly decreased in response to stress (Ghi et al., 1995; Endou et al., 2001; Westerink et al., 2002). Following the development of other histaminergic PET probes, non-invasive measurement of neuronal histamine release would be feasible in humans by PET ligand-activation study in the future.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was performed at the Cyclotron and Radioisotope Center, Tohoku University. This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (nos. 21390171 and 21650088) and the Japan Society of Technology (“Molecular Imaging”). We thank Profs. R. Iwata, S. Furumoto, and N. Okamura for encouraging us to publish this study. We appreciate the technical assistance provided by Y. Ishikawa and K. Takeda in the PET studies.
References
Abe, H., Honma, S., Ohtsu, H., and Honma, K. (2004). Circadian rhythms in behavior and clock gene expressions in the brain of mice lacking histidine decarboxylase. Brain Res. Mol. Brain Res. 124, 178–187.
Boecker, H., Henriksen, G., Sprenger, T., Miederer, I., Willoch, F., Valet, M., Berthele, A., and Tölle, T. R. (2008). Positron emission tomography ligand activation studies in the sports sciences: measuring neurochemistry in vivo. Methods 45, 307–318.
Edison, P., Brooks, D. J., Turkheimer, F. E., Archer, H. A., and Hinz, R. (2009). Strategies for the generation of parametric images of [11C]PIB with plasma input functions considering discriminations and reproducibility. Neuroimage 48, 329–338.
Endou, M., Yanai, K., Sakurai, E., Fukudo, S., Hongo, M., and Watanabe, T. (2001). Food-deprived activity stress decreased the activity of the histaminergic neuron system in rats. Brain Res. 891, 32–41.
Eriksson, K. S., Sergeeva, O., Brown, R. E., and Haas, H. L. (2001). Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J. Neurosci. 21, 9273–9279.
Ferretti, C., Blengio, M., Ghi, P., Adage, T., Portaleone, P., and Ricci Gamalero, S. (1998). Hypothalamic histamine release in normal and stressed rats is affected by sex and aging. Pharmacol. Biochem. Behav. 59, 255–260.
Ghi, P., Ferretti, C., and Blengio, M. (1995). Effects of different types of stress on histamine-H3 receptors in the rat cortex. Brain Res. 690, 104–107.
Ghi, P., Orsetti, M., Gamalero, S. R., and Ferretti, C. (1999). Sex differences in memory performance in the object recognition test. Pharmacol. Biochem. Behav. 64, 761–766.
Haas, H., and Panula, P. (2003). The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 4, 241–251.
Haas, H. L., Sergeeva, O. A., and Selbach, O. (2008). Histamine in the nervous system. Physiol. Rev. 88, 1183–1241.
Hommer, D. W., Bjork, J. M., and Gilman, J. M. (2011). Imaging brain response to reward in addictive disorders. Ann. N.Y. Acad. Sci. 1216, 50–61.
Huang, Z. L., Qu, W. M., Li, W. D., Mochizuki, T., Eguchi, N., Watanabe, T., Urade, Y., and Hayaishi, O. (2001). Arousal effect of orexin A depends on activation of the histaminergic system. Proc. Natl. Acad. Sci. U.S.A. 98, 9965–9970.
Iwabuchi, K., Ito, C., Tashiro, M., Kato, M., Kano, M., Itoh, M., Iwata, R., Matsuoka, H., Sato, M., and Yanai, K. (2005). Histamine H1 receptors in schizophrenic patients measured by positron emission tomography. Eur. Neuropsychopharmacol. 15, 185–191.
Jacobs, E. H., Yamatodani, A., and Timmerman, H. (2000). Is histamine the final neurotransmitter in the entrainment of circadian rhythms in mammals? Trends Pharmacol. Sci. 21, 293–298.
Kano, M., Fukudo, S., Tashiro, A., Utsumi, A., Tamura, D., Itoh, M., Iwata, R., Tashiro, M., Mochizuki, H., Funaki, Y., Kato, M., Hongo, M., and Yanai, K. (2004). Decreased histamine H1 receptor binding in the brain of depressed patients. Eur. J. Neurosci. 20, 803–810.
Kim, J. S., Ichise, M., Sangare, J., and Innis, R. B. (2006). PET imaging of serotonin transporters with [11C]DASB: test-retest reproducibility using a multilinear reference tissue parametric imaging method. J. Nucl. Med. 47, 208–214.
Koepp, M. J., Gunn, R. N., Lawrence, A. D., Cunningham, V. J., Dagher, A., Jones, T., Brooks, D. J., Bench, C. J., and Grasby, P. M. (1998). Evidence for striatal dopamine release during a video game. Nature 393, 266–268.
Lin, J. S. (2000). Brain structures and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Med. Rev. 4, 471–503.
Mochizuki, T., Yamatodani, A., Okakura, K., Horii, A., Inagaki, N., and Wada, H. (1992). Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol. Behav. 51, 391–394.
Narendran, R., Mason, N. S., May, M. A., Chen, C. M., Kendro, S., Ridler, K., Rabiner, E. A., Laruelle, M., Mathis, C. A., and Frankle, W. G. (2011). Positron emission tomography imaging of dopamine D2/3 receptors in the human cortex with [11C]FLB 457: reproducibility studies. Synapse 65, 35–40.
Sakai, K., Takahashi, K., Anaclet, C., and Lin, L. S. (2010). Sleep-waking discharge of ventral tuberomammillary neurons in wild-type and histidine decar-boxylase knock-out mice. Front. Behav. Neurosci. 4:53. doi: 10.3389/fnbeh.2010.00053
Suzuki, A., Tashiro, M., Kimura, Y., Mochizuki, H., Ishii, K., Watabe, H., Yanai, K., Ishiwata, K., and Ishii, K. (2005). Use of reference tissue models for quantification of histamine H1 receptors in human brain by using positron emission tomography and [11C]doxepin. Ann. Nucl. Med. 19, 425–433.
Tagawa, M., Kano, M., Okamura, N., Higuchi, M., Matsuda, M., Mizuki, Y., Arai, H., Iwata, R., Fujii, T., Komemushi, S., Ido, T., Itoh, M., Sasaki, H., Watanabe, T., and Yanai, K. (2001). Neuroimaging of histamine H1 receptor occupancy in human brain by positron emission tomography (PET): a comparative study of ebastine, a second-generation antihistamine, d-chlorpheniramine, a classical antihistamine. Br. J. Clin. Pharmacol. 52, 501–509.
Takahashi, K., Lin, J. S., and Sakai, K. (2008). Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience 153, 860–870.
Tuomisto, L., Lozeva, V., Valjakka, A., and Lecklin, A. (2001). Modifying effects of histamine on circadian rhythms and neuronal excitability. Behav. Brain Res. 124, 129–135.
Watanabe, T., and Yanai, K. (2001). Studies on functional roles of the histaminergic neuron system by using pharmacological agents, knockout mice and positron emission tomography. Tohoku J. Exp. Med. 195, 197–217.
Westerink, B. H., Cremers, T. I., De Vries, J. B., Liefers, H., Tran, N., and De Boer, P. (2002). Evidence for activation of histamine H3 autoreceptors during handling stress in the prefrontal cortex of the rat. Synapse 43, 238–243.
Yanai, K., Rogala, B., Chugh, K., Paraskakis, E., Pampura, A. N., and Boev, R. (2012). Safety considerations in the management of allergic diseases: focus on antihistamines. Curr. Med. Res. Opin. 28, 623–642.
Yanai, K., and Tashiro, M. (2007). The physiological and patho-physiological roles of neuronal histamine: an insight from human PET studies. Pharmacol. Ther. 113, 1–15.
Yanai, K., Watanabe, T., Meguro, K., Yokoyama, H., Sato, I., Sasano, H., Itoh, M., Iwata, R., Takahashi, T., and Ido, T. (1992). Age-dependent decrease in histamine H1 receptor in human brains revealed by PET. Neuroreport 3, 433–436.
Yanai, K., Zhang, D., Tashiro, M., Yoshikawa, T., Naganuma, F., Harada, R., Nakamura, T., Shibuya, K., and Okamura, N. (2011). Positron emission tomography evaluation of sedative properties of antihistamines. Expert Opin. Drug Saf. 10, 613–622.
Yasuno, F., Sanabria, S. M., Burns, D., Hargreaves, R. J., Ghose, S., Ichise, M., Chin, F. T., Morse, C. L., Pike, V. W., and Innis, R. B. (2007). PET imaging of neurokinin-1 receptors with [18F]SPA-RQ in human subjects: assessment of reference tissue models and their test-retest reproducibility. Synapse 61, 242–251.
Keywords: H1 receptor, attentive waking, circadian rhythm, histamine release, positron emission tomography (PET), test–retest reliability, human brain
Citation: Shibuya K, Funaki Y, Hiraoka K, Yoshikawa T, Naganuma F, Miyake M, Watanuki S, Sato H, Tashiro M and Yanai K (2012) [11C]Doxepin binding to histamine H1 receptors in living human brain: reproducibility during attentive waking and circadian rhythm. Front. Syst. Neurosci. 6:45. doi: 10.3389/fnsys.2012.00045
Received: 27 January 2012; Accepted: 21 May 2012;
Published online: 11 June 2012.
Edited by:
Pertti Panula, University of Helsinki, FinlandReviewed by:
Bela Szabo, Albert Ludwigs University, GermanyConstance Hammond, Institut National de la Recherche Médicale et de la Santé Inserm, France
Copyright: © 2012 Shibuya, Funaki, Hiraoka, Yoshikawa, Naganuma, Miyake, Watanuki, Sato, Tashiro and Yanai. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Kazuhiko Yanai, Department of Pharmacology, Tohoku University School of Medicine, 2-1 Seiryo-machi, Aoba-ku, Sendai 980-8575, Japan. e-mail:eWFuYWlAbWVkLnRvaG9rdS5hYy5qcA==
 Katsuhiko Shibuya1,2
Katsuhiko Shibuya1,2