
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Syst. Neurosci. , 26 March 2012
Volume 6 - 2012 | https://doi.org/10.3389/fnsys.2012.00014
This article is part of the Research Topic Histamine in the Brain View all 11 articles
Novel fluorescent chalcone-based ligands at human histamine H3 receptors (hH3R) have been designed, synthesized, and characterized. Compounds described are non-imidazole analogs of ciproxifan with a tetralone motif. Tetralones as chemical precursors and related fluorescent chalcones exhibit affinities at hH3R in the same concentration range like the reference antagonist ciproxifan (hH3R pKi value of 7.2). Fluorescence characterization of our novel ligands shows emission maxima about 570 nm for yellow fluorescent chalcones and ≥600 nm for the red fluorescent derivatives. Interferences to cellular autofluorescence could be excluded. All synthesized chalcone compounds could be used to visualize hH3R proteins in stably transfected HEK-293 cells using confocal laser scanning fluorescence microscopy. These novel fluorescent ligands possess high potential to be used as pharmacological tools for hH3R visualization in different tissues.
Histaminergic receptors belong to class A of membrane bound G-protein-coupled receptors (GPCRs). They consist of four subtypes, the histamine H1, H2, H3, and H4 receptors (Walter and Stark, 2012). The H3 receptor has a neurotransmitter function. High receptor densities could be found in different areas of the central nervous system (Martinez-Mir et al., 1990; Sander et al., 2008). Possible indications of H3 receptor antagonists/inverse agonists could be the treatment of cognitive and sleep disorders as well as schizophrenia, epilepsy, adipositas, and neuropathic pain (Girard et al., 2004). To get information on etiopathology and accumulation or depletion of human histamine H3 receptors (hH3R) and to accelerate the clinical development of pharmaceuticals in the screening of drugs it is interesting to design labeled H3 receptor ligands. To date several different techniques have been used to measure receptor occurrence in tissues. Radioactive competition is often used for instance in brain slices with [3H](R)-alpha-methylhistamine (Martinez-Mir et al., 1990), [3H]Nα-methylhistamine (Le et al., 2009), [3H]-A-349821 (Miller et al., 2009), [125I]iodoproxyfan (Ligneau et al., 1994; Stark et al., 1996), and [125I]iodophenpropit (Jansen et al., 2000). Adversely, analysis of ex vivo autoradioactivity often takes longer time (Le et al., 2009) and synthesis and storage of radioligands causes high costs and special equipment/rooms. Radioactive exposure as generally known is very harmful. Fluorescent ligands are preferred over radioligands in terms of safety precautions and often applicability. Fluorescent ligands and their use for the localization and detection of GPCRs is still a topical area of investigation (Kuder and Kiec-Kononowicz, 2008). Attempts to design fluorescent human hH3R were established with motif structure elements of Sangers reagent, dansyl, NBD, cyanoisoindol, and tetramethylrhodamine groups (Amon et al., 2006, 2007; Cowart et al., 2006; Kuder et al., 2010). Most of these compounds showed high affinity at histamine H3 receptors (hH3R Ki: 0.1–10 nM), but their fluorescence absorption and emission wavelengths were mainly between 300 and 500 nm. In this wavelength range interactions with cellular autofluorescence occur and in addition to this problem, most of these ligands possess low fluorescence intensities. A PhD thesis from the working group of Prof. Buschauer (University Regensburg, Erdmann, 2010) presented further fluorescent human histamine H3 ligands. Applied fluorophores possess good fluorescent features but are expensive or difficult to synthesize. The aim of this study was the optimization of the fluorescent properties taking advantage of already published fluorescent ligands as lead structures. A literature survey indicated bio-active fluorescent benzylidine tetralones (Kamakshi et al., 2010) possessing different antibacterial activity and useful physicochemical data (Al-Ansari, 1998). Tomecková et al. (2004) reported on related cyclic chalcone analogs demonstrating biological effects on mitochondrial outer membrane via fluorescence microscopy. These results motivated us to use chalcones as fluorescent element for labeling of histamine H3 receptor ligands to generate novel fluorescent pharmaceutical tools (Figure 1).
All reagents and solvents were purchased from VWR (Darmstadt, Germany), Sigma-Aldrich (Steinheim, Germany), Alfa Aesar (Ward Hill, MA, USA), Perkin Elmer Life and Analytical Sciences (Rodgau, Germany), and Acros Organics (Geel, Belgium), and were used without further purification (unless otherwise stated). 1H and 13C NMR spectra were recorded on a AV 250 Spektrometer (5.9 T; 1H: 250 MHz; 13C: 63 MHz), AV 300 Spektrometer (7.1 T; 1H: 300 MHz; 13C: 75 MHz), or AV 400 Spektrometer (0.4 T; 1H: 400 MHz, 13C: 100 MHz): Bruker (Rheinstetten, Germany). Electro-spray-ionization MS (ESI MS) was performed on a: VG Platform II: Fisons Instruments (Manchester, GB) and nano-ESI (nESI): Mariner Workstation TOF: Applied Biosystems (Carlsbad, CA, USA). High resolution MS (HRMS) was recorded on a LTQ Orbitrap XL: Thermo Fisher Scientific (Waltham, MA, USA). Elemental analyses (C, H, N) were measured on a Vario MicroCube: Elementar Heraeus (Hanau, Deutschland) and were within ±0.4% of the theoretical values for all final compounds. Preparative column chromatography was performed on silica gel 60 F254, coat thickness: 0.2 mm (VWR, Darmstadt, Germany). The microwave oven used was a Biotage Initiator 2.0 (400 W): Biotage (Uppsala, Sweden). For detailed synthesis procedures and analytical data see Section “Appendix.”
The initializing precursor 3-(piperidin-1-yl)propan-1-ol hydrochloride was synthesized by alkylation of piperidine with 3-chloropropan-1-ol as described in the literature (Apelt et al., 2005) and chlorinated (Sander et al., 2010). 6-Hydroxy-1-tetralone was commercially available whereas its regioisomer 7-hydroxy-1-tetralone was prepared from the corresponding methoxy derivative (Scheme 1). Ether cleavage has been carried out with para-toluenesulfonic acid and 1-butyl-3-methyl-1H-imidazolium bromide as ionic liquid under microwave condition with yields greater than 90% as described before (Boovanahalli et al., 2003). Synthesis of compound 1 and 2 started with the alkylation of 6- and 7-hydroxy-1-tetralone with 1-(3-chloropropyl)piperidine hydrochlorid (Scheme 1) by Williamson ether reaction, respectively (Williamson, 1851). Compound 1 and 2 were converted into fluorescent chalcones (compound 3, 4, 5, and 6) via aldol condensation with appropriate aldehyde.
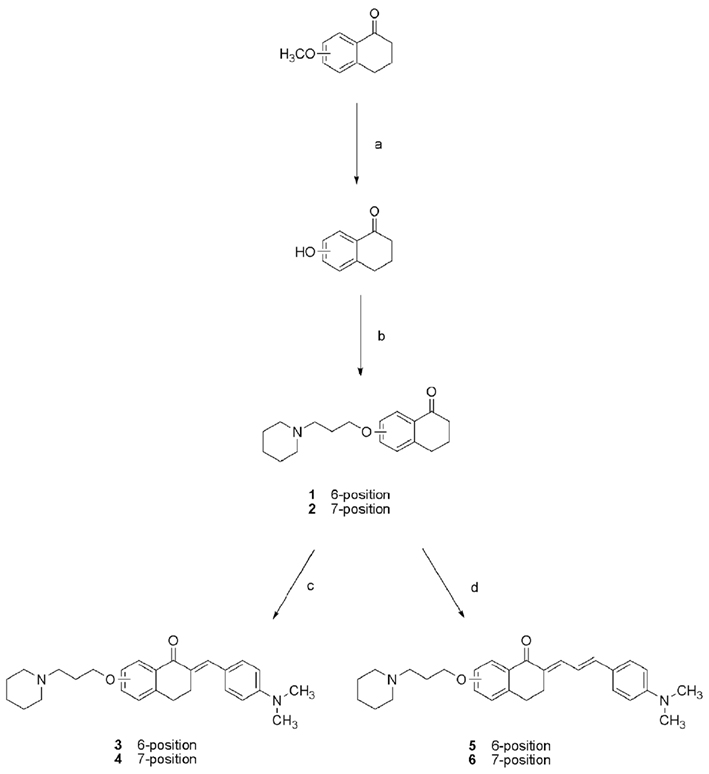
Scheme 1. Synthesis of compounds 1–6. (a) para-Toluenesulfonic acid, 1-butyl-3-methyl-1H-imidazolium bromide, microwave irradiation, 160°C, 1 h, η = 97%; (b), 1-(3-chloropropyl)piperidine hydrochlorid, K2CO3, KI, acetone, 60°C, 48 h, η = 71–94%; (c) 4-(dimethylamino)benzaldehyde, ethanol/water, NaOH, RT, over night, η = 63–85%; (d) 4-(dimethylamino)cinnamic aldehyde, ethanol/water, NaOH, RT, over night, η = 69–89%.
Alkylation of 4-(hydroxymethyl)phenol with 1-(3-chloropropyl)piperidine hydrochlorid was the initializing step in the synthesis of compounds with elongated spacer B (Scheme 2). Resulting (4-(3-(piperidin-1-yl)propoxy)phenyl)methanol was chlorinated with thionylchloride. After alkylation with 6- and 7-hydroxy-1-tetralone fluorescent chalcones were synthesized with corresponding aldehydes (compounds 9–12), respectively. All tetralone and chalcone derivatives were purified by column chromatography. Oily products were crystallized as salts of oxalic acid in ethanol.
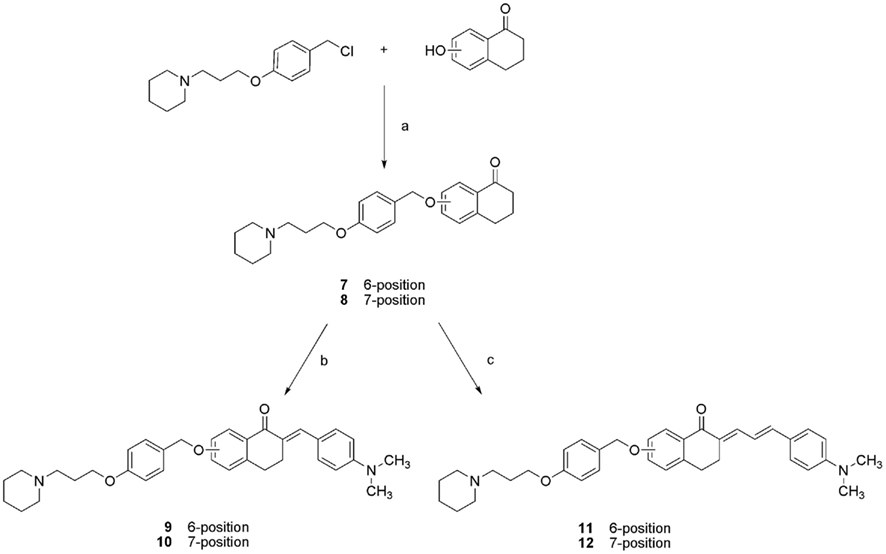
Scheme 2. Synthesis of compound 7–12. (a) K2CO3, KI, acetone, 60°C, 48 h, η = 65–71%; (b) 4-(dimethylamino)benzaldehyde, ethanol/water, NaOH, RT, over night, η = 85–91%; (c) 4-(dimethylamino)cinnamic aldehyde, ethanol/water, NaOH, RT, over night, η = 93–95%.
For fluorescence characterization all corresponding histamine H3 receptor ligands were dissolved in buffer (12.5 mM MgCl2, 100 mM NaCl, and 75 mM Tris/HCl, pH 7.4) at a concentration of 10 mM. Fluorescence absorption and emission spectra were recorded on a Fluorolog HORIBA JOBIN YVON fluorometer (HORIBA scientific, Kyoto, Japan) at room temperature. Spectra were analyzed with FluorEssence™ (HORIBA scientific, Kyoto, Japan) for Windows®.
HEK-293 cells stably expressing the recombinant human histamine H3 receptor were used for membrane extraction. In brief, membrane protein concentration was determined by the method of Bradford (1976). Competition binding experiments were carried out as described before (Kottke et al., 2011). Membranes were incubated with [3H]Nα-methylhistamine (2 nM) and test ligand in a concentration range between 0.01 nM and 100 μM. For determination of non-specific binding 10 μM of pitolisant was used. All values are means of at least three independent measurements, each in triplicates and on seven different concentrations. Binding data were analyzed by the software GraphPad Prism™ (2000, version 3.02, San Diego, CA, USA).
CHO-K1 cells expressing the human histamine H1 receptor were used for membrane extraction. In brief, human histamine H1 receptor radioligand competition binding assay was performed as described before (Rossbach et al., 2011). Membranes were incubated with 1 nM of [3H]pyrilamine and test ligand in a concentration range from 100 nM to 100 μM. For determination of non-specific binding 10 μM of chlorpheniramine was used. All values are means of at least two independent measurements, each in triplicates and four different concentrations. Binding data were analyzed by the software GraphPad Prism™ (2000, version 3.02, San Diego, CA, USA).
Sf9 cells transiently expressing the human histamine H4 receptor and co-expressed with G-protein Gαi/o and Gβ1γ2 subunits were used for membrane extraction. Competition binding experiments were carried out as described before (Kottke et al., 2011). Membranes were incubated with 10 nM [3H]histamine and test ligand in a concentration range from 0.01 nM to 100 μM. Non-specific binding was determined by using 10 μM JNJ-7777120. All values are means of at least two independent measurements, each in triplicates and four different concentrations. Binding data were analyzed by the software GraphPad PrismTM (2000, version 3.02, San Diego, CA, USA).
Slides were coated with poly-D-lysine (50 μg/mL, Sigma-Aldrich, Steinheim, Germany) and cooled for 2 h. HEK-293 cells stably expressing the recombinant human histamine receptor (mean protein content: 10 μg/mL; Bmax: 300–900 fmol/mg) were seeded (100,000 cells/mL) and grown in 2 mL of Dulbecco’s modified Eagle’s medium (without phenol red) with 2 mM glutamine, 10 mM HEPES, 10% fetal bovine serum, and 10 μL/mL penicillin/streptomycin in an atmosphere of 5% CO2 at 37°C for 48 h in six-well plates with poly-D-lysine coated slides. The medium was removed, and the slides were incubated with 3% BSA in buffer (12.5 mM MgCl2, 100 mM NaCl, and 75 mM Tris/HCl, pH 7.4) for 30 min at 37°C. Afterward the slides were washed with PBS buffer and incubated with 2 mL 200 nM of appropriate fluorescent H3 ligand in buffer (12.5 mM MgCl2, 100 mM NaCl, and 75 mM Tris/HCl, pH 7.4) for 1 h at 37°C. The ligand solution was removed, washed with PBS buffer, and the cells were fixed in methanol for 20 min at a temperature of −20°C. After equilibration with PBS buffer the nucleus was stained with 100 nM blue fluorescent DAPI (4′,6-diamidino-2-phenylindole; Kapuscinski, 1995). The slides were mounted on cover slips with 30 μL Mowiol. For reasons of comparison HEK-293 cells were cultured as blind reference and treated with respective ligand in a similar manner.
Cell preparations were fluorescently visualized with a confocal laser scanning microscope (Leica TCS SP5, Wetzlar, Germany). Fluorescence intensities were adjusted minimizing autofluorescence and all slices were measured with the same intensity to generate comparable images. All images were recorded with a 60× oil immersions objective. Fluorescence colors were adapted to appropriate fluorescence emission wavelengths. Fluorescent ligands were excited with a 488 nm multiline argon laser and emission was detected between 520 and 790 nm. DAPI-stained nuclei of the HEK-293 cells were excited with a 405 nm diode laser and emission was measured between 420 and 500 nm. Images were recorded in sequential mode to avoid interferences.
Historically the first potent histamine H3 receptor ligands were derivatized from the endogenous ligand histamine. Ciproxifan as parent compound for these lead structures possesses a basic imidazole ring as core moiety (Figure 1). Spacer A is a propyl group, followed by a polar ether functionality linking the phenyl group which is then connected to structurally different affinity enhancing elements. The imidazole-containing ciproxifan exerts an affinity at the human histamine H3 receptor in the medium nanomolar concentration range (Table 1; Ligneau et al., 2000). Since the imidazole group may potentially be associated with interaction to the cytochrome P450 enzyme system, the change from imidazole to more robust piperidine systems in the western part of the molecule has been performed resulting in an improved pharmacological profile (Lazewska et al., 2006, 2008; Sander et al., 2008). In 2001 the piperidine analog of ciproxifan, UCL-2190 (Figure 1), was published (Meier et al., 2001). Likewise we introduced the piperidine core element in our newly designed compounds, kept spacer A constant with three methylene groups as well as the benzyl ether spacer. For compound 7–12 spacer B was elongated by the benzyl ether. The keto group in the lipophilic residue of ciproxifan was incorporated into a tetrahydronaphthalene system (1, 2, 7, and 8) which were converted to fluorescent chalcones (3, 4, 5, 6, 9, 10, 11, and 12).
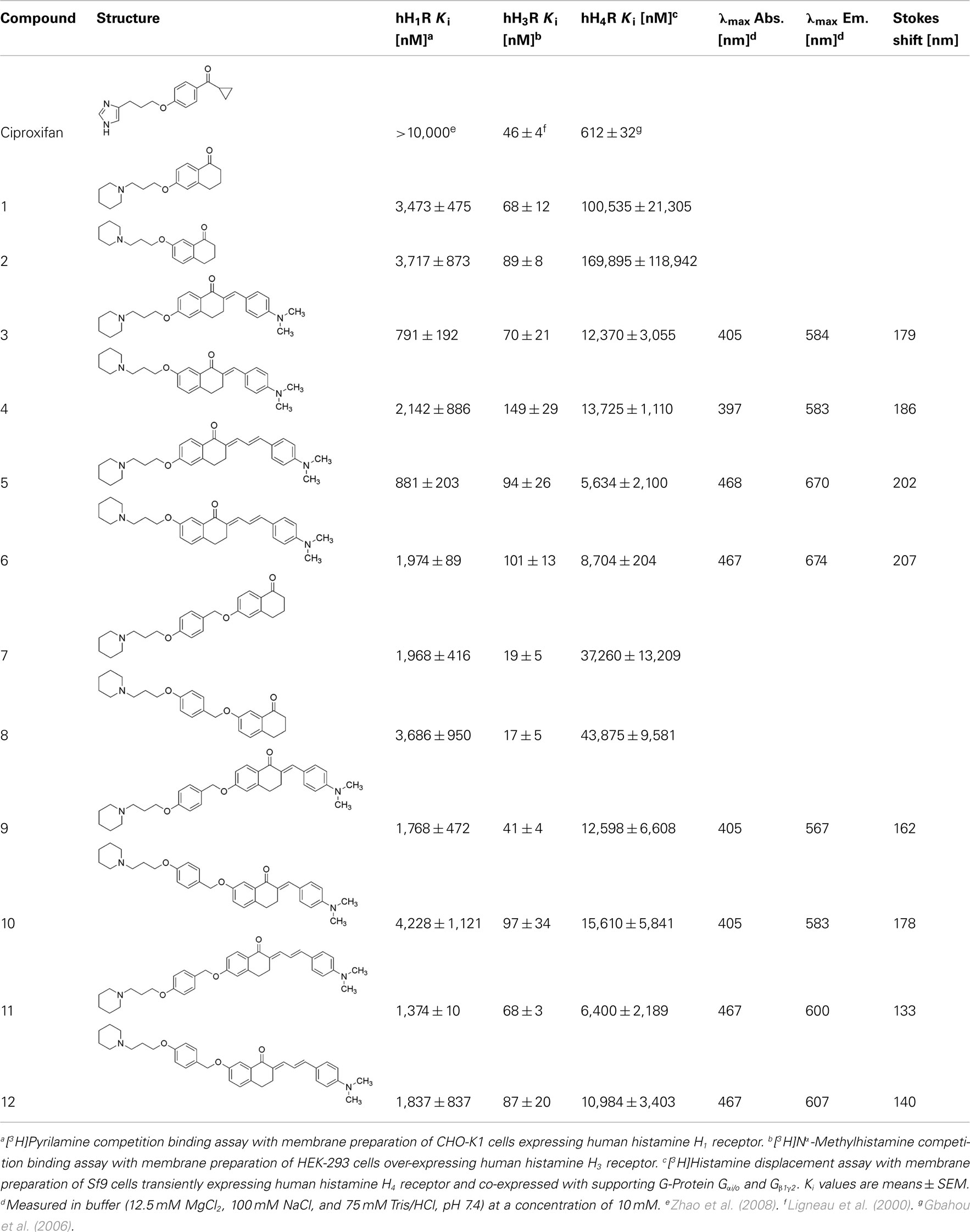
Table 1. Human histamine H1, H3, and H4 receptor affinities and fluorescence absorption and emission maximum wavelengths of novel non-imidazole ligands.
During design of novel fluorescent pharmacological tools it is important to generate compounds with high affinity at the target receptor, high fluorescence intensities, and emission wavelengths near the infrared wavelength range. It is difficult to retain binding affinity and low molecular weight with structurally larger fluorophoric elements in small molecule GPCR ligands. Fluorophores emitting with high wavelengths frequently consist of bulky structures. Al-Ansari (1998) and Kamakshi et al. (2010) described the synthesis of unbulky, fluorescent benzylidene tetralones which have been used as fluorophore lead element (Figure 1).
Affinities of the newly designed fluorescent chalcones (3, 4, 5, 6, 9, 10, 11, and 12) at the human histamine H3 receptor are in a comparable nanomolar concentration range like that of ciproxifan (Table 1). Tetralones with elongated aromatic spacer B exhibit slightly lower Ki values than those of the shorter tetralones (1 → 7; 2 → 8) most probably due to increased lipophilic interactions. Elongation of the tetralones with appropriate aldehydes gives no further improvement in binding properties.
The variation from 6- to 7-hydroxy-1-tetralone derivatives causes no remarkable difference in binding and does not result in higher affinities at the human histamine H3 receptor.
For selectivity validation the new compounds have been screened on affinity at related human histamine H1 and H4 receptors. All ligands possess highest affinity at the human histamine H3 receptor demonstrating their receptor preference (Table 1). Affinity at the human H1 receptor is about one log unit and at the human histamine H4 receptor about two log units higher than that at the human histamine H3 receptor.
Compound 8 demonstrated one of the highest selectivity over all 12 compounds. Compound 9 is the most selective compound in the fluorescent chalcone series whereas compound 5 the less selective chalcone. These data further prove the beneficial effects of elongation of spacer B via benzyl ether concerning selectivity and affinity.
Kamakshi et al. (2010) described the fluorophore lead structure (Figure 1) as compound with intense emission signals. Absorption maximum was found at 406 nm. The fluorophore lead is described as charge transfer compound where the N,N-dimethyl group behaves as the electron donor moiety and the carbonyl group as the electron acceptor moiety. These two groups form a donor–acceptor complex upon excitation. Fluorescence measurement confirmed the absorption maxima (compounds 3, 9, and 10: 405 nm; cf. Appendix).
Introduction of an additional vinyl group resulted in an increased maximum in excitation and emission wavelength. (cf. 3 → 5: Δλmax Abs.: 63 nm, Δλmax Em.: 102 nm). Compounds with propenone element (3, 4, 9, 10) emit in the yellow area of the fluorescence spectrum and compounds with pentadienone elements (5, 6, 11, 12) in the red one. It is important to design fluorophores which emit fluorescence higher than 500 nm. In the blue spectroscopic area (300–450 nm) high autofluorescence occurs and causes difficulties in detection of the actual target. In fluorescence visualization it is the aim to achieve emission in the near infrared wavelength area to improve the signal-to-noise ratio. Therefore it is a need to use fluorophores with high quantum yields and low quenching properties. To avoid self-quenching it is important to synthesize fluorophores with large Stokes shifts. All compounds fulfill this requirement (Table 1).
Elongation of spacer B via benzyl ether group causes decrease of emission wavelengths of red emitting chalcones. Introduction of a phenylether group can influence the donor–acceptor complex of the fluorophore. Elongated compounds show higher fluorescence emission intensities (Figure 2; cf. Appendix).
For the measurement of fluorescence absorption and emission buffer (12.5 mM MgCl2, 100 mM NaCl, and 75 mM Tris/HCl, pH 7.4) has been used to simulate assay conditions and simultaneously find the ideal settings for confocal laser microscope studies.
To minimize unspecific binding cells were incubated for 30 min with 3% BSA solution. Fluorescent histamine H3 receptor ligands were added in a concentration range of two up to three times of Ki-value at the human histamine H3 receptor (200 nM) on hH3-HEK-293 and HEK-293 cells. After washing steps and staining of the nucleus with blue fluorescent DAPI as reported in literature (Kapuscinski, 1995), slides were fixed with Mowiol and analyzed with a confocal laser scanning microscope (Figure 3).
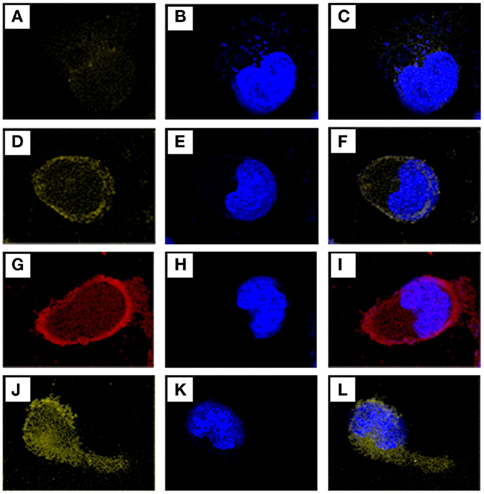
Figure 3. Confocal laser scanning microscope images of fluorescent human histamine H3 ligands on HEK-293 cells and hH3-HEK-293. All images were taken on a Leica TCS SP5 microscope. Fluorescent chalcones were incubated for 1 h and after washing steps nuclei were stained with blue fluorescent DAPI. (A) Shows a HEK-293 cell labeled with compound 3 and (B) shows the DAPI-stained nucleus of the same cell. (C) Is an overlay of image (A,B). (D) Shows compound 3 on an hH3-HEK-293 cell, (E) the DAPI-stained nucleus of the same cell and (F) an overlay of (D) and (E). Red fluorescent compound5 on an hH3-HEK-293 cell is shown in (G), cell nucleus in (H), and the overlay of(G) and(H) in (I). (J) Shows an hH3-HEK-293 cell stained with compound 9, (K) its nucleus, and (L) the overlay of (J,K).
Chalcones synthesized from 4-(dimethylamino)benzaldehyde result in yellow fluorescent ligands like compound3 and 9. Chalcones synthesized from 4-(dimethylamino)cinnamic aldehyde in red emitting ligands like compound5. Due to their maximum emission wavelengths ligand color on the confocal laser scanning microscope was adjusted. Fluorescence images were recorded in sequential mode to avoid interfering of emission wavelengths of ligand and DAPI fluorescence. In the first step DAPI-stained nuclei were recorded (Figures 3B,E,H,K) between 420 and 500 nm, then fluorescent ligand emission was measured between 520 and 790 nm (Figures 3A,D,G,J). Appropriate images were over-laid to co-localize fluorescent ligand and fluorescent nucleus (Figures 3C,F,I,L). Figure 3D shows the enrichment of the yellow emitting compound3 in the outer membrane of hH3-HEK-293 cells. Figure 3G shows the enrichment in same regions in hH3-HEK-293 cells of the red emitting compound5 which emits more intensive (Figure 2). The most human histamine H3 receptor selective and most intense compound (9) is shown in Figures 3J–L. Fluorescent human histamine H3 ligands stain the outer membrane of hH3-HEK-293 cells where human hH3R are mainly expressed. In HEK-293 cells which do not have human hH3R, no enrichment of ligand in the outer cell membrane can be identified (Figures 3A,C; cf. Appendix) indicating selectivity to the aimed receptor (Table 1). The fluorescent ligands on HEK-293 cells are diffusely distributed and emit in a low manner if compared to images 3D, 3G, and 3J where outer membranes emit strong fluorescence (cf. Appendix). Differences between hH3- and HEK-293 cells are facile to detect. Fluorescence emission of chalcone derivatives on HEK-293 cells is in the same range as autofluorescence (cf. Appendix) and therefore negligible.
In our work we achieved the design of selective and fluorescent human histamine H3 receptor ligands. Affinities at the human histamine H3 receptor of all newly synthesized compounds are in the nanomolar concentration range. All 12 compounds showed high H3 receptor preference in comparison to their binding properties at H1 and H4 receptors.
The fluorescent chalcone ligands facilitate visualization of human hH3R on hH3–HEK cells. Their fluorescence characteristics (fluorescence emission maxima higher than 500 nm) avoid appearance of interference with autofluorescence. Intense light signals label the localization of targeted receptor. Reference HEK-293 cells which do not over-express human hH3R show negligible binding of fluorescent ligands demonstrating low unspecific binding.
Compounds 3, 5, and 9 showed excellent, but slightly diverse properties concerning absorption, emission, Stokes shift, intensity and H3 receptor affinity and selectivity. Best compound concerning wavelength represents compound 5 (λmax Abs./Em.: 468/670 nm) but it is not as selective as compound 9 which emits with highest intensity of all synthesized compounds in the yellow area of spectrum. Compound 9 has the highest affinity at the human histamine H3 receptor compared to the other chalcone derivatives in this series.
Our new fluorescent ligands open new possibilities for non-radioactive alternatives for novel binding assays and novel visualization tools. They are precious tools for detailed pharmacological analyses on detection of receptors in cells and tissues. Studies on different tissues with these novel ligands are envisaged to provide further evidence of their convenience as pharmacological tools.
More detailed information is available in the Appendix.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was kindly supported by the Hesse LOEWE Schwerpunkte NeFF, OSF, and AFA as well as by the FP7 EU COST Actions BM0806, BM1007 and CM1103. We greatly thank Prof. J.-C. Schwartz (Bioprojet, Saint-Grégoire, France) for providing HEK-293 cells stably expressing the recombinant human histamine H3 receptor. The CHO-K1 cells stably expressing the human histamine H1 receptor were generously gifted by Prof. H. Timmermann and Prof. R. Leurs (Amsterdam, The Netherlands). We grateful thank Prof. R. Seifert (Hannover, Germany) for providing Sf9 cells and baculovirus stock solutions encoding for the human histamine H4 receptor and G-protein Gαi2 and Gβ1γ2 subunits.
Al-Ansari, I. A. Z. (1998). Ground- and excited-state properties of some 3,4-dihydro-1-(2-p-substituted benzylidene)naphtalenones: substituent and environmental effects. J. Phys. Org. Chem. 10, 687–696.
Amon, M., Ligneau, X., Camelin, J.-C., Berrebi-Bertrand, I., Schwartz, J.-C., and Stark, H. (2007). Highly potent fluorescence-tagged nonimidazole histamine H3 receptor ligands. ChemMedChem. 2, 708–716.
Amon, M., Ligneau, X., Schwartz, J.-C., and Stark, H. (2006). Fluorescent non-imidazole histamine H3 receptor ligands with nanomolar affinities. Bioorg. Med. Chem. Lett. 16, 1938–1940.
Apelt, J., Grassmann, S., Ligneau, X., Pertzt, H. H., Ganellin, C. R., Arrang, J.-M., Schwartz, J.-C., Schunack, W., and Stark, H. (2005). Search for histamine H3 receptor antagonists with combined inhibitory potency at Nτ-methyltransferase: ether derivatives. Pharmazie 60, 97–106.
Boovanahalli, S. K., Kim, D. W., and Chi, D. Y. (2003). Application of ionic liquid halide nucleophilicity for the cleavage of ethers: a green protocol for the regeneration of phenols from ethers. J. Org. Chem. 69, 3340–3344.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Cowart, M., Gfesser, G. A., Bhatia, K., Esser, R., Sun, M., Miller, T. R., Krueger, K., Witke, D., Esbenshade, T. A., and Hancock, A. A. (2006). Fluorescent benzofuran histamine H3 receptor antagonists with sub-nanomolar potency. Inflamm. Res. 55, S47–S48.
Erdmann, D. (2010). Histamine H2 and H3 Receptor Antagonists: Synthesis and Characterization of Radiolabelled and Fluorescent Pharmacological Tools. Ph.D. thesis, University Regensburg, Regensburg.
Gbahou, F., Vincent, L., Humbert-Claude, M., Tardivel-Lacombe, J., Chabret, C., and Arrang, J.-M. (2006). Compared pharmacology of human histamine H3 and H4 receptors: structure-activity relationships of histamine derivatives. Br. J. Pharmacol. 147, 744–754.
Girard, P., Pansart, Y., Coppe, M. C., Verniers, D., and Gillardin, J. M. (2004). Role of the histamine system in nefopam-induced antinociception in mice. Eur. J. Pharmacol. 503, 63–69.
Jansen, F. P., Michizuki, T., Maeyama, K., Leurs, R., and Timmermann, H. (2000). Characterization of histamine H3 receptors in mouse brain using the H3 antagonist [125I]iodophenpropit. Naunyn Schmiedebergs Arch. Pharmacol. 362, 60–67.
Kamakshi, R., Swarna Latha, S., and Reddy, B. S. R. (2010). An efficient synthesis of bio-active fluorescent benzylidine tetralones. Indian J. Chem. 49B, 944–947.
Kottke, T., Sander, K., Weizel, L., Schneider, E. H., Seifert, R., and Stark, H. (2011). Receptor-specific functional efficacies of alkyl imidazoles as dual histamine H3/H4 receptor ligands. Eur. J. Pharmacol. 654, 200–208.
Kuder, K. J., and Kiec-Kononowicz, K. (2008). Fluorescent GPCR ligands as new tools in pharmacology. Curr. Med. Chem. 21, 2132–2143.
Kuder, K. J., Kottke, T., Stark, H., Ligneau, X., and Camelin, J.-C. (2010). Search for novel, high affinity histamine H3 receptor ligands with fluorescent properties. Inflamm. Res. 59(Suppl. 2), S247–S248.
Lazewska, D., Kuder, K., Ligneau, X., Schwart, J.-C., Schunack, W., Stark, H., and Kiec-Kononowicz, K. (2008). Piperidine variations in search for non-imidazole histamine H3 receptor ligands. Bioorg. Med. Chem. Lett. 16, 8729–8736.
Lazewska, D., Ligneau, X., Schwartz, J.-C., Schunack, W., Stark, H., and Kiec-Kononowicz, K. (2006). Ether derivatives of 3-piperidinopropan-1-ol as non-imidazole histamine H3 receptor ligands. Bioorg. Med. Chem. 14, 3522–3529.
Le, S., Finn, J. P., Larijani, M. E., Marino, M. J., and Schaffhauser, H. (2009). Detection of low level histamine H3 receptor occupancy by autoradiography. J. Neurosci. Methods 185, 70–75.
Ligneau, X., Garbarg, M., Vizuete, M. L., Diaz, J., Purand, K., Stark, H., Schunack, W., and Schwartz, J.-C. (1994). [125I]iodoproxyfan, a new antagonist to label and visualize cerebral histamine H3 receptors. J. Pharmacol. Exp. Ther. 271, 452–459.
Ligneau, X., Morisset, S., Tardivel-Lacombe, J., Gbahou, F., Ganellin, C. R., Stark, H., Schunack, W., Schwartz, J.-C., and Arrang, J. M. (2000). Distinct pharmacology of rat and human histamine H3 receptors: role of two amino acids in the third transmembrane domain. Br. J. Pharmacol. 131, 1247–1250.
Martinez-Mir, M. I., Pollard, H., Moreau, J., Arrang, J. M., Ruat, M., Traiffort, E., Schwartz, J.-C., and Palacios, J. M. (1990). Three histamine receptors (H1, H2 and H3) visualized in the brain of human and non-human primates. Brain Res. 526, 322–327.
Meier, G., Apelt, J., Reichert, U., Graßmann, S., Ligneau, X., Elz, S., Leurquin, F., Ganellin, C. R., Schwartz, J.-C., Schunack, W., and Stark, H. (2001). Influence of imidazole replacement in different structural classes of histamine H3-receptor antagonists. Eur. J. Pharm. Sci. 13, 249–259.
Miller, T. R., Milicic, I., Bauch, J., Du, J., Surber, B., Browman, K. E., Marsh, K., Cowart, M., Brioni, J. D., and Esbenshade, T. A. (2009). Use of the H3 receptor antagonist radioligand [3H]-A-349821 to reveal in vivo receptor occupancy of cognition enhancing H3 receptor antagonists. Br. J. Pharmacol. 157, 139–149.
Rossbach, K., Nassenstein, C., Gschwandtner, M., Schnell, D., Sander, K., Seifert, R., Stark, H., Kietzmann, M., and Bäumer, W. (2011). Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience 190, 89–102.
Sander, K., Kottke, T., and Stark, H. (2008). Histamine H3 receptor antagonists go to clinics. Biol. Pharm. Bull. 31, 2163–2181.
Sander, K., Kottke, T., Weizel, L., and Stark, H. (2010). Kojic acid derivatives as histamine H3 receptor ligands. Chem. Pharm. Bull. 58, 1353–1361.
Stark, H., Purand, K., Hüls, A., Ligneau, X., Garbarg, M., Schwartz, J.-C., and Schunack, W. (1996). [125I]iodoproxyfan and related compounds: a reversible radioligand and novel classes of antagonists with high affinity and selectivity for the histamine H3 receptor. J. Med. Chem. 15, 1220–1226.
Tomecková, V., Perjési, P., Guzy, J., Kušnír, J., Chovanová, Z., Chavková, Z., and Mareková, M. (2004). Comparison of effect of selected synthetic chalcone analogues on mitochondrial outer membrane determined by fluorescence microscopy. J. Biochem. Biophys. Methods 61, 135–141.
Walter, M., and Stark, H. (2012). Histamine receptor subtypes: a century of rational drug design. Front. Biosci. (Schol. Ed.) 4, 461–488.
Zhao, C., Sun, M., Bennani, Y. L., Gopalakrishnan, S. M., Witte, D. G., Miller, T. R., Krüger, K. M., Browman, K. E., Thiffault, C., Wetter, J., Marsh, K. C., Hancock, A. A., Esbenshade, T. A., and Cowart, M. D. (2008). The alkaloid conessine and analogues as potent histamine H3 receptor antagonists. J. Med. Chem. 51, 5423–5430.
NMR chemical shifts (δ) are reported in ppm downfield from tetramethylsilane as internal reference. Multiplicities of peaks have following abbreviations: br, broad; s, singlet; d, doublet; dd, double doublet; t, triplet; m, multiplet; q, quintet. Coupling constants are given in Hertz (Hz). Numbers and assignments of protons or carbon atoms are named: ox, oxalate; pip, piperidine; ph, phenyl; prop, propyl; tetr, tetralone; dmab, 4-(dimethylamino)benzyl; dmap, 4-(dimethylamino)phenyl; benzylid, benzylidene; allylid, allyllidene.
Piperidine (47.4 g, 0.56 mol), 3-chloropropan-1-ol (35.1 g, 0.37 mol), potassium carbonate (77.0 g, 0.56 mol) and potassium iodide (61.6 g, 0.37 mol) were refluxed in absolute acetone (300 mL) for 72 h under inert atmosphere. The mixture was allowed to cool to room temperature, inorganic components were removed by filtration and the filtrate was concentrated to dryness. The crude oil was further purified by distillation (10 mbar, 88°C). The oily product was crystallized as hydrochloride from isopropanolic HCl. White solid, 54.1 g, 81%.
C8H17NO × HCl
Molecular weight: 179.7
1H NMR (300 MHz, DMSO-d6) δ 10.59 (br s, 1H, NH+), 4.39 (s, 1H, OH), 3.46 (t, J = 5.9, 2H, prop-1H2), 3.36–3.31 (m, 2H, pip-2,6Heq), 3.01–2.94 (m, 2H, prop-3H2), 2.84–2.80 (m, 2H, pip-2,6Hax), 1.86–1.66 (m, 7H, prop-2H2, pip-3,5H2, pip-4Heq), 1.42–1.28 (m, 1H, pip-4Hax).
13C NMR (75 MHz, DMSO-d6) δ 57.99 (prop-1C), 53.49 (prop-3C), 52.99 (pip-2,6C), 26.36 (prop-2C), 22.18 (pip-3,5C), 21.33 (pip-4C).
ESI MS: 143.8 [M + H+] (100).
3-(Piperidin-1-yl)propan-1-ol hydrochloride (35.3 g, 0.2 mol) was suspended in toluene (100 mL) an excess of thionyl chloride (28.5 mL, 0.4 mol) was added dropwise at a temperature of 0°C under inert atmosphere. After beginning of the exothermic reaction the mixture was stirred for 3 h at 60°C. Thionyl chloride and toluene were distilled off. The beige product crystallized during the reaction and could be filtered off. Beige solid, 38.1 g, 98%.
C8H16ClN × HCl
Molecular weight: 198.1
1H NMR (300 MHz, DMSO-d6) δ 10.69 (br s, 1H, NH+), 3.68 (t, J = 6.4, 2H, prop-3H2), 3.41–3.36 (m, 2H, pip-2,6Heq), 3.11–3.04 (m, 2H, prop-1H2), 2.94–2.81 (m, 2H, pip-2,6Hax), 2.31–2.22 (m, 2H, prop-2H2), 1.77–1.60 (m, 5H, pip-3,5H2, pip-4Heq), 1.39–1.28 (m, 1H, pip-4Hax).
13C NMR (75 MHz, DMSO-d6) δ 54.14 (prop-3C), 52.01 (pip-2,6C), 41.44 (prop-1C), 25.19 (prop-2C), 23.08 (pip-3,5C), 22.31 (pip-4C).
ESI MS: 161.6 [M + H+] (100).
6-(3-(Piperidin-1-yl)propoxy)-1-tetralone. 1-(3-Chloropropyl)piperidine hydrochloride (1 g, 5.1 mmol), 6-hydroxy-1-tetralone (0.9 g, 5.6 mmol), potassium carbonate (2.3 g, 16.7 mmol) and potassium iodide (0.9 g, 5.6 mmol) were refluxed in absolute acetone (100 mL) for 72 h under inert atmosphere. After cooling to room temperature inorganic salts were filtered off. The filtrate was concentrated to dryness and resuspended in dichloromethane. The organic layer was washed three times with 2 M NaOH solution and brine, dried with MgSO4, and concentrated to dryness. Resulting brown oil was crystallized with waterfree oxalic acid in absolute ethanol. Beige crystals were filtered off. Beige solid, 1.4 g, 71%.
C18H25NO2 × (COOH)2 × 0.25 H2O
Molecular weight: 381.9
1H NMR (400 MHz, DMSO-d6) δ 7.83 (d, J = 8, 1H, tetr-8H), 6.90 (m, J = 12, 2H, tetr-5,7H), 4.09 (t, J = 8, 2H, prop-3H2), 2.91 (t, J = 8, 2H, tetr-2H2), 2.52 (t, J = 9, 2H, tetr-4H2), 2.38 (t, 2H, J = 8, prop-1H2), 2.36–2.34 (m, 4H, pip-2,6H2), 2.01 (q, 2H, J = 8, tetr-3H2), 1.88 (q, 2H, J = 7, prop-2H2), 1.54–1.46 (m, 4H, pip-3,5H2), 1.43–1.35 (m, 2H, pip-4H2).
13C NMR (100 MHz, DMSO-d6) δ 196.04 (tetr-1C), 164.40 (ox-COOH), 162.53 (tetr-7C), 147.18 (tetr-8aC), 128.67 (tetr-8C), 125.63 (tetr-4aC), 113.58 (tetr-5C), 113.08 (tetr-7C), 66.24 (prop-3C), 54.96 (prop-1C), 54.08 (pip-2,6C), 38.38 (tetr-2C), 29.27(tetr-4C), 26.13 (prop-2C), 25.57 (pip-3,5C), 24.10 (pip-4C), 22.93 (tetr-3C).
ESI MS: 288.52 [M + H+] (100).
Anal. calc.: C, 62.89; H, 7.26; N, 3.67. Found: C, 62.91; H, 7.41; N, 3.58.
7-Hydroxy-1-tetralone. 7-Methoxy-1-tetralone (1 g, 5.7 mmol), para-toluenesulfonic acid (3.2 g, 17.0 mmol), and 1-butyl-3-methyl-1H-imidazol-3-ium bromide (12.2 g, 34.1 mmol) were mixed in a 20 mL microwave vial. Mixture was heated for 1 h for 160°C under microwave condition. After cooling down to room temperature the reaction mixture was diluted with water and extracted three times with ethyl acetate. The organic phase was washed with brine, dried with MgSO4, and concentrated to dryness. Resulting yellowish crystals were used without further purification. Yellowish solid, 0.9 g, 97%.
C10H10O2
Molecular Weight: 162.1
1H NMR (250 MHz, DMSO-d6) δ 9.58 (s, 1H, OH), 7.25 (s, 1H, tetr-8H), 7.19 (d, J = 10, 1H, tetr-5H), 6.98 (d, J = 10, 1H, tetr-6H), 2.83 (t, J = 7.5, 2H, tetr-4H2), 2.55 (t, J = 7.5, 2H, tetr-2H2), 2.00 (q, J = 6.3, 2H, tetr-3H2).
13C NMR (63 MHz, DMSO-d6) δ 197.48 (tetr-1C), 155.86 (tetr-7C), 135.27 (tetr-4aC), 133.06 (tetr-8aC), 130.18 (tetr-5C), 121.10 (tetr-6C), 111.80 (tetr-8C), 38.30 (tetr-2C), 28.12 (tetr-4C), 23.25 (tetr-3C).
ESI MS: 160.5 [M − H+] (100).
7-(3-(Piperidin-1-yl)propoxy)-1-tetralone. 1-(3-Chloropropyl)piperidine hydrochloride (1.55 g, 7.9 mmol), 7-hydroxy-1-tetralone (1.4 g, 8.6 mmol), potassium carbonate (3.6 g, 25.9 mmol), and potassium iodide (1.4 g, 8.6 mmol) were refluxed in absolute acetone (50 mL) for 72 h under inert atmosphere. After cooling to room temperature inorganic salts were filtered off. The filtrate was concentrated to dryness and resuspended in dichloromethane. The organic layer was washed three times with 2 M NaOH solution and brine, dried with MgSO4, and concentrated to dryness. Beige crystals, 2.1 g, 94%.
C18H25NO2
Molecular Weight: 287.4
1H NMR (250 MHz, DMSO-d6) δ 7.34 (s, 1H, tetr-8H), 7.29 (d, J = 7.5, 1H, tetr-5H), 7.16 (d, J = 7.5, 1H, tetr-6H), 4.02 (t, J = 5, 2H, prop-1H2), 2.87 (t, J = 6.25, 2H, tetr-4H2), 2.59 (t, J = 6.25, 2H, tetr-2H2), 2.38 (t, 2H, J = 5, prop-3H2), 2.35–2.31 (m, 4H, pip-2,6H2), 2.02 (q, 2H, J = 5, tetr-3H2), 1.86 (q, 2H, J = 7, prop-2H2), 1.54–1.48 (m, 4H, pip-3,5H2), 1.41–1.34 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 197.38 (tetr-1C), 157.35 (tetr-7C), 137.14 (tetr-4aC), 133.18 (tetr-8aC), 130.60 (tetr-5C), 122.08 (tetr-6C), 109.99 (tetr-8C), 66.39 (prop-1C), 55.09 (prop-3C), 54.10 (pip-2,6C), 38.45 (tetr-2C), 27.94 (tetr-4C), 26.56 (prop-2C), 25.60 (pip-3,5C), 24.10 (pip-4C), 23.09 (tetr-3C).
ESI MS: 288.6 [M + H+] (100).
HRMS: calc.: 288.19581; found: 288.19633.
(E)-2-(4-(Dimethylamino)benzylidene)-6-(3-(piperidin-1-yl)propoxy)-1-tetralone. Compound 1 (500 mg, 1.7 mmol) and 4-(dimethylamino)benzaldehyde (260 mg, 1.7 mmol) were dissolved in 5 mL ethanol. 15 M NaOH (0.25 mL) was added. The mixture was stirred over night at room temperature, concentrated to dryness, and purified by column chromatography (CH2Cl2/MeOH, 9/1). The oily product was crystallized with waterfree oxalic acid in absolute ethanol. Crystals were filtered off. Yellow solid, 582 mg, 63%.
C27H34N2O2 × (COOH)2 × 1.25 H2O
Molecular weight: 530.78
1H NMR (400 MHz, DMSO-d6) δ 7.92 (d, J = 8, 1H, tetr-8H), 7.63 (s, 1H, benzylid-2H), 7.45 (d, J = 8, 2H, dmab-2,6H), 6.95 (d, J = 8, 1H, tetr-7H), 6.90 (s, 1H, tetr-5H), 6.79 (d, J = 8, 2H, dmab-3,5H), 4.16 (t, J = 12, 2H, prop-3H2), 3.12–3.08 (m, 6H, prop-1H2, pip-2,6H2), 3.00 (s, 6H, dmab-N(CH3)), 2.96 (t, J = 9, 2H, tetr-4H2), 2.92 (q, J = 8, 2H, tetr-3H2), 2.15 (q, J = 7, 2H, prop-2H2), 1.80–1.69 (m, 4H, pip-3,5H2), 1.61–1.41 (m, 2H, pip-4H2).
13C NMR (100 MHz, DMSO-d6) δ 196.12 (tetr-1C), 164.32 (ox-COOH), 161.87 (tetr-7C), 150.52 (dmab-4C), 145.45 (tetr-8aC), 136.37 (benzylid-2C), 132.05 (tetr-2C), 131.84 (dmab-2,6C), 129.67 (tetr-8C), 126.64 (tetr-4aC), 122.64 (dmab-1C), 113.77 (tetr-5C), 112.72 (tetr-7C), 111.68 (dmab-3,5C), 65.30 (prop-3C), 53.40 (prop-1C), 52.28 (pip-2,6C), 40.12 (dmab-N(CH3)2), 28.18(tetr-4C), 26.82 (prop-2C), 23.54 (pip-4C), 22.78 (pip-3,5C), 21.53 (tetr-3C).
ESI MS: 419.6 [M + H+] (100).
Anal. calc.: C, 65.58; H, 7.31; N, 5.27. Found: C, 65.63; H, 6.92; N, 5.27.
(E)-2-(4-(Dimethylamino)benzylidene)-7-(3-(piperidin-1-yl)propoxy)-1-tetralone. Compound 2 (500 mg, 1.7 mmol) and 4-(dimethylamino)benzaldehyde (260 mg, 1.7 mmol) were dissolved in 7 mL ethanol. 15 M NaOH (0.25 mL) was added. The mixture was stirred over night at room temperature, concentrated to dryness, and purified by column chromatography (CH2Cl2/MeOH, 9/1). Yellow solid, 619 mg, 85%.
C27H34N2O2
Molecular Weight: 418.6
1H NMR (250 MHz, DMSO-d6) δ 7.67 (s, 1H, benzylid-2H), 7.47 (d, J = 10, 2H, dmab-3,5H), 7.42 (s, 1H, tetr-8H), 7.30 (d, J = 7.5, 1H, tetr-5H), 7.16 (d, J = 8.75, 1H, tetr-6H), 6.79 (d, J = 7.5, 2H, dmab-2,6H), 4.06 (t, J = 7.5, 2H, prop-3H2), 3.09 (t, J = 6.25, 2H, tetr-3H2), 2.99 (s, 6H, dmab-N(CH3)), 2.86 (t, J = 7.5, 2H, tetr-4H2), 2.62–2.52 (m, 6H, prop-3H2, pip-2,6H2), 1.99 (q, 2H, J = 9, prop-2H2), 1.65–1.56 (m, 4H, pip-3,5H2), 1.48–1.41 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 186.24 (tetr-1C),157.44 (tetr-7C), 150.68 (dmab-4C), 137.24 (benzylid-2C), 134.30 (tetr-8aC), 131.91 (dmab-2,6C), 130.96 (tetr-4aC), 130.34 (tetr-5C), 122.63 (dmab-1C), 117.11 (tetr-6C), 111.93 (dmab-3,5C), 110.60 (tetr-8C), 65.94 (prop-1C), 54.54 (prop-3C), 53.21 (pip-2,6C), 39.53 (dmab-N(CH3)2), 27.37 (pip-3,5C), 26.12 (tetr-4C), 25.47 (prop-2C), 24.32 (pip-4C), 23.18 (tetr-3C).
ESI MS: 419.7 [M + H+] (100).
HRMS: calc.: 419.26930; found: 419.26971.
(E)-2-((E)-3-(4-(Dimethylamino)phenyl)allylidene)-6-(3-(piperidin-1-yl)propoxy)-1-tetralone. Compound 1 (500 mg, 1.7 mmol) and 4-(dimethylamino)cinnamic aldehyde (305 mg, 1.7 mmol) were dissolved in 3 mL ethanol. 15 M NaOH (0.5 mL) was added. The mixture was stirred over night at room temperature, concentrated to dryness, and purified by column chromatography (CH2Cl2/MeOH, 9/1). The oily product was crystallized with ethanol. Crystals were filtered off. Red solid, 545 mg, 69%.
C29H36N2O2×0.5 H2O
Molecular weight: 453.6
1H NMR (400 MHz, DMSO-d6) δ 7.90 (d, J = 8, 1H, tetr-8H), 7.52 (d, J = 16, 1H, allylid-2H), 7.31 (d, J = 8, 2H, dmap-2,6H), 6.95 (d, J = 8, 1H, tetr-7H), 6.94 (d, J = 16, 1H, allylid-4H), 6.91 (s, 1H, tetr-5H), 6.80 (d, J = 8, 2H, dmap-3,5H), 6.50 (t, J = 16, 1H, allylid-3H), 4.13 (t, J = 12, 2H, prop-3H2), 2.99 (s, 6H, dmap-N(CH3)), 2.97–2.93 (m, 6H, prop-1H2, pip-2,6H2), 2.47–2.31 (m, 4H, tetr-3H2, tetr-4H2), 1.96 (q, 2H, J = 7, prop-2H2), 1.56–1.48 (m, 4H, pip-3,5H2), 1.46–1.36 (m, 2H, pip-4H2).
13C NMR (100 MHz, DMSO-d6) δ 196.08 (tetr-1C), 162.37 (tetr-7C), 149.33 (dmap-4C), 144.58 (tetr-8aC), 141.31 (allylid-2C), 138.02 (allylid-4C), 135.99 (tetr-2C), 133.04 (dmap-2,6C), 129.06 (tetr-8C), 127.02 (tetr-4aC), 125.56 (allylid-3C), 121.97 (dmap-1C), 112.57 (tetr-5C), 112.21 (tetr-7C), 111.08 (dmap-3,5C), 64.98 (prop-3C), 52.27 (prop-1C), 51.98 (pip-2,6C), 40.32 (dmab-N(CH3)2), 28.88 (tetr-4C), 26.31 (prop-2C), 23.40 (pip-4C), 22.18 (pip-3,5C), 21.55 (tetr-3C).
ESI MS: 445.74 [M + H+] (100).
Anal. calc.: C, 76.79; H, 8.22; N, 6.18. Found: C, 76.71; H, 8.47; N, 5.90.
(E)-2-((E)-3-(4-(Dimethylamino)phenyl)allylidene)-7-(3-(piperidin-1-yl)propoxy)-1-tetralone. Compound 2 (500 mg, 1.74 mmol) and 4-(dimethylamino)cinnamic aldehyde (305 mg, 1.74 mmol) were dissolved in 3 mL ethanol. 15 M NaOH (0.5 mL) was added. The mixture was stirred over night at room temperature, concentrated to dryness, and purified by column chromatography (CH2Cl2/MeOH, 9/1). Red solid, 689 mg, 89%.
C29H36N2O2
Molecular Weight: 444.6
1H NMR (250 MHz, DMSO-d6) δ 7.53 (d, J = 7.5, 1H, allyl-2H), 7.43–7.36 (m, 3H, dmap-2,6H, tetr-8H), 7.28 (d, J = 7.5, 1H, tetr-5H), 7.18–7.12 (m, 2H, tetr-6H, allylid-4H), 6.80 (t, J = 16, 1H, allyl-3H), 6.74 (d, J = 10, 2H, dmap-3,5H), 4.07 (t, J = 6.25, 2H, prop-1H2), 3.09 (t, J = 6.25, 2H, tetr-3H2), 2.98 (s, 6H, dmap-N(CH3)), 2.87 (t, J = 7.5, 2H, tetr-4H2), 2.74–2.60 (m, 6H, prop-3H2, pip-2,6H2), 2.01 (q, J = 9, 2H, prop-2H2), 1.68–1.54 (m, 4H, pip-3,5H2), 1.52–1.40 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 185.72 (tetr-1C), 157.42 (tetr-7C), 151.24 (dmap-4C), 142.62 (allylid-2C), 137.31 (allylid-4C), 134.41 (tetr-8aC), 130.92 (tetr-4aC), 129.71 (tetr-5C), 128.98 (dmap-2,6C), 124.22 (allylid-3C), 120.54 (dmap-1C), 118.97 (tetr-6C), 112.03 (dmap-3,5C), 110.64 (tetr-8C), 65.97 (prop-1C), 54.54 (prop-3C), 53.41 (pip-2,6C), 40.29 (dmap-N(CH3)2), 28.86 (tetr-4C), 27.17 (prop-2C), 25.86 (pip-3,5C), 24.74 (pip-4C), 23.24 (tetr-3C).
ESI MS: 445.7 [M + H+] (100).
HRMS: calc.: 445.28495; found: 445.28500.
(4-(3-(Piperidin-1-yl)propoxy)phenyl)methanol. 1-(3-Chloropropyl)piperidine hydrochloride (4 g, 20.2 mmol), 4-hydroxybenzyl alcohol (2.5 g, 22.2 mmol), potassium carbonate (8.3 g, 60.6 mmol), and potassium iodide (1.7 g, 20.2 mmol) were refluxed in absolute acetone (100 mL) for 72 h under inert atmosphere. After cooling to room temperature inorganic salts were filtered off. The filtrate was concentrated to dryness and resuspended in dichloromethane. The organic layer was washed three times with 2 M NaOH solution and brine, dried with MgSO4, and concentrated to dryness. Yellowish solid, 3.6 g, 72%.
C15H23NO2
Molecular weight: 249.4
1H NMR (250 MHz, DMSO-d6) δ 7.29 (d, J = 10, 2H, ph-2,6H), 6.96 (d, J = 10, 2H, ph-3,5H), 5.12 (t, J = 7, 1H, OH), 4.49 (d, J = 10, 2H, CH2-OH), 4.03 (t, J = 7.5, 2H, prop-1H2), 2.42 (t, J = 7.5, 2H, prop-3H2), 2.37–2.28 (m, 4H, pip-2,6H2), 1.93 (q, J = 6.26, 2H, prop-2H2), 1.60–1.49 (m, 4H, pip-3,5H2), 1.49–1.38 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 157.45 (ph-4C), 134.30 (ph-1C), 127.95 (ph-2,6C), 113.93 (ph-3,5C), 66.01 (prop-1C), 62.75 (CH2-OH), 55.25 (prop-3C), 54.11 (pip-2,6C), 26.57 (prop-2C), 25.26 (pip-3,5C), 23.80 (pip-4C).
ESI MS: 250.46 [M + H+] (100).
1-(3-(4-(Chloromethyl)phenoxy)propyl)piperidine hydrochloride. (4-(3-(Piperidin-1-yl)propoxy)phenyl)methanol (3.6 g, 14.5 mmol) was dissolved in 100 mL toluene and an excess of thionyl chloride (3.2 mL, 43.6 mmol) was added dropwise at 0°C under inert atmosphere. Once the exothermic reaction had decayed the mixture was stirred for 3 h at 60°C. Afterward toluene and thionylchloride was distilled off. Crude product was re-crystallized in ethanol. Beige solid, 4.1 g, 93%.
C15H22ClNO × HCl
Molecular weight: 304.3
1H NMR (250 MHz, DMSO-d6) δ 10.62 (br s, 1H, NH+), 7.42 (d, J = 15, 2H, ph-2,6H), 6.99 (d, J = 15, 2H, ph-3,5H), 4.73 (s, 2H, CH2-Cl), 4.07 (t, J = 10, 2H, prop-1H2), 3.45 (t, J = 10, 2H, prop-3H2), 3.21–3.11 (m, 2H, pip-2,6Heq), 2.94–2.81 (m, 2H, pip-2,6Hax), 1.91 (q, J = 6.26, 2H, prop-3H2), 1.69–1.50 (m, 5H, pip-3,5H2, pip-4Heq), 1.49–1.38 (m, 1H, pip-4Hax).
13C NMR (63 MHz, DMSO-d6) δ 158.38 (ph-4C), 130.35 (ph-2,6C), 130.33 (ph-1C), 114.58 (ph-3,5C), 65.35 (prop-1C), 53.44 (prop-3C), 51.86 (pip-2,6C), 46.08 (CH2-Cl), 23.30 (prop-2C), 22.43 (pip-3,5C), 21.55 (pip-4C).
ESI MS: 268.42 [M + H+] (100), 571.78 [2M + HCl] (19).
6-(4-(3-(Piperidin-1-yl)propoxy)benzyloxy)-1-tetralone. 1-(3-(4-(Chloromethyl)phenoxy)propyl)piperidine hydrochloride (1.7 g, 5.6 mmol), 6-hydroxy-1-tetralone (1.0 g, 6.2 mmol), potassium carbonate (2.6 g, 18.5 mmol) and potassium iodide (1.0 g, 6.2 mmol) were refluxed in absolute acetone (70 mL) for 72 h under inert atmosphere. After cooling to room temperature inorganic salts were filtered off. The filtrate was concentrated to dryness and resuspended in dichloromethane. The organic layer was washed three times with 2 M NaOH solution and brine, dried with MgSO4, and concentrated to dryness. Beige solid, 1.4 g, 65%.
C25H31NO3
Molecular weight: 393.5
1H NMR (250 MHz, DMSO-d6) δ 7.84 (d, J = 7.5, 1H, tetr-8H), 7.42 (d, J = 7.5, 2H, ph-2,6H), 6.99–6.93 (m, 4H, ph-3,5H, tetr-5H, tetr-7H), 5.11 (s, 2H, ph-CH2), 4.06 (t, J = 6.3, 2H, prop-1H2), 3.11–3.04 (m, 6H, prop-3H2, pip-2,6H2), 2.90 (t, J = 6.3, 2H, tetr-2H2), 2.53 (t, J = 6.3, 2H, tetr-4H2), 2.15–1.96 (m, 4H, tetr-3H2, prop-2H2), 1.73–1.69 (m, 4H, pip-3,5H2), 1.55–1.53 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 196.37 (tetr-1C), 162.30 (tetr-6C), 158.39 (ph-4C), 147.23 (tetr-8aC), 130.00 (ph-2,6C), 128.63 (tetr-8C, ph-1C), 126.08 (tetr-4aC), 114.93 (ph-3,5C), 113.56 (tetr-5C), 113.54 (tetr-7C), 69.50 (prop-1C), 65.39 (ph-CH2), 53.84 (prop-3C), 52.67 (pip-2,6C), 38.93 (tetr-2C), 29.17 (tetr-4C), 24.28 (prop-2C), 23.11 (pip-3,5C), 23.09 (pip-4C), 21.73 (tetr-3C).
ESI MS: 394.7 [M + H+] (100).
HRMS: calc.: 394.23767; found: 394.23800.
7-(4-(3-(Piperidin-1-yl)propoxy)benzyloxy)-1-tetralone. 1-(3-(4-(Chloromethyl)phenoxy)propyl)piperidine hydrochloride (2.4 g, 7.9 mmol), 7-hydroxy-1-tetralone (1.4 g, 8.6 mmol), potassium carbonate (3.6 g, 25.9 mmol) and potassium iodide (1.4 g, 8.6 mmol) were refluxed in absolute acetone (300 mL) for 72 h under inert atmosphere. After cooling to room temperature inorganic salts were filtered off. The filtrate was concentrated to dryness and resuspended in dichloromethane. The organic layer was washed three times with 2 M NaOH solution and brine, dried with MgSO4, and concentrated to dryness. Beige solid, 2.2 g, 71%.
C25H31NO3
Molecular Weight: 393.5
1H NMR (250 MHz, DMSO-d6) δ 7.34 (s, 1H, tetr-8H), 7.33 (d, J = 10, 2H, ph-2,6H), 7.23 (d, J = 7.5, 1H, tetr-5H), 7.15 (d, J = 7.5, 1H, tetr-6H), 6.90 (d, J = 10, 2H, ph-3,5H), 5.00 (s, 2H, ph-CH2), 3.97 (t, J = 5, 2H, prop-1H2), 2.80 (t, J = 7.5, 2H, tetr-4H2), 2.50 (t, J = 7.5, 2H, tetr-2H2), 2.46–2.40 (m, 6H, prop-3H2, pip-2,6H2), 2.02 (q, 2H, J = 5, tetr-3H2), 1.92 (q, 2H, J = 7, prop-2H2), 1.68–1.61 (m, 4H, pip-3,5H2), 1.52–1.43 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 197.29 (tetr-1C), 157.88 (ph-4C), 156.80 (tetr-7C), 137.10 (tetr-4aC), 133.03 (tetr-8aC), 130.88 (ph-1C), 129.17 (ph-2,6C), 129.10 (tetr-5C), 121.67 (tetr-6C), 114.60 (ph-3,5C), 110.11 (tetr-8C), 69.41 (prop-1C), 67.55 (ph-CH2), 57.84 (prop-3C), 56.07 (pip-2,6C), 38.34 (tetr-2C), 30.42 (tetr-4C), 27.88 (prop-2C), 26.09 (pip-3,5C), 24.95 (pip-4C), 23.14 (tetr-3C).
ESI MS: 394.7 [M + H+] (100).
HRMS: calc.: 394.23767; found: 394.23767.
(E)-2-(4-(Dimethylamino)benzylidene)-6-(4-(3-(piperidin-1-yl)propoxy)benzyloxy)-1-tetralone. Compound 7 (300 mg, 0.8 mmol) and 4-(dimethylamino)benzaldehyde (113, 0.8 mmol) were dissolved in 3 mL ethanol. 15 M NaOH (0.2 mL) was added. The mixture was stirred over night at room temperature, concentrated to dryness, and purified by column chromatography (CH2Cl2/MeOH, 9/1). Yellow solid, 357 mg, 85%.
C34H40N2O3
Molecular Weight: 524.7
1H NMR (250 MHz, DMSO-d6) δ 7.89 (d, J = 7.5, 1H, tetr-8H), 7.61 (s, 1H, benzylid-2H), 7.43–7.37 (m, 4H, dmab-2,6H, ph-2,6H), 7.04–6.96 (m, 4H, tetr-7H, ph-3,5H, tetr-5H), 6.74 (d, J = 7.5, 2H, dmab-3,5H), 5.10 (s, 2H, ph-CH2), 4.02 (t, J = 5, 2H, prop-1H2), 3.07 (t, J = 7.5, 2H, tetr-3H2), 2.97 (s, 6H, dmab-N(CH3)2), 2.85 (t, J = 7.5, 2H, tetr-4H2), 2.68–2.61(m, 6H, prop-3H2, pip-2,6H2), 1.99 (q, J = 5, 2H, pip-2H2), 1.62–1.58 (m, 4H, pip-3,5H2), 1.48–1.38 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 185.13 (tetr-1C), 162.03 (tetr-6C), 158.31 (ph-4C), 150.38 (dmab-4C), 145.37 (tetr-2C), 136.27 (benzylid-2C), 132.01 (tetr-8aC), 131.79 (dmab-2,6C), 130.70 (tetr-8C), 130.55 (ph-2,5C), 129.61 (ph-1C), 126.79 (tetr-4aC), 122.64 (dmab-1C), 114.40 (ph-3,5C), 113.06 (tetr-5C), 111.65 (dmab-3,5C), 110.94 (tetr-7C), 69.22 (prop-1C), 65.50 (ph-CH2), 54.35 (prop-3C), 53.21 (pip-2,6C), 40.13 (N(CH3)2), 28.17 (tetr-4C), 26.79 (prop-2C), 24.97 (pip-4C), 24.22 (pip-3,5C), 22.86 (tetr-3C).
ESI MS: 525.5 [M + H+] (100).
HRMS: calc.: 525.31117; found: 525.31034.
(E)-2-(4-(Dimethylamino)benzylidene)-7-(4-(3-(piperidin-1-yl)propoxy)benzyloxy)-1-tetralone. Compound 8 (60 mg, 0.2 mmol) and 4-(dimethylamino)benzaldehyde (23 mg, 0.2 mmol) were dissolved in 1.5 mL ethanol. 15 M NaOH (0.04 mL) was added. The mixture was stirred over night at room temperature, concentrated to dryness, and purified by column chromatography (CH2Cl2/MeOH, 9/1). Yellow solid, 72 mg, 91%.
C34H40N2O3
Molecular weight: 524.7
1H NMR (250 MHz, DMSO-d6) δ 7.58 (s, 1H, benzylid-2H), 7.41 (d, J = 5, 2H, dmab-3,5H), 7.36 (d, J = 5, 2H, ph-2,6H), 7.31 (s, 1H, tetr-8H), 7.23 (d, J = 7.5, 1H, tetr-5H), 7.14 (d, J = 7.5, 1H, tetr-6H), 6.90 (d, J = 7.5, 2H, ph-3,5H), 6.72 (d, J = 10, 2H, dmab-3,5H), 5.01 (s, 2H, ph-CH2), 3.97 (t, J = 5, 2H, prop-1H2), 3.02 (t, J = 5, 2H, tetr-3H2), 2.92 (s, 6H, dmab-N(CH3)2), 2.89 (t, J = 7.5, 2H, tetr-4H2), 2.84–2.78 (m, 6H, prop-3H2, pip-2,6H2), 1.99 (q, J = 5, 2H, prop-2H2), 1.66–1.60 (m, 4H, pip-3,5H2), 1.42–1.33 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 185.93 (tetr-1C), 158.30 (ph-4C), 157.28 (tetr-7C), 150.98 (dmab-4C), 137.16 (benzylid-2C), 135.54 (tetr-2C), 134.52 (tetr-8aC), 132.01 (dmab-2,6C), 130.43 (tetr-4aC), 129.75 (tetr-5C), 129.48 (ph-2,6C), 129.13 (ph-1C), 122.73 (dmab-1C), 120.70 (tetr-6C), 114.81 (ph-3,5C), 111.69 (dmab-3,5C), 110.80 (tetr-8C), 69.50 (prop-1C), 65.43 (ph-CH2), 55.73 (prop-3C), 53.04 (pip-2,6C), 39.62 (dmab-N(CH3)3), 28.24 (tetr-4C), 27.78 (prop-2C), 27.03 (pip-3,5C), 24.58 (pip-4C), 23.94 (tetr-3C).
ESI MS: 525.8 [M + H+] (100).
HRMS: calc.: 525.31117; found: 525.31129.
(E)-2-((E)-3-(4-(Dimethylamino)phenyl)allylidene)-6-(4-(3-(piperidin-1-yl)propoxy)benzyloxy)-1-tetralone. Compound 7 (300 mg, 0.8 mmol) and 4-(dimethylamino)cinnamic aldehyde (133 mg, 0.8 mmol) were dissolved in 5 mL ethanol. 15 M NaOH (0.22 mL) was added. The mixture was stirred over night at room temperature, concentrated to dryness, and purified by column chromatography (CH2Cl2/MeOH, 9/1). Yellow solid, 398 mg, 95%.
C36H42N2O3
Molecular Weight: 550.7
1H NMR (250 MHz, DMSO-d6) δ 7.96 (d, J = 10, 1H, tetr-8H), 7.57 (d, J = 7.5, 2H, ph-2,6H), 7.48 (d, J = 7.5, 2H, dmap-2,6H), 7.40 (d, J = 16, 1H, allylid-2H), 7.14–7.01 (m, 6H, ph-3,5H, dmap-3,5H, tetr-5H, tetr-7H), 6.84 (t, J = 16, 1H, allylid-3H), 6.79 (d, J = 16, 1H, allylid-4H), 5.19 (s, 2H, ph-CH2), 4.11 (t, J = 6.3, 2H, prop-1H2), 3.56–3.42 (m, 6H, prop-3H2, pip-2,6H2), 3.12–3.05 (m, 2H, tetr-3H2), 3.03 (s, 6H, N(CH3)2), 2.18 (q, J = 4.7, 2H, pip-2H2), 1.81–1.76 (m, 4H, pip-3,5H2), 1.65–1.59 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 184.91 (tetr-1C), 162.19 (tetr-6C), 158.09 (ph-4C), 151.02 (dmap-4C), 145.99 (tetr-8aC), 141.89 (allylid-2C), 136.49 (allylid-4C), 131.56 (tetr-2C), 129.79 (ph-2,6C), 128.89 (ph-1C),128.86 (tetr-8C), 127.37 (tetr-4aC), 124.39 (dmap-1,2,6C), 119.17 (allylid-3C), 114.89 (ph-3,5C), 114.15 (dmap-3,5C), 113.40 (tetr-5C), 111.91 (tetr-7C), 69.46 (prop-1C), 65.36 (ph-CH2), 53.81 (prop-3C), 52.70 (pip-2,6C), 40.22 (N(CH3)2), 28.30 (tetr-4C), 25.70 (pip-3,5C), 24.21 (prop-2C), 23.09 (pip-4C), 21.60 (tetr-3C).
ESI MS: 551.4 [M + H+] (100).
HRMS: calc.: 551.32682; found: 551.32618.
(E)-2-((E)-3-(4-(Dimethylamino)phenyl)allylidene)-7-(4-(3-(piperidin-1-yl)propoxy)benzyloxy)-1-tetralone. Compound 8 (60 mg, 0.2 mmol) and 4-(dimethylamino)cinnamic aldehyde (27 mg, 0.2 mmol) were dissolved in 1.5 mL ethanol. 15 M NaOH (0.04 mL) was added. The mixture was stirred over night at room temperature, concentrated to dryness, and purified by column chromatography (CH2Cl2/MeOH, 9/1). Yellow solid, 77 mg, 93%.
C36H42N2O3
Molecular Weight: 550.7
1H NMR (250 MHz, DMSO-d6) δ 7.59 (d, J = 16, 1H, allylid-2H), 7.54 (d, J = 7.5, 2H, dmap-2,6H), 7.47 (d, J = 5, 2H, ph-2,6H), 7.38 (s, 1H, tetr-8H), 7.35 (d, J = 5, 1H, tetr-5H), 7.29 (d, J = 10, 1H, tetr-6H), 7.17 (d, J = 16, 1H, allylid-3H), 7.04 (d, J = 10, 2H, ph-3,5H), 6.86 (t, J = 16, 1H, allylid-3H), 6.80 (d, J = 10, 2H, dmap-3,5H), 5.14 (s, 2H, ph-CH2), 4.11 (t, J = 6.25, 2H, prop-1H2), 3.18 (t, J = 5, 2H, tetr-3H2), 3.03 (s, 6H, dmap-N(CH3)2), 2.97 (t, J = 7.5, 2H, tetr-4H2), 2.95–2.81 (m, 6H, prop-3H2, pip-2,6H2), 2.15 (q, J = 5, 2H, prop-2H2), 1.85–1.77 (m, 4H, pip-3,5H2), 1.60–1.45 (m, 2H, pip-4H2).
13C NMR (63 MHz, DMSO-d6) δ 183.03 (tetr-1C), 158.34 (ph-4C), 157.83 (tetr-7C), 150.38 (dmap-4C), 141.18 (allylid-2C), 138.52 (allylid-4C), 135.46 (tetr-2C), 133.62 (tetr-8aC), 131.08 (dmap-2,6C), 129.63 (tetr-4aC), 129.88 (tetr-5C), 129.84 (ph-2,6C), 128.31 (ph-1C), 125.34 (allylid-3C), 124.71 (dmap-1C), 120.63 (tetr-6C), 114.62 (ph-3,5C), 114.92 (tetr-8C), 111.76 (dmap-3,5C), 73.58 (prop-1C), 70.44 (ph-CH2), 56.35 (prop-3C), 54.47 (pip-2,6C), 39.89 (dmap-N(CH3)3), 28.56 (tetr-4C), 27.85 (prop-2C), 25.83 (pip-3,5C), 24.83 (pip-4C), 23.45 (tetr-3C).
ESI MS: 551.8 [M + H+] (100).
HRMS: calc.: 551.32682; found: 551.32705.
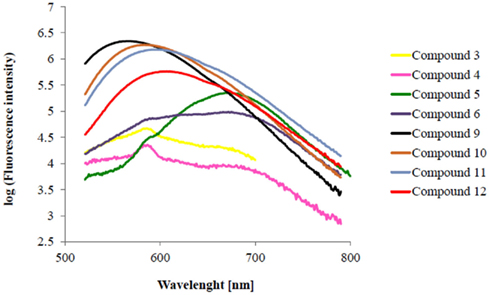
Figure A1. Fluorescence emission spectra of chalcone derivatives in logarithmic delineation (Excitation wavelength: 405 nm).
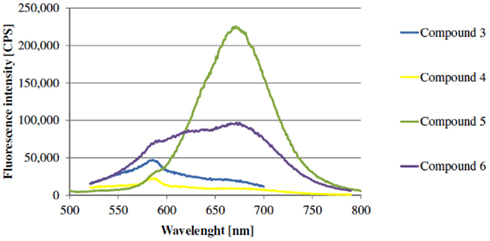
Figure A3. Fluorescence emission spectra of chalcone derivatives with O-alkyl-ether as spacer B (Excitation wavelength: 405 nm).
Keywords: human histamine H3 receptor, fluorescent ligand, fluorescence confocal laser scanning microscopy, pharmacological tool
Citation: Tomasch M, Schwed JS, Weizel L and Stark H (2012) Novel chalcone-based fluorescent human histamine H3 receptor ligands as pharmacological tools. Front. Syst. Neurosci. 6:14. doi: 10.3389/fnsys.2012.00014
Received: 25 January 2012; Paper pending published: 10 February 2012;
Accepted: 01 March 2012; Published online: 26 March 2012.
Edited by:
Maria Beatrice Passani, Universita’ di Firenze, ItalyReviewed by:
Marco De Amici, Università degli Studi di Milano, ItalyCopyright: © 2012 Tomasch, Schwed, Weizel and Stark. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Holger Stark, Biocenter, Institute of Pharmaceutical Chemistry, Johann Wolfgang Goethe University, Max-von-Laue-Str. 9, 60438 Frankfurt am Main, Germany. e-mail:aC5zdGFya0BwaGFybWNoZW0udW5pLWZyYW5rZnVydC5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.