- Department of Veterinary Integrative Biosciences, Texas A&M University, College Station, TX, USA
Brain activity differs in the various sleep stages and in conscious wakefulness. Awakening from sleep requires restoration of the complex nerve impulse patterns in neuronal network assemblies necessary to re-create and sustain conscious wakefulness. Herein I propose that the brain uses rapid eye movement (REM) to help wake itself up after it has had a sufficient amount of sleep. Evidence suggesting this hypothesis includes the facts that, (1) when first going to sleep, the brain plunges into Stage N3 (formerly called Stage IV), a deep abyss of sleep, and, as the night progresses, the sleep is punctuated by episodes of REM that become longer and more frequent toward morning, (2) conscious-like dreams are a reliable component of the REM state in which the dreamer is an active mental observer or agent in the dream, (3) the last awakening during a night’s sleep usually occurs in a REM episode during or at the end of a dream, (4) both REM and awake consciousness seem to arise out of a similar brainstem ascending arousal system (5) N3 is a functionally perturbed state that eventually must be corrected so that embodied brain can direct adaptive behavior, and (6) cortico-fugal projections to brainstem arousal areas provide a way to trigger increased cortical activity in REM to progressively raise the sleeping brain to the threshold required for wakefulness. This paper shows how the hypothesis conforms to common experience and has substantial predictive and explanatory power regarding the phenomenology of sleep in terms of ontogeny, aging, phylogeny, abnormal/disease states, cognition, and behavioral physiology. That broad range of consistency is not matched by competing theories, which are summarized herein. Specific ways to test this wake-up hypothesis are suggested. Such research could lead to a better understanding of awake consciousness.
Herein is presented the notion that rapid eye movement (REM) sleep is a sleeping brain’s attempt to recover consciousness from the disruption of deep sleep. The rationale derives from the following self-evident fact: awakening from deep sleep requires the brain to become “less sleepy” by re-organizing its circuitry toward the threshold for conscious wakefulness. REM may well be a mechanism for moving the brain state in that direction. REM may be a spontaneously triggered state that occurs when the brain has had enough slow-wave sleep (SWS).
Rapid eye movement normally occurs only after long sequence of SWS stages, initially dominated by deep Stage N3 [the new equivalent for the older term, Stage IV, according to the classification scheme of 2004 from the American Academy of Sleep Medicine (Iber et al., 2007)]. Hereafter, I will use N3 any time I am referring to the more familiar term, Stage IV. In N3 the complex neural network inter-relationships necessary to create and sustain wakefulness must surely be obliterated. REM also promotes numerous conscious-like dreams. Also, the last awakening from a night’s sleep typically occurs in a REM episode during a dream, suggesting that the threshold for wakefulness has been reached. These observations lead to the hypothesis that REM might be the brain’s way to awaken itself after it has had a sufficient amount of SWS.
Rapid eye movement and dreaming could be a self-start mode to re-construct and sustain consciousness after that capability has been wiped out in Stage N3. Stage N3 is so deep it even shuts down basic breathing reflexes in people with sleep apnea. Without REM and in the absence of external stimulus, the brain might stay in Stage N3 coma continuously until starvation and dehydration cause death. You could say, REM is the brain’s way of “booting up” its consciousness in the absence of an “external trigger.”
From the Stage N3 pit, the whole neural representation of conscious self has to be reconstructed from procedural memory, and the brain has to re-start the process from a zero baseline. The aborted REM episodes early in a night’s sleep may reflect early attempts to help the brain recover the capacity for creating consciousness. These attempts may have to reach a certain set-point threshold, and may even be probabilistic, involving trial and error. Sustained awakening might require a series of REM episodes throughout the night.
The inherent speculation in any hypothesis needs to meet four criteria. The hypothesis must,
(1) have evidence to suggest it
(2) yield predictive and explanatory power
(3) compare favorably with competing theories
(4) be testable
How this wake-up hypothesis meets these criteria is summarized below.
Evidence
All mammals (and birds and a few reptilian species) have a recurrent cyclic pattern of sequential stages of SWS and REM (Weitzman, 1972). The cycle reflects distinct state changes with likely corresponding changes in unit activity and associated field potentials. Certain well-established facts about this cyclic sleep pattern suggest that the REM stage of sleep serves to awaken a sleeping brain.
(1) A normal human nightly sleep is a progression of unconscious sleep stages, punctuated by episodes of brain activation (REM) in which brain activity resembles that seen in wakefulness and dreams occur in which events seem to be consciously perceived (Figure 1). Early in the night, the human brain undergoes a state change of so-called “SWS,” so-called because of the dominance of slow-frequency brain field potentials. The brain then soon plunges into what is known as Stage N3, a relatively long and deep abyss of sleep as far removed from consciousness as a normal, un-drugged brain can get. Obviously, whatever patterns of nerve impulses and network temporal–spatial activity were required to construct and sustain consciousness during wakefulness were obliterated upon falling asleep. The large slow electrical waves that permeate the neocortex during mammalian sleep presumably reflect slow synchronous activity of neurons that is preventing emergence of consciousness. How does the brain get itself out of this abyss in the absence of external stimulation?
(2) Dreaming, a kind of awake state in which the dreamer is an active mental observer or agent in the dream, is a reliable component of the REM state. The wake-up hypothesis regards dreaming as a consequence of REM. Like the consciousness of wakefulness, the ability to dream in REM requires highly ordered and orchestrated neuronal population activity. Though the brain is activated in REM, it lacks the corrective feedback from external reality of wakefulness and that of course could help explain why the dreaming brain constructs images and story lines that may often be bizarre. The state change progression to REM is also a progression toward consciousness.
(3) Slow-wave sleep and REM have apparently co-evolved, being most conspicuous in mammals. Suppose the brain mechanisms that evolved to produce awareness in dreams are the same as those that evolved to produce human consciousness. Suppose rudiments of those same dreaming and consciousness processes occur in lower mammals. Of course we cannot prove non-humans are conscious. But, since REM and dreams coincide in humans, there is the possibility that other mammals that also have REM may also dream and have a degree of consciousness. Anybody who seen dogs sleep is hard pressed to explain in any other way how the feet paddling, nose twitching, change in facial expression, and barking that often occur during their REM sleep occur without a cognitive component.
(4) Most people awaken after a normal night’s sleep in the morning at some point in a REM episode, often in the midst of a dream. This suggests that there is a threshold for awakening and consciousness and that it is REM that drives the sleeping brain to reach that threshold.
(5) Rapid eye movement most likely arises from some of the same ascending brainstem arousal influences that create and sustain wakefulness. In awake subjects, conscious arousal is enhanced by activity in the brainstem’s ascending reticular arousal system (ARAS). Sleeping animals are awakened by stimuli that activate the ARAS. Conscious experiences and thinking interfere with sleep, because sensory input activates the ARAS, which in turn activates the neocortex to produce wakefulness and consciousness (Figure 2).
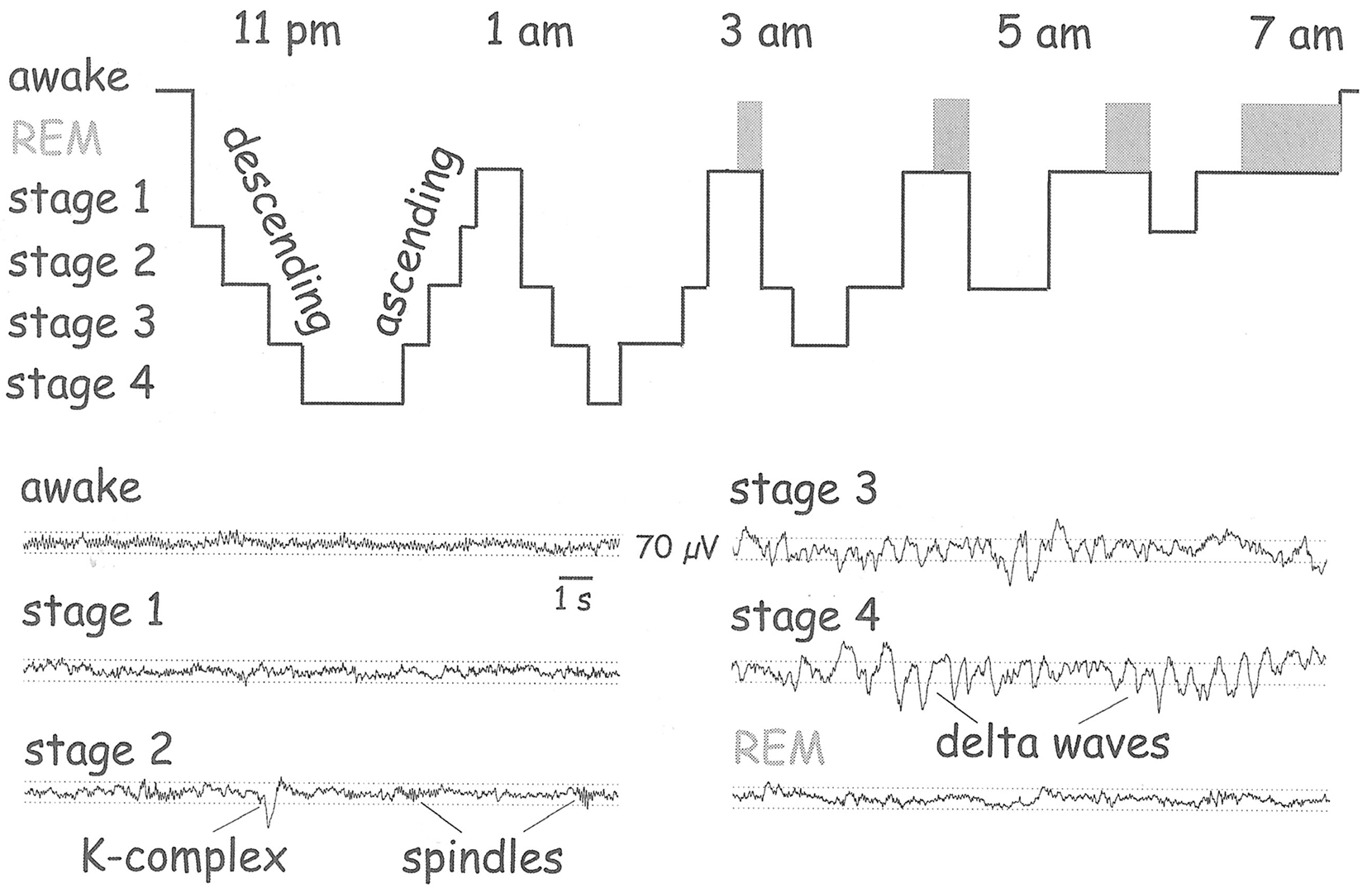
Figure 1. Schematic illustration of the sequence of stages in normal human sleep. For the first hour or so, the brain is plunged into a deep functional abyss. As the night progresses, SWS episodes (Stages 1-4) become shorter, and REM episodes appear and become longer, culminating in wakefulness in the morning. The EEG (lower half of figure) reflects these state changes and reveals similarity between wakefulness and REM. (From Buzsáki, 2006).
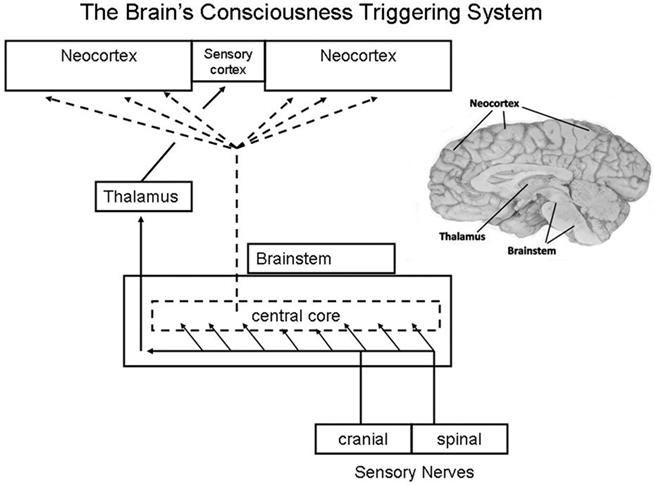
Figure 2. Diagram of the traditional view of the function of the ascending reticular arousal system (ARAS), which provides a global activation of neocortex at the same time that somatic sensations are topographically routed via specific thalamic nuclei to the sensory cortex. Typically over-looked (and not illustrated here) are the feedback influences from neocortex to thalamus and brainstem reticulum. See comments below it item #6. (From Klemm, 2011).
Though there are many discrete and heterogeneous neuronal clusters in the reticular formation, widespread activation can be produced from focal, low-level electrical stimulation at most levels along the central reticular core. Activation responses are not limited to the cortex and EEG, but also include limbic system (onset of hippocampal theta activity) and both ascending and descending muscle tone (Klemm, 1972). The brainstem reticular formation was originally thought to be a homogeneous polysynaptic net that indiscriminatingly generated consciousness from sleep or enhanced arousal of the awake state. One could get that impression reinforced from the way arousal is represented in Figure 2. We now know (see Hobson and Brazier, 1980) that the reticular core contains numerous nuclear groups that have their own distinct functions, many of which may not be necessary or sufficient for consciousness even though many of these neurons project extensively to the neocortex and modulate consciousness. Nonetheless, the functional neuroanatomy of even the defined nuclei in the brainstem reticular formation constitute the basic set of somato-sensing structures necessary for core consciousness and its core self to emerge Parvizi and Damasio (2001). While there is a reticular origin of fibers that cause diffuse cortical desynchronization, the different neurotransmitters released from brainstem nuclei into cortex have modulatory functions such as adjusting local patterns of synchronization and coherence. The classical arousal response seems to be engendered mainly from cholinergic neurons in the upper brainstem (Steriade, 1993). Nonetheless, we should not infer that these nuclei function independently of each other, because neurons in this region are surrounded by interlacing fibers, which gave the region the appearance of a reticulum that is a web. Cortical activation and consciousness are not likely to depend on one single brainstem nucleus or one single family of nuclei, but rather on a network formed by several families of nuclei Parvizi and Damasio (2001).
Though the reticular arousal system idea fell somewhat out of favor in the face of discoveries of reticular formation heterogeneity, a modern pioneer in cortical activation research, Mircea Steriade said that “it is encouraging that the concept of brainstem activation of the cortical processes has been rescued from oblivion and substantiated” (Steriade, 1996).
Recent MRI studies confirm a relatively global inactivation of brain during non-REM and a reactivation during REM that is greater in some areas than during wakefulness (Maquet et al., 1996; Braun et al., 1997; Nofzinger et al., 1997).
Neuronal activity in brainstem and hypothalamus varies considerably across the sleep–wakefulness continuum, and it is not clear which of these neurons have necessary and sufficient causal relations to a given state. Some brainstem neurons are only active during REM, some brainstem and hypothalamic neurons are mostly active during wakefulness, while others are mostly active during both SWS and REM, and still others are mostly active during wakefulness and REM (Steridae and Hobson, 1976; Datta, 1995; McGinty and Szymusiak, 2003; Sakai and Crochet, 2003). This confused picture suggests significant network organizational differences along the sleep–wakefulness continuum and that state transitions are not trivial processes.
Comparable identification of neuron activity in the neocortex in the various sleep stages is not available. But it seems possible that REM sleep is not simply a kind of wakefulness, but rather an intermediate stage between SWS and wakefulness, once that reflects an attempt to “recover” from SWS. Circuit activity in REM may reflect an incomplete attempt to re-organize. Even so, a key commonality is the release of acetylcholine in the cortex. Acetylcholine release in the neocortex is particularly important to alert consciousness, and microdialysis measurements of its release reveal an increase of approximately 100% during both quiet waking and REM, while the amount released during active waking was still larger, on the order of 175% during active waking, compared with the amount released in SWS Marrosu et al. (1995).
In going to sleep, external source of arousal abates and consciousness disappears. The deep Stage N3 sleep interferes most profoundly with the brain’s ability to generate consciousness. SWS sleep abolishes whatever temporal pattern of action potentials and their flow through circuits and networks necessary for consciousness. To awaken, this impulse flow pattern must be regenerated.
Stage N3 sleep is the deepest stage of sleep and occupies almost half of the first hour of sleep and has a significant presence for the next 2 h (Webb and Agnew, 1971). Whatever the function of SWS, at some point the brain has had enough of it. REM may be the signature that enough SWS has occurred.
Do dreams play a role? Dream content, as in nightmares, might contribute to an arousal level sufficient for awakening, just as stimulating thought during wakefulness can keep us aroused to the point of making it difficult to go to sleep. But more fundamental are the neuronal processes that create REM and in turn a level of activity that can produce and sustain wakefulness.
Rapid eye movement is commonly regarded to emerge from auto-excitatory mechanisms arising from increasing activity in certain neuron clusters in the pontine region of the reticular formation (Steriade and McCarley, 2005). The switching among waking, REM, and non-REM states has been attributed to certain pontine neurons: “REM-off” neurons which are active in waking and silent in REM, and “REM-on” neurons that are active during wakefulness and inactive in REM (reviewed by Hobson, 2009).
These neurons not only enable the signs of REM, such as REMs and suppression of bodily movement, but they probably also cooperate with adjacent reticular neurons to turn on the ARAS, cortical activity, and the consciousness of dreams. These pontine neurons increase their firing during dreaming and cease it with the transition to wakefulness. Thus the main difference between wakefulness and dreaming is that these special pontine neurons are only active during dreaming, while most of the other reticular formation neurons are active in both REM and wakefulness. Certainly, the profound re-organizing of neuronal networks and likely dynamic instabilities during REM could be expected to distort dream content. In any case, it would seem easier for the brain to switch instantly from REM consciousness to wakefulness than from Stage N3 sleep to wakefulness.
(6) Rapid eye movement sleep shares many, but not all, of the properties of wakefulness and thus may be a transitional state between SWS and wakefulness. SWS is a functionally perturbed state that eventually must be corrected so that embodied brain can direct adaptive behavior that can only occur in wakefulness. REM is likely an intermediary step between SWS and wakefulness, a notion that is supported by unit recordings of pedunculopontine neurons in various stages of the sleep–wakefulness cycle (Datta and Siwek, 2002). Aside from the obvious fact that REM is a more activated brain state than SWS, it is obviously a state that more closely resembles alert wakefulness During SWS, particularly the deepest Stage N3, large, slow field potentials arise from within cortical columns (Contreras and Steriade, 1995), reflecting a major disruption of cortical function. Awake consciousness and presumably REM occur with circuit reorganizations that not only antagonize SWS but also according to numerous studies in recent years create multiple new higher frequencies.
Most recently, emphasis is being placed on high-frequency oscillations in cortex in the beta and gamma range as the index and probable cause of the enhanced thinking capabilities that occur concurrently with the fast waves (Uhlhaas et al., 2009). Unfortunately for theories of REM, spectral coherence studies have thus far mostly focused on the role of frequency-specific coherences during different wakefulness conditions, rather than on a rigorous comparison across the sleep cycle and its state transitions. The few field-potential coherence studies of sleep cycle do not yield a coherent understanding, but they clearly suggest an importance to consciousness for coherence of activity among neocortical regions.
High-frequency oscillation and inter-area coherences seem to be fundamental properties of both wakefulness and REM. Electrical stimulation of the caudal midbrain reticular formation can abolish large slow EEG waves and at the same time enhance and synchronize high-frequency rhythms in intracortical and corticothalamic networks (Steriade et al., 1996). Within the REM state when dreams become lucid, frontal EEG exhibits more 40 Hz activity and at the same time frontal and posterior EEG activity become more coherent (Voss et al., 2009).
Widespread gamma synchrony appears during wakefulness and REM, but not during SWS (Llinás and Ribrary, 1993). In both states there is a fronto-occipital phase shift of 12–13 ms. But such activity is not identical, in that sensory stimuli can re-set the oscillation in wakefulness but not in REM (or SWS). The activity in this study was filtered around 40 Hz, so it is possible that other frequencies, especially higher ones, are also synchronized during wakefulness and dreaming.
In another study (Cantero, et al., 2002), EEG coherence was strengthened between fronto-temporal cortical regions within a broad frequency range during SWS, but to the detriment of the coherence between temporal and parieto-occipital areas, suggesting underlying compensatory mechanisms between temporal and other cortical regions. Coherence built up progressively across the night, although no changes were observed within a given SWS period. No electrophysiological changes were found within REM sleep epochs.
This lab reported another study (Cantero et al., 2003) in which there was no evidence of theta-band activity coherence in the hippocampus and the cerebral cortex in any brain state. But at sites within the hippocampus and cortex, theta activity was coherent during various stages of alert wakefulness and REM, but not SWS.
In a subsequent study from this lab (Cantero et al., 2004), gamma power exhibited greater variation across cortical regions during wakefulness than during either slow-wave or REM sleep. Additionally, gamma coherence was higher in wakefulness than during sleep.
EEG analysis of eyes-closed wakefulness revealed widespread nearest-neighbor positive synchronous interactions, similar to the results with magnetoencephalography, though less consistent across subjects. REM sleep demonstrated positive synchronous interactions akin to wakefulness but weaker. SWS showed strong positive interactions in a large left fronto-temporal–parietal cluster markedly more consistent across subjects (Langheim et al., 2011).
Coherences within the hippocampus may relate to the memory consolidation processes that occur during sleep. In Buzsáki’s lab (Montgomery et al., 2008), researchers simultaneously recorded from 96 implanted silicon probes in the hippocampus. Synchrony of the theta and gamma field potential activity and their underlying impulse activity was much greater among certain parts of the hippocampus during REM than during regular alert wakefulness, while the opposite was true in other regions of the hippocampus.
These findings suggest that various stages of the sleep–wakefulness cycle have distinct differences in their underlying neural network activity. REM may be a process for combating the activity disarray occurring in SWS by increasing ordered unit activity and promoting regional (and perhaps inter-frequency) coherences.
(7) Cortico-fugal projections to brainstem arousal areas provide a way for increased cortical activity in REM to progressively raise the sleeping brain to the activity level required for wakefulness. Terminal degeneration studies in the brainstem after fronto-parietal neocortical lesions in cats revealed cortico-fugal terminals scattered throughout both sides of the pontine and medullary reticular formation, especially prominent in the region immediately dorsal to the rostral half of the inferior olive Rossi and Brodal (1956). The pontine projection occurs in the rostral reticular zone extending dorsally to just in front of the abducens nerve. This projection is especially relevant because the control neurons for REM are found in pontine RF. This study also revealed cortico-fugal projections to the medullary reticular formation known to mediate global motor inhibition, which is prominent during SWS and most prominent during REM except for punctuated bursts of twitching. Most cortico-fugal fiber terminate in reticular regions that do not have long ascending projections. Thus, external stimuli may more readily cause awakening than can cortico-fugal drive during REM. Nonetheless, neurons in the nucleus gigantocellularis, a major brainstem component of the ARAS, do project rostrally and also receive dense cortico-fugal input. Rossi and Brodal suggested this is a path whereby neocortex can modulate the ARAS. Cortico-fugal input also goes to the nucleus of the solitary tract, a well-known SWS “center.”
Anterograde and retrograde degeneration methods used in monkeys yielded further support for the idea that neocortex can modulate the ARAS Kuypers and Lawrence (1967). Parietal pre- and post-central neocortex project to the dorsolateral midbrain reticular formation. Frontal neocortex projects to all levels of the reticular formation.
Electrophysiological approaches revealed ample excitatory cortico-fugal projections to the midbrain reticular formation from broad expanses of neocortex (Jasper et al., 1952; Bremer and Terzuolo, 1953; French, et al., 1955). In monkeys, neurons in the ARAS revealed short-latency activation by stimulation of the oculomotor frontal area, cingulate gyrus, orbital surface of the frontal lobe, sensorimotor neocortex, posterior parietal regions, and the superior temporal gyrus (French et al., 1955). The authors explicitly ventured the conclusion that the ARAS is regulated by cortico-fugal inputs.
Corticifugal projections to the brainstem could influence the cortical activation of REM sleep via the thalamic reticular nucleus (TRN), a thin GABAergic layer of neurons that has bidirectional connections with the neocortex. Clear evidence of a TRN role during various states of consciousness has been amply documented (Yingling and Skinner, 1977; Pinault, 2004). While a possible role in mediating cortical activation of the ARAS via REM has not been studied, a need for such studies is suggested by this “wake-up” hypothesis and the fact that the cortico-fugal projections to TRN are strongly activating (Gentet and Ulrich, 2003; Evrard and Ropert, 2009; Lam and Sherman, 2010).
The ARAS exerts a powerful activating influence on the neocortex via ascending projections via both reticular and intra-laminar regions of the thalamus (Nieuwenhuys et al., 2008). Thus, these areas of the thalamus and the cortico-fugal projections that they receive must be important in any consciousness recovery process involving the triggering of REM. Electrical stimulation evokes the characteristic signs of consciousness, namely, field-potential gamma waves in widespread areas of the neocortex (MacDonald et al., 1998).
The TRN is a nexus that mediates the interaction of topographically specific thalamic areas and the cortex in the processing of visual, auditory, and somatosensory inputs, movement, and limbic system operations (Guillery et al., 1998).The TRN is implicated in attentiveness, particularly for visual stimuli (Crick, 1984; Guillery, et al., 1998), which is not surprising given that the TRN has primary inputs from the lateral geniculate body as well as the brainstem. It receives inputs from the cortex and sends outputs back to the thalamus (Jones, 1975).
The intra-laminar portion of the thalamus contains neurons that have characteristic impulse firing patterns during wakefulness (Steriade and Glenn, 1982). During transitions to wakefulness, intra-laminar thalamic neurons exhibit marked increases in firing, which lag the initial increase in brainstem and basal forebrain cholinergic neurons. Thus, it may be that while brainstem neurons trigger consciousness, intra-laminar thalamic neurons may be needed to sustain it and regulate attention shifts (reviewed by Schiff, 2008).
Fitting Common Experience into the New Explanation
How do we reconcile this wake-up view with the obvious fact that arousing stimuli during sleep, as with an alarm clock, can cause awakening without the need for preceding REM? First, if one is in Stage N3 sleep when the alarm goes off, arousal is hard to accomplish, because the depth of sleep in that stage is quite profound (reviewed by Dement and Mitler, 1974). Second, even when awakened in Stage N3, most people are still groggy and not at their peak level of conscious function.
Stimulus-induced awakening is not the same as a sleep situation where the brain arouses itself. In spontaneous awakening from sleep, the brain has to find a way to engage these arousal processes.
Those consciousness-generating processes are not trivial. Non-human brains cannot do it at all or only minimally at best. Even with an adequate anatomical substrate, brains have to know how to generate the right network impulse patterns throughout vast regions of brain. They have to know what oscillatory frequencies to use and where, and how to synchronize them. After Stage N3, all this has to be done from a zero baseline. No wonder it takes all night of repeated REM for the brain to do this if it is unperturbed by external stimuli. The store of memories for how to activate the ARAS without external stimuli during REM may well be a key requirement to prod the ARAS to get back into the arousal loop.
Why do we resume a normal REM dream pattern after being awakened in the middle of the night? When one is awakened in the middle of the night, for whatever reason, the brain still needs more sleep, which is why a person goes back to sleep. The fact that REM and dreaming resume indicates the wake-up effect of REM has not yet been accomplished. Although dreaming is not needed to create the capacity for consciousness as such, the REM that causes dreaming may be needed to complete the used for optimal conscious function.
We should ask why the mammalian brain needs the deep stages of sleep. Two schools of thought prevail: (1) an evolutionary adaptation forces an organism to conserve energy and (2) restoration of physiological functions depleted during wakefulness (reviewed by Vertes, 1984). Why don’t lower animals need SWS? Except for a few reptiles and birds, they don’t exhibit SWS during their “sleep.” Presumably, SWS (and REM) are essential accompaniments of the brain development that enables consciousness.
There is some consolidation of memories going on in sleep (Rauchs, 2011; Wilhelm et al., 2011). Sleep provides a relative protection from external stimuli accessible in consciousness that could interfere with consolidation. Also, perhaps some regenerative “rest” occurs for neurotransmitter systems. Supporting the notion that SWS is a state of brain rest, neurons in virtually every brain area fire less during SWS than in any other state (Steridae and Hobson, 1976). What Stage N3 accomplishes for brain doesn’t matter as far as answering the question of why we need REM. What does matter is recovering from N3.
Other reasonably supported reasons for SWS sleep include thermal regulation and psychological equilibrium (reviewed by Hobson, 2009).
Wake-Up Hypothesis Predictive and Explanatory Power
Ontogeny/age Effects
(1) Why does so much REM occur in the fetus (Roffwarg, et al., 1966; Parmelee, et al., 1967), when few would contend that a fetus needs or is capable of consciousness?
Recordings from sheep fetuses reveal the presence of REM, and signs of REM are evident in human fetuses by 7 months of pregnancy (Schwab et al., 2009). REM occurs because the brain needs it. Fetuses and infants need heightened neural activity to stimulate brain development. About 50% of infant sleeping is spent in REM, compared to only about 20% for adults.
In addition to needing REM for brain development, I suggest that moving up out of the Stage N3 pit is hard for young, immature brains, because they are still learning how to optimize consciousness-generating processes.
How can one know whether a fetus has consciousness, especially late in gestation? In any case, fetal brain probably benefits from the stimulus of REM in promoting maturation. A great deal of REM may be needed to help fetal brain acquire the capacity for achieving consciousness after birth.
(2) Wake-up hypothesis predicts a learning component to a brain’s ability to generate and sustain consciousness. Is it not possible that a developing brain has to learn how to arouse itself from sleep or at least perfect endogenous mechanisms? Think about babies. They sleep most the time and typically have only short periods of wakefulness necessitated no doubt by the need for feeding (or the discomfort of soiled diapers). Their sleep is frequently punctuated by short episodes of REM (body twitches, EEG activation, and even smiling; Petre-Quadens, 1974). Adults have less REM as they get older. These age relationships exist in all species that have SWS and REM capability. Is it possible that REM is a process for helping to train the brain how to generate consciousness from a sleeping state? After all, because of physical limitations, a baby has very limited opportunity to hone its consciousness-generating skills through conscious interaction in the outside world. The inner-world conscious engagement provided by REM and dreaming may be nature’s way for the brain to master one of its most important capabilities, trigger consciousness in the absence of external stimulation. Perhaps older brains need less REM because they have learned efficient ways to awaken.
There is also the matter that the brain is still developing in the very young. REM provides longer periods of neocortical stimulation to help promote neuronal growth and synapse formation. Further, REM may be helping the infant brain learn how to generate consciousness.
(3) The wake-up hypothesis predicts that SWS and REM would be ontologically linked (Roffwarg et al., 1966; de Benedictis et al., 2007). Babies may need more REM sleep to help construct the capability for consciousness. Also, REM provides stimulation to an undeveloped brain.
Seminal studies of infants have been reported by Kahn (pp. 204–205, in Weitzman, 1972). He electrographically monitored 2-year-olds who slept at night and napped in the daytime and 5-year-olds who slept only at night. Both groups showed the first non-REM period (dominated by Stage N3) was the longest sleep stage of the night. The successive duration of Stage N3 sleep in 2-year-olds was 39, 13, 11, 8, 5, 2, 1 min. In 5-year-olds, it was 75, 15, 8, 5, 0, 1 min. REM periods had about the same duration (~20–25 min) throughout the night. Both groups revealed that children awakened within the last REM episode. Likewise, napping in the 2-year-olds was terminated in REM. It is almost as if SWS and REM were competing processes and as a night’s sleep progresses, REM starts to win.
Rapid eye movement occurs because the baby’s brain needs it. Infants and fetuses need heightened neural activity to stimulate brain development. Moving up out of the Stage N3 pit is hard for young, immature brains, because they are still learning what the consciousness-generating process is all about.
(4) Wake-up hypothesis explains the claim that REM and dreaming are not necessarily related. Young children are said to have REM but do not dream” (Goodenough, 1978; Foulkes, 1999). This conclusion was based on studies of 3- to 5-year-olds. Very young children may not be good at remembering dreams. Even adults typically remember very few of their dreams. This is a skill that is best developed with specific effort and experience.
(5) Wake-up hypothesis predicts that less REM would occur in older adults who of course have had years learning how to “boot up” the brain. Even after learning any task, the task still has to be performed. It should get easier over time, of course, but we have no physiological metrics to indicate how hard or easy it is to awaken spontaneously. Also, the elderly have less SWS than younger people and therefore may have less need for REM (Prinz et al., 1982).
Phylogeny
(6) Why do all species sleep and awaken, even those that never show REM (Cirelli and Tononi, 2008)? We need to distinguish mammalian sleep from behavioral quiescence. Reports of EEG in lower animal species, like fish and amphibians, show what seems to be an “activated” EEG even during behavioral quiescence that superficially looks like sleep (Klemm, 1973). What is not clear is the frequency band of such “activated” EEGs. Such data were obtained before the age of digital EEG and frequency analysis. We know little about the full frequency band and spatio-temporal coherences in the EEG of any non-primate species. Moreover, the degree and topography of coherences have never been subjected to examination in any lower species. One index of degree of consciousness could well be the ratio of gamma activity to beta activity. Another index could be frequency-band-specific differences in the level and topographic distribution of coherence within and between frequencies.
The important point as it relates to mammalian sleep is that fish, amphibians, and many reptiles do not have the capacity for generating large, slow EEG waves during behavioral quiescence (i.e., SWS) with interspersed epochs of REM. Mammalian sleep is presumably an evolved capacity, as is consciousness, and likely is a physiologically demanding state that requires long periods of brain “rest.”
(7) The wake-up hypothesis predicts that REM would only occur in advanced species (more recently evolved reptiles and mammals and in humans). The previous answer applies here as well. But to expand the explanation only these species have the capacity (and apparently need) for SWS.
I should add that primitive species lack the neocortex piece of the ARAS neural machinery to produce consciousness in the first place. Moreover, EEGs suggest that the brains of these animals don’t have the mechanisms for the Stage N3 kind of sleep either. Stage N3 may be a needed recovery process in species that can generate full consciousness, and REM and dreaming could be needed recovery processes for Stage N3.
The neocortex is the seat of consciousness, and the primitive cortex of lower animals (reviewed by Mountcastle, 1998) is not likely to generate the full-blown consciousness of humans. There is thus less need for SWS, and likewise less need for REM.
In a few reptile species, birds, and lower mammals, REM epochs can occur, but they are short and don’t occur often during a night’s sleep. But the fact that these species have REM at all suggests that they have some degree of consciousness during REM and their wakefulness. They may be marginally sentient beings. This has to be seriously considered in higher mammals such as domesticated animals and non-human primates. Anyone who has had close relationships with animals such as dogs, cats, or horses already appreciates this possibility.
Prey species, such as ruminants, rabbits, and guinea pigs, sleep very little and likewise have little REM (reviewed in Campbell and Tobler, 1984). Long bouts of SWS and REM increase the vulnerability time for predation, and thus prey species cannot afford to sleep much. This is what you might expect if REM is needed to antagonize SWS. With less SWS, there is less need for REM.
Capacity for SWS and REM appeared early in mammalian evolution. For example, modern armadillos, anatomically little changed from their 65-million-year-old Xenarthran ancestors, show electrographic REM signs approximately 8.9–21.5% of their behaviorally quiescent sleep time, depending on criteria and data interpretation (Prudom and Klemm, 1972). The typical slow-wave EEG pattern occurred about 50% of the total sleep time, with durations of a given period ranging from 0.75 to 375 min. Sleep in armadillos is fragmented because their poor thermoregulatory capability leads to the disruptive effects of shivering.
(8) How does one explain the sleep without REM that occurs in the “Immobility Reflex” (also known as Tonic Immobility or Animal Hypnosis)? Again, one should not confuse quiescence with sleep. Immediately after induction into this state, which is normally produced by physical restraint and manipulation, the EEG is activated and muscle tone is sustained. Soon afterward, the EEG may show large slow-waves, but the state is spontaneously terminated then or even earlier (Klemm, 1990b).
(9) The wake-up hypothesis explains the atypical REM sleep of ruminants. Originally, scientists assumed that ruminants never slept, just dozed, in order to keep from regurgitating their regurgitated cud. However, we now know that ruminants do exhibit SWS and REM, and they shut down rumination to do so (Klemm, 1966). Total time is SWS is short, so not surprisingly, REM is short.
(10) How do we explain lack of sleep and REM in marine mammals? They can’t sleep or they would drown. Actually, they do sleep, but only one hemisphere at a time (Mukhametov, 1984, 1987). While clear signs of REM have not been reported in sleeping whales, dolphins, or fur seals (Lyamin et al., 2004), an explanation may be that their brains never totally shut down during sleep. One hemisphere is always wide awake.
(11) Wake-up hypothesis predicts that the most developed REM capability should occur in humans. A species that has the highest level of consciousness has farther for the brain to go to lift brain function out of the Stage N3 pit. It may also be true, but has never been tested to my knowledge, that Stage N3 is deeper and more profound in humans than in lower mammals.
Abnormal/disease Conditions
(12) How does the wake-up hypothesis fit changes in REM incidence in various psychiatric diseases? It is not surprising that cognitive disorders would affect (and may be affected by) REM. In clinical depression, for example, REM sleep incidence increases (Coble et al., 1981). This might be a response to the need of the brain to antagonize the depression; that is, the brain may find it more difficult to arouse a depressed brain.
(13) Since anti-depressants decrease REM, they should also decrease SWS so there is less need for REM. to regenerate consciousness. These drugs decrease Stage N3 sleep and increase the lighter Stages I and II sleep (Cohen et al., 1982). Thus, there is less need for REM and less difficulty for REM helping brain to regenerate consciousness. The depression of REM only occurs for the first few nights, then it rebounds to become prolific (Lewis and Oswald, 1969). Also, it seems inappropriate to use an abnormal state to explain what happens in normal sleep. In this case, the drug itself may have stimulus properties that facilitate spontaneous awakening. Note also that these drugs take several days to start showing their effect.
(14) The wake-up hypothesis posits that REM contributes to achieving consciousness, but does not necessarily affect the quality of that state. For example, drug-free schizophrenics have sleep and dream patterns similar to normals (Lauer et al., 1997). Therefore, the role of REM seems confined to generating consciousness per se without necessarily determining the quality of that consciousness.
(15) How can one explain why people can awaken from anesthesia without preceding REM? First, anesthesia is not normal sleep. It may be too much to expect any hypothesis of normal function to explain all abnormal function. Second, the impression that consciousness just pops up from anesthetization may be an illusion. The anesthetized brain must struggle mightily to overcome the drug’s depression of network activity needed for consciousness. We know that struggle goes on because it gets expressed in physical thrashing about. That is why orderlies strap surgical patients to a gurney and “hide” them in a recovery room while the brain is fighting the body to wake-up. Do we know that REM is absent during such times? One study showed that 44% of patients actually dreamed during surgical anesthesia and two-thirds of these could recall the dream content (Brice et al., 1970).
Also, there is also a lot of poking and prodding stimulation during recovery from vital-sign checking, clean-up, bandaging, etc. As for absence of dream recollection, dreams, and hallucinations may well be occurring during recovery from anesthesia but can’t be remembered because the drug prevents consolidation of the dream memories.
Cognition
(16) The wake-up hypothesis predicts that REM would promote dreaming, because dreaming is an imperfect simulation of the awake state. Eventually, REM should be expected to drive the brain to wakefulness threshold, which is consistent with the common observation that people often awake while dreaming.
(17) The wake-up hypothesis also predicts that because everyone has SWS, everyone should have REM and dreams, even those who say they don’t dream in normal sleeping. When sleepers are awakened and queried during physiological signs of REM, they often reveal that they had been dreaming at the time of awakening, Under normal sleep conditions, people don’t remember all their dreams. Skill at remembering dreams can be developed through training.
(18) Because REM is an abortive attempt to wake-up, wake-up hypothesis predicts that the dreaming of REM and conscious wakefulness are cognitively and neurophysiologically similar. Both states are egoistic and a “sense of I” is operative. Dreaming is a simulation of the conscious life of wakefulness. Dreams can be bizarre, because there is no constraint of external stimuli and reality and perhaps because completely normal neural activity is not yet established. As mentioned, both states are dependent on brainstem reticular formation activity. The EEGs are similar in the two states (Dement, 1958), as are the fundamental excitatory and inhibitory thalamocortical processes (Steriade et al., 1979).
(19) The wake-up hypothesis predicts that REM episodes would be short and choppy, at least early in a night’s sleep. Why doesn’t the brain try to recover from Stage N3 with just one long REM episode instead of having a series of choppy short bouts of REM? If a long, sustained REM episode in early evening could cause awakening, a person might not get enough SWS. For whatever reason, a short sleep cycle is not acceptable to human brain. The repeated episodes of short REM probably reflect the difficulty the brain has to fight its way out of stupor.
(20) How can we explain why cognitive activity (“dreaming”) can occur in non-REM sleep, and it does not cause awakening (Hobson et al., 2000)? First, by definition, NREM dreaming is clearly not moving the brain to awakening to the same extent REM does. Also, non-REM dreaming apparently only occurs to a limited extent in light sleep stages (Foulkes, 1962; Nielsen, 2000). Wake-up hypothesis posits that it is not dreaming per se that wakes people up, but the intrinsic triggering of the ARAS.
Behavioral physiology
(21) The wake-up hypothesis predicts that REM would only appear in lighter stages of sleep (see Figure 1). If REM is triggered by cortico-fugal projections to the ARAS, then these could be too suppressed in deep Stage N3 sleep to trigger REM.
(22) The wake-up hypothesis predicts that REM should increase toward morning. As the night and REM episodes progress, the brain is gaining capacity to awaken itself. Each successive REM epoch should make it easier to move into REM and eventual self-induced awakening.
(23) The wake-up hypothesis helps to explain why the brain needs to make up lost REM. Both animal and human experiments confirm that the brain needs REM and, if REM-deprived, will devote extra time to REM. Though sleep may be cut short by external arousal stimuli, the next time the brain goes to sleep, it is lacking the complete “rehearsal” needed to awaken itself. REM effects are apparently cumulative. The brain intuitively “knows” that REM is an important tool it must nurture. It has learned how important REM is.
(24) How do people wake-up on their own after a night of being deprived of REM by a sleep-research experimenter? Of course, the only way such experimental subjects can be deprived of REM is for the experimenter to jostle them into wakefulness every time the REM physiological signs first appear. All that external stimulation must have a cumulative arousal effect that is likely to be much greater than any alarm clock.
In rats, a common way of preventing REM is to make rats sleep on a flower pot inverted in a tub of water. When they fall into REM, the postural atonia causes them to fall into the water. Isn’t that a strong external stimulus? In other words, the way awakening occurs in REM-deprived subjects is through strong external stimulation.
(25) How does this idea apply to lucid dreams? A lucid dream is a dream in which one is aware that one is dreaming. I do not know of any studies that have tracked whether or not a dream was more likely to be lucid at a given point in sleep. Perhaps the quasi-conscious participation of self in lucid dreams is moving the brain closer to awakening threshold. It would be useful to query people as they wake-up and tally the percentage of the immediately antecedent dreams that were lucid.
(26) Why do we awaken at the end of REM? In a study of young adults (Feinberg, et al., 1967), awakening always occurred within a REM episode. Vertes (1990) surmised that the role of REM is to counteract SWS (p. 565). What form might such counteraction take? How would REM activate the ARAS? Earlier, I suggested that excitatory cortico-fugal projections to the ARAS could do the job.
Consciousness disappears during SWS, and there is minimal excitation coming from the outside world. As a result the ARAS receives little-to-no excitation from the periphery or from feedback from the neocortex. Neocortex and ARAS presumably have a reciprocal relationship. ARAS triggers neocortical activation and an activated neocortex supplies feedback to help keep the ARAS active. But in sleep, neocortex neurons that supply input to the ARAS slow their impulse firing drastically, thus removing excitatory drive to the ARAS. If a sleeping brain is to become conscious without external stimuli, it must have a self-generating mechanism, and that mechanism probably requires the neocortex to activate the ARAS, perhaps by releasing it from inhibition. Reticular neurons in the midbrain may be critical in this activation, because they receive converging excitatory input not only from reticular neurons in the medulla and collaterals from sensory fibers, but also from cerebral neocortex, the preoptic “sleep center” area, and several nuclei in the thalamus.
The sleeping brain’s arousal problem is made more difficult when consciousness has to be dredged up out of the deepest stage of sleep.
Comparison with Competing Theories
Competing theories focus on either REM or the dreaming that occurs in REM. One REM hypothesis, currently in great vogue, is that the activated neuronal activity has the purpose of helping consolidate memories of the day’s experiences, especially for procedural memories (Stickgold et al., 2000a,b; Wilson and Louie, 2001; Maquet et al., 2002). Experimental disruption of dreams does interfere with long-term memory formation. The problem is that such off-line processing and memory consolidation also occur in non-REM sleep.
The consolidation hypothesis could have it backward. Maybe we don’t have REM to consolidate the day’s memories but that consolidation is a consequence of the brain continuing to operate in sleep, and, in the absence of external stimulation, resumes processing of the day’s experiences because that is the information most readily at hand.
Rapid eye movement may occur as a way to mobilize the brain for the next day’s activity, constituting a “dress rehearsal” for the next day. These factors were an integral part of the idea that the brainstem enables an ARAS-mediated behavioral “readiness response” to stimuli to assure that appropriate behavior and cognition occur (Klemm, 1990). REM could help the brain rehearse how to reactivate readiness when the time comes in the morning. A related explanation for young, immature brains is that REM provides a source of endogenous stimulation that is useful in promoting brain maturation and the capability for consciousness.
This way of looking at the issue is the perspective of an animal physiologist. A similar explanation for REM was also proposed by the physiologist, Vertes (1984, 1986). He suggested that REM sleep provided endogenous stimulation to prevent the brain from being shut down too long in SWS and that such sustained suppression might make it difficult to awaken. I agree.
Psychologists like to focus on dreams, as opposed to the REM that enables dreaming. Ancient shaman priests and religious prophets have thought of dreams as God’s way of communicating with us (of course these folks did not know about REM or that other mammals also have REM that might support dreaming. If they had, they might have concluded that God talks to animals too). Then came Freud and psychiatrists, who focused on the dreams of REM (REM had not been discovered in Freud’s time). They thought of dreaming as the brain’s release of subconscious thinking into dream consciousness. Among the early ideas that have been mentioned is the possibility that dreaming acts as an escape valve for letting off the “psychic steam” that accumulates from emotional stresses during the day. Dream content does have symbolic meaning, as Freud showed. People become anxious, irritable, and sometimes borderline psychotic, if they are experimentally deprived of REM and its dreaming.
Yet another hypothesis is that REM is said to be caused by random impulse activity, and as a result the brain dreams and makes up stories to try and make sense of all the erratic activity Hobson and McCarley (1977). But dreams have a major conscious aspect. How could that be a trivial and random process? Some dream content, for example, is highly structured, not likely to be created and sustained by random action-potential firing. Such an explanation could not apply to the consciousness of wakefulness, and it seems unlikely to be applicable to the consciousness of dreaming. If consciousness were that easy to generate, why don’t more species show it?… and why do we need a whole night’s sleep to achieve it?
We should be skeptical of cavalier claims the neuron firing patterns during dreaming are random. Have rigorous tests of randomness have been made?
In REM, almost all neurons in all parts of the brain increase firing rate during REM, compared with SWS and often compared with alert wakefulness (Evarts, 1967; Hobson and McCarley, 1971). Since firing rate is generally considered indicative of biologically significant information processing, to regard it as noise and random seems unjustified. Paradoxically, it was Hobson and McCarley who helped prove that impulse discharge increases in REM.
Other lines of evidence argue against REM resulting from random activity. Certain neurons in the pons, the seat of REM genesis, fire in clusters to create so-called PGO waves during REM (Sakai, 1980). Likewise, neurons controlling the REMs of REM fire selectively during REM and not SWS. The same can be said for brainstem neurons that cause the postural atonia of REM.
Some neurons fire less during REM. For instance, locus coeruleus cells not only fire at higher rates during SWS, but are virtually silent during REM (Foote et al., 1980; Aston-Jones and Bloom, 1981). Raphe nuclei discharge at progressively lower rates from wakefulness to SWS to REM (Jacobs et al., 1974; Jacobs and Jones, 1978). Septal neurons fire in discontinuous “theta-rhythm” bursts throughout REM, but not SWS. All these regional disparities are inconsistent with the idea that REM comes from random unit activity.
The sleeping brain may be using REM to re-construct the highly ordered combinatorial coding and temporal coherences of neocortical circuitry needed to facilitate awakening into full consciousness competency. This re-organization may even include adjusting the sleep–wakefulness servo-system’s set-point circuit to make consciousness easier to generate and sustain, being fortified against drowsing off to sleep during boring parts of the day.
One problem common to each of these theories is the presupposition of purpose. REM and dreaming surely have neurophysiological effects, but assigning purpose may not be appropriate. What if REM is a consequence of something else and its correlates such as emotional venting and memory consolidation are coincidental by-products of intrinsic REM processes?
What might that “something else” be? I suggest it is the cumulative effect of satisfying the need for SWS. Wakefulness, as the biologically most adaptive state, may be the brain’s default mode. Once the need for SWS is satisfied the brain needs to wake-up.
A more recent hypothesis has been proposed by Hobson (2009). He suggests that REM is a “protoconscious state… that is of functional use to the development and maintenance of waking consciousness.” No way to test the idea is proposed. The present wake-up hypothesis specifies what this “functional use” may be. Additionally, the wake-up hypothesis is testable, and several approaches are herein suggested.
Testing REM Theories
To be accepted, any hypothesis should be testable. Since the wake-up hypothesis is that REM moves the brain closer to awakening threshold, the obvious approach is to compare awakening threshold in SWS with that in REM. However, in a dream during REM, disruption might be difficult because of focused attention on dream content. Therefore, one might need to compare awakening threshold in SWS with that during REM when human subjects report existence of a dream and REM when subjects were not aware of a dream.
Wake-up hypothesis is testable if one builds upon the so-called “reciprocal interaction model” described by Steriade and McCarley(2005; pp. 513–560). This model posits a REM oscillator circuit intrinsic to certain regions of the brainstem that ultimately control neocortical activity. A necessary first step would seem to examine the network dynamics of ARAS, pontine REM circuits, thalamus, and neocortex. Unit activity in the ARAS is the most conspicuous sign so far identified for transition from SWS to REM or wakefulness (Steriade et al., 1982). But past research has usually involved recording from one or a few neurons in a given area at a time. Testing the wake-up hypothesis requires simultaneous recording in the several relevant brain areas and comparing the activity in wakefulness, SWS, and REM.
Ideally, such studies should use combinatorial mathematics to evaluate unit activity coding among simultaneously recorded neurons within defined circuits (Klemm, 2011). This approach has yet to be applied in neuroscience in general, and the sleep cycle would be an excellent place to start.
If REM occurs because of interactions with neocortex, then sleep cycle studies are needed in which the ARAS circuitry is evaluated, particularly the cortico-reticular projections (Leichnetz et al., 1987). Recordings from intra-laminar and reticular thalamus are also needed. Such studies should also include field-potential observations, because significant state-dependent frequency coherence patterns are likely, as existing literature indicates. Field-potential studies would probably benefit from use wavelet analysis, which is less affected by the non-stationarity of sleep cycle state changes than power spectral approaches.
Finally, a possibility usually completely ignored is the role of ultra-slow activity and associated molecular events, much of which is probably generated by glia cells (Barres et al., 1990; Fields and Stevens-Graham, 2002). These potentials could create a background that profoundly alters thresholds for transitioning among various sleep stages, REM, and awake consciousness. Technical difficulties abound in the study of glia, but it seems clear that glial cells are functionally coupled to neurons, both electrotonically (Alvarez-Maubecin, 2000) and electrochemically.
This wake-up hypothesis invites re-examination of the role of the ARAS in awake consciousness. First, it could help confirm and identify the set-point threshold for consciousness that the ARAS has to reach to trigger consciousness. The idea of threshold for awakening comes from our subjective experience that awakening from sleep (or even anesthesia) seems to be “switched on.” Just what happens when this “switch” is thrown may only seem to be instantaneous, but in any case a distinct transition occurs that has to be caused by specific changes in the network dynamics of the relevant brain areas.
Of course REM sleep has functions other than the wake-up hypothesis and these need not be mutually exclusive. The point advocated here is that a major function of REM is to help a sleeping brain wake-up.
Conclusion
Rapid eye movement sleep has several benefits in addition to “re-booting” a sleeping brain. Just getting ready for the next day’s conscious activity has its value, even if there were no need to overcome the neural disruption of Stage N3 sleep. There is also a potential benefit in consolidating certain memories that perhaps cannot be accomplished as well in other stages of sleep.
Then there is the likelihood that REM and dream content can have reward properties. Dog owners, for example, may be justified in the suspicion that their dog sleeps so much because it is bored and looks forward to the adventures, such as chasing deer and catching critters that it has learned can be experienced without effort in REM and its dreams. Dreams are nature’s way of enhancing the life experience.
This also relates to a basic propensity of brain: stimulus seeking. Advanced animals have an evolved brain that feeds on stimulus. REM is a very convenient way to address that need.
Rapid eye movement sleep’s cortical stimulus role is at the heart of the wake-up hypothesis. Since Stage N3 sleep cortical-column dynamics drive the brain out of the mode required for consciousness, the column circuits have to be re-set, and that apparently can only be accomplished via the ARAS, which in stimulus-absent sleep must be switched on by a much less efficient processes generated within corticothalamic and corticobulbar systems. REM episodes may be difficult to sustain in early evening because corticifugal influences are apparently less robust than external stimulation. As the night’s sleep progresses, the brain becomes more facile at triggering and sustaining REM to the set-point threshold for awakening. The existence of such supposed changes could be tested with the appropriate electrophysiological approaches.
The wake-up view supports the obvious impression that there is some sort of antagonism between SWS and REM. The dreaming that occurs during human REM could be considered incidental to a REM process that is auto-triggered by SWS. REM could serve to help consciousness-generating systems to reach threshold in the absence of external stimulation. In short, whatever triggers REM may do so via the ARAS, which otherwise remains dormant without external stimulation.
Of all REM theories, the wake-up hypothesis comes closest to explaining all the key characteristics of REM.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnote
- ^A preliminary version of this paper was presented at the 2010 annual meeting of the Society for Neuroscience.
References
Alvarez-Maubecin, V. (2000). Functional coupling between neurons and glia. J. Neurosci. 20, 4091–4098.
Aston-Jones, G., and Bloom, R. E. (1981). Activity of the norepinephrine-containing locus ceruleus neurons in behaving rats anticipate fluctuations in the sleep-waking cycle. J. Neurosci. 1, 876–886.
Barres, G. A., Chun, L. L. Y., and Corey, D. P. (1990). Ion channels in vertebrate glia. Annu. Rev. Neurosci. 13, 441–474.
Braun, A. R., Balkin, T. J., Wesenten, N. J., Carson, R. E., Varga, M., Baldwin, P., Selbie, S., Belenky, G., and Herscovitch, P. (1997). Regional cerebral blood flow throughout the sleep-wake cycle. Brain 120, 1173–1197.
Bremer, F., and Terzuolo, C. (1953). Interaction de l’écorce cérébrale et de la formation réticularée du tronc cerebral dans le mechanism de l’éveil et du maintien de l’activité vigile. J. Physiol. Pathol. Gén. 45, 56–57.
Brice, D. D., Hetherington, R. R., and Utting, J. E. (1970). A simple study of awareness and dreaming during anesthesia. Br. J. Anaesth. 42, 535–542.
Campbell, S. S., and Tobler, I. (1984). Animal sleep, a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8, 269–300.
Cantero, J. L., Atienza, M., Madsen, J. R., and Stickgold, R. (2004). Gamma EEG dynamics in neocortex and hippocampus during human wakefulness and sleep. Neuroimage 22, 1271–1280.
Cantero, J. L., Atienza, M., Salas, R. M., and Dominguez-Marin, E. (2002). Effects of prolonged waking-auditory stimulation on electroencephalogram synchronization and cortical coherence during subsequent slow-wave sleep. J. Neurosci. 22, 4702–4708.
Cantero, J. L., Atienza, M., Stickgold, R., Kahana, M. J., Madsen, J. R., and Kocsis, B. (2003). Sleep-dependent theta oscillation in the human hippocampus and neocortex. J. Neurosci. 23, 10897–10903.
Cirelli, C., and Tononi, G. (2008). “Is sleep essential?” (essay). PLoS Biol 6, e216. doi: 10.1371/journal.pbio.0060216 [Public Library of Science].
Coble, P. A., Kupfer, D. J., and Shaw, D. H. (1981). Distribution of REM latency in depression. Biol. Psychiatry 16, 453–466.
Cohen, R. M., Pickar, D., Garnett, D., Lipper, S., Gillin, J. C., and Murphy, D. L. (1982). REM sleep suppression induced by selective monoamine oxidase inhibitors. Psychopharmacology (Berl.) 78, 137–140.
Contreras, D., and Steriade, M. (1995). Cellular basis of EEG slow rhythms, a study of dynamic corticothalamic relationships. J. Neurosci. 15, 604–622.
Crick, F. (1984). Function of the thalamic reticular complex: the searchlight hypothesis. Neurobiology 81, 4586–4590.
Datta, S. (1995). Neuronal activity in the peribrachial area: relationship to behavioral state control. Neurosci. Biobehav. Rev. 19, 67–84.
Datta, S., and Siwek, D. F. (2002). Single cell activity patterns of pedunculopontine tegmentum neurons 642 across the sleep-wake cycle in the freely moving rats. J. Neurosci. Res. 70, 611–621.
de Benedictis, T., Larson, H., Kemp, G., Barston, S., and Segal, R. (2007). Understanding Sleep, Sleep Needs, Cycles, and Stages. Available at: http://www.helpguide.org/life/sleeping.htm
Dement, W. C. (1958). The occurrence of low voltage, fast electroencephalogram patterns during behavioral sleep in the rat. Electroencephalogr. Clin. Neurophysiol. 10, 291–296.
Dement, W. C., and Mitler, M. M. (1974). “An introduction to sleep,” in Basic Sleep Mechanisms, eds O. Petre-Quadens and J. D. Schlag (New York: Academic Press), 271–296.
Evarts, E. V. (1967). “Unit activity in sleep and wakefulness,” in The Neurosciences – A Study Program, eds G. C. Quarton, T. Melnechuk and F. O. Schmitt (New York: Rockefeller University Press), 545–556.
Evrard, A., and Ropert, N. (2009). Early development of the thalamic inhibitory feedback loop in the primary somatosensory system of the newborn mice. J. Neurosci. 29, 9930–9940.
Feinberg, L., Koresko, R. L., and Heller, N. (1967). EEG sleep patterns as a function of normal and pathological aging in man. J. Psychiatr. Res. 5, 107–144.
Fields, R. D., and Stevens-Graham, B. (2002). New insights into neuron-glia communication. Science 298, 558–562.
Foote, S. L., Aston-Jones, G., and Bloom, F. E. (1980). Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. U.S.A. 77, 3033–3037.
Foulkes, D. (1999). Children’s Dreaming and the Development of Consciousness. Cambridge, MA: Harvard University Press.
Foulkes, W. D. (1962). Dream reports from different stages of sleep. J. Abnorm. Soc. Psychol. 65, 14–25.
French, J. D., Hernández-Peón, R., and Livingston, R. B. (1955). Projections from neocortex to cephalic brain stem (reticular formation) in monkey. J. Neurophysiol. 18, 74–95.
Gentet, L. J., and Ulrich, D. (2003). Strong, reliable and precise synaptic connections between thalamic relay cells and neurones of the nucleus reticularis in juvenile rats. J. Physiol. 546, 801–811.
Goodenough, D. R. (1978). “Dream recall, history and current status of the field,” in The Mind in Sleep, Psychology and Psychophysiology, eds A. M. Arkin, J. S. Antrobous and S. J. Ellman (Hillsdale, NJ: Lawrence Erlbaum Associates), 113–140.
Guillery, R. W., Feig, S. L., and Lozsadi, D. A. (1998). Paying attention to the thalamic reticular nucleus. Trends Neurosci. 21, 28–32.
Hobson, I. A., and Brazier, M. A. B. (eds). (1980). The Reticular Formation Revisited. New York: Raven Press, 564.
Hobson, J. A. (2009). REM sleep and dreaming, towards a hypothesis of protoconsciousness. Nat. Neurosci. Rev. 10, 803–813.
Hobson, J. A., and McCarley, R. W. (1971). Cortical unit activity in sleep and waking. Electroencephalogr. Clin. Neurophsiol. 30, 97–112.
Hobson, J. A., and McCarley, R. W. (1977). The brain as a dream state generator, an activation-synthesis hypothesis of the dream process. Am. J. Psychiatry 1324, 1335–1348.
Hobson, J. A., Pace-Schott, E. F., and Stickgold, R. (2000). Dreaming and the brain, toward a cognitive neuroscience of conscious states. Behav. Brain Sci. 23, 793–1121.
Iber, C., Ancoli-Israel, S., Chesson, A., and Quan, S. F. (2007). The AASM Manual for the Scoring of Sleep and Associated Events. Westchester, IL: American Academy of Sleep Medicine.
Jacobs, B. L., and Jones, B. E. (1978). “The role of central monoamines and acetylcholine systems in sleep-wakefulness states, mediation or modulation?” in Cholinergic-Monoaminergic Interactions in the Brain, ed. L. L. Butcher (Orlando: Academic Press), 271–290.
Jacobs, B. L., Wise, W. D., and Taylor, K. M. (1974). Differential behavioral and neurochemical effects following lesions of the dorsal and median raphe nuclei in rats. Brain Res. 79, 3563–3363.
Jasper, H., Ajmone_Marsan, C., and Stoll, J. (1952). Corticofugal projections to the brain stem. Arch. Neurol. Psychiatry 67, 155–172.
Jones, E. G. (1975). Some aspects of the organization of the thalamic reticular complex. J. Comp. Neurol. 162, 285–308.
Klemm, W. R. (1966). Sleep and paradoxical sleep in ruminants. Proc. Soc. Exp. Biol. Med. 121, 635–638.
Klemm, W. R. (1972). Ascending and descending excitatory influences in the brain stem reticulum, a reexamination. Brain Res. 36, 444–452.
Klemm, W. R. (1973). “Typical electroencephalograms, vertebrates,” in Biology Data Book, Vol. II, 2nd Edn, eds P. L. Altman and D. S. Dittmer (Bethesda, MD: Federation of American Societies for Experimental Biology), 254–260.
Klemm, W. R. (1990a). “The behavioral readiness response,” in Brainstem Mechanisms of Behavior, eds W. R. Klemm and R. P. Vertes (New York: John Wiley and Sons), 105–145.
Klemm, W. R. (1990b). “Behavioral inhibition,” in Brainstem Mechanisms of Behavior, eds W. R. Klemm and R. P. Vertes (New York, NY: Wiley and Sons), 497–533.
Klemm, W. R. (2011). Atoms of Mind. The “Ghost in the Machine” Materializes. New York, NY: Springer.
Kuypers, H. G. J. M., and Lawrence, D. G. (1967). Cortical projections to the red nucleus and the brain stem in the Rhesus monkey. Brain Res. 4, 151–188.
Lam, Y. W., and Sherman, S. M. (2010). Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cereb. Cortex 20, 13–24.
Langheim, F. J. P., Murphy, M., Riedner, B. A., and Tononi, G. (2011). Functional connectivity in slow-wave sleep, identification of synchronous cortical activity during wakefulness and sleep using time series analysis of electroencephalographic data. J. Sleep Res. doi: 10.1111/j.1365-2869.2011.00911.x. [Epub ahead of print].
Lauer, C., Schreiber, W., Pollmächer, T., Holsboer, F., and Krieg, J. C. (1997). A polysomnographic study on drug-naive patients. Neuropsychopharmacology 16, 51–60.
Leichnetz, G. R., Gonzalo-Ruiz, A., DeSalles, A. A. F., and Hayes, R. I. (1987). The origin of brainstem afferents of the paramedian pontine reticular formation in the cat. Brain Res. 422, 389–397.
Lewis, S. A., and Oswald, I. (1969). Overdose of tricyclic anti-depressants and deductions concerning their cerebral action. Br. J. Psychiatry 115, 1403–1410.
Llinás, R., and Ribary, U. (1993). Coherent 40-Hz oscillation characterizes dream state in humans. Proc. Nat. Acad. Sci. U.S.A. 90, 2078–2081.
Lyamin, O. I., Mukhametov, L. M., and Siegel, J. M. (2004). Relationships between sleep and eye state in cetaceans and pinnipeds. Arch. Ital. Biol. 142, 557–568.
MacDonald, K. D., Fifkova, E., Jones, M. S., and Barth, D. S. (1998). Focal stimulation of the thalamic reticular nucleus induces focal gamma waves in cortex. J. Neurophysiol. 79, 474–477.
Maquet, P., Peigneux, P., Laureys, S., and Smith, C. (2002). Be caught napping, you’re doing more than resting your eyes. Nat. Neurosci. 5, 618–619.
Maquet, P., Péters, J., Aerts, J., Delfiore, G., Degueldre, C., Luxen, A., and Franck, G. (1996). Functional neuroanatomy of human of rapid-eye-movement sleep and dreaming. Nature 383, 163–166.
Marrosu, F., Portas, C., Mascia, M. S., Casu, M. A., Fà, M., Giagheddu, M., Imperato, A., and Gessa, G. L. (1995). Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 671, 329–332.
McGinty, D., and Szymusiak, R. (2003). Hypothalamic regulation of sleep and arousal. Front. Biosci. 8, 1074–1083.
Montgomery, S. M., Sirota, A., and Buzsáki, G. (2008). Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J. Neurosci. 28, 6731–6741.
Mukhametov, L. M. (1987). Unihemispheric slow-wave sleep in Amazonian dolphin, Inia geoffrensis. Neurosci. Lett. 79, 128–132.
Nielsen, T. A. (2000). A review of mentation in REM and NREM sleep, “covert” REM sleep as a possible reconciliation of two opposing models. Behav. Brain Sci. 23, 851–66; discussion 904–1121.
Nieuwenhuys, R., Voogd, J., and van Huijzen, C. (2008). The Human Nervous System, 4th Edn. Berlin: Springer.
Nofzinger, E. A., Mintun, M. A., Wiseman, M., Kupfer, D. J., and Moore, R. Y. (1997). Forebrain activation in REM sleep, an FDG PET study. Brain Res. 770, 192–201.
Parmelee, A., Wenner, W., Akiyama, Y., Schulz, M., and Stern, E. (1967). Sleep states in premature infants. Dev. Med. Child Neurol. 9, 70–77.
Petre-Quadens, O. (1974). “Sleep in the human newborn,” in Basic Sleep Mechanisms, eds O. Petre-Quadens and J. D. Schlag (New York: Academic Press), 355–376.
Pinault, D. (2004). The thalamic reticular nucleus: structure, function and concept. Brain Res. Brain Res. Rev. 46, 1–31.
Prinz, P.N., Peskind, E. R., Vitaliano, P. P., Raskind, M. A., Eisdorfer, C., Zemcuznikov, N., and Gerber, C. J. (1982). Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J. Am. Geriatr. Soc. 30, 86–93.
Prudom, A. E., and Klemm, W. R. (1972). Electrographic correlates of sleep behavior in a primitive mammal, the armadillo Dasypus novemcinctus. Physiol. Behav. 10, 275–282.
Rauchs, G. (2011). Sleep contributes to the strengthening of some memories over others, depending on hippocampal activity at learning. J. Neurosci. 31, 2563–2568.
Roffwarg, H. P., Muzio, N. N., and Dement, W. C. (1966). Ontogenetic development of the human sleep-dream cycle. Science 152, 604–619.
Rossi, G. F., and Brodal, A. (1956). Corticofugal fibres to the brain-stem reticular formation. An experimental study in the cat. J. Anat. 90, 42–62.
Sakai, K. (1980). “Some anatomical and physiological properties of ponto-mesencephalic tegmental neurons with special reference to PGO waves and postural atonia during paradoxical sleep,” in The Reticular Formation Revisited, eds J. A. Hobson and M. A. B. Brazier (New York: Raven Press), 427–447.
Sakai, K., and Crochet, S. (2003). A neural mechanism of sleep and wakefulness. Sleep Biol. Rhythms 1, 29–42.
Schiff, N. D. (2008). Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann. N. Y. Acad. Sci. 1129, 105–118.
Schwab, K., Groh, T., Schwab, M., and Witte, H. (2009). Nonlinear analysis and modeling of cortical activation and deactivation patterns in the immature fetal electrocorticogram. Chaos 19, 015111.
Steriade, M. (1993). Central core modulation of spontaneous oscillations and sensory transmission in thalamocortical systems. Curr. Opin. Neurobiol. 3, 619–625.
Steriade, M., Contreras, D., Amzica, F., and Timofeev, I. (1996). Synchronization of fast (30-40) spontaneous oscillations in intrathalamic and thalmo-cortical networks. J. Neurosci. 16, 2788–2808.
Steriade, M., and Glenn, L. L. (1982). Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. J. Neurophysiol. 48, 352–371.
Steriade, M., Kitsikis, A., and Oakson, G. (1979). Excitatory and inhibitory processes in parietal association neurons during reticular activation and sleep-waking cycle. Sleep 1, 339–335.
Steriade, M., and McCarley, R. W. (2005). Brain Control of Wakefulness and Sleep, 2nd Edn. New York: Springer.
Steriade, M., Oakson, G., and Ropert, N. (1982). Firing rates and patterns of midbrain reticular neurons during steady and transitional studies of the sleep-waking cycle. Exp. Brain Res. 46, 37–51.
Steridae, M., and Hobson, J. A. (1976). Neuronal activity during the sleep-waking cycle. Prog. Neurobiol. 6, 155–376.
Stickgold, R., Malia, A., Maguire, D., Roddenberry, D., and O’Connor, M. (2000a). Replaying the game, hypnagogic images in normals and amnesics. Science 290, 350–353.
Stickgold, R., Whidbee, D., Schirmer, B., Patel, V., and Hobson, J. A. (2000b). Visual discrimination task improvement, a multi-step process occurring during sleep. J. Cogn. Neurosci. 12, 246–254.
Uhlhaas, P. J., Pipa, G., Lima, B., Melloni, L., Neuenschwander, S., Nikolić, D., and Singer, W. (2009). Neural synchrony in cortical networks, history, concept and current status. Front. Integr. Neurosci. 3:17. doi: 10.3389/neuro.07017.2009
Vertes, R. P. (1986). A life-sustaining function for REM sleep, A Hypothesis. Neurosci. Biobehav. Rev. 10, 371–376.
Vertes, R. P. (1990). “Brainstem mechanisms of slow-wave sleep and REM sleep,” in Brainstem Mechanisms of Behavior, eds W. R. Klemm and R. P. Vertes (New York: Wiley and Sons), 535–584.
Voss, U., Holzmann, R., Tuin, I., and Hobson, J. A. (2009). Lucid dreaming, a state of consciousness with features of both waking and non-lucid dreaming. Sleep 32, 1191–1200.
Webb, W. B., and Agnew, H. W. Jr. (1971). Stage 4 sleep, influence of time course variables. Science 174, 1351–1356.
Weitzman, E. (1972). “Periodicity in sleep and waking states,” in The Sleeping Brain. Perspectives in the Brain Sciences, Vol. 1, ed. M. H. Chase (Los Angeles: University of California), 193–238.
Wilhelm, I., Diekelmann, S., Molzow, I., Ayoub, A., Mölle, M., and Born, J. (2011). Sleep selectively enhances memory expected to be of future relevance. J. Neurosci. 31, 1563–1569.
Keywords: REM, dreaming, sleep, stage IV sleep, stage N3 sleep, ascending reticular activating system, consciousness, arousal
Citation: Klemm WR (2011) Why does REM sleep occur? A wake-up hypothesis. Front. Syst. Neurosci. 5:73. doi: 10.3389/fnsys.2011.00073
Received: 18 May 2011; Paper pending published: 01 June 2011;
Accepted: 08 August 2011; Published online: 06 September 2011.
Edited by:
Federico Bermudez-Rattoni, Universidad Nacional Autónoma de México, MexicoReviewed by:
Subimal Datta, Boston University School of Medicine, USAJohn F. Araujo, Federal University of Rio Grande do Norte, Brazil
Copyright: © 2011 Klemm. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: W. R. Klemm, Department of Veterinary Integrative Biosciences, Texas A&M University, 4458 TAMU, College Station, TX 77843-4458, USA. e-mail:d2tsZW1tQGN2bS50YW11LmVkdQ==
