- 1Department of Experimental Pathology, Immunology, and Microbiology, Faculty of Medicine, American University of Beirut, Beirut, Lebanon
- 2Faculty of Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Background: The increasing incidence of inflammatory bowel diseases (IBD) over the last two decades has prompted the need to create new types of therapeutic interventions. The gut microbiome has emerged as a key component in the prognosis and pathophysiology of IBDs. The alteration or dysbiosis of the gut microbiome has been shown to exacerbate IBDs. The bacterial composition of the gut microbiome can be modulated through the usage of probiotics, prebiotics, and synbiotics. These interventions induce the growth of beneficial bacteria. Additionally, these interventions could be used to maintain gut homeostasis, reduce the inflammation seen in these morbidities, and strengthen the gut epithelial barrier.
Methods: The literature review was conducted in October 2024 using PubMed, Scopus, and Google Scholar screening for recent clinical trials in addition to reviews relevant to the topic.
Aims: This review aims to summarize the recent clinical trials of probiotics, prebiotics, and synbiotics in IBD patients highlighting their potential benefits in alleviating symptoms and enhancing the quality of life.
Conclusion: Certain probiotic formulations such as single strain ones consisting of Lactobacillus, or mixed-strain combinations of Lactobacillus and Bifidobacterium, prebiotic compounds such as fructooligosaccharides, and synbiotic combinations of both have proven effective in improving the clinical, immunological, and symptomatic aspects of the disease course. While promising, these findings remain inconclusive due to inconsistent study designs, small sample sizes, and varying patient responses. This emphasizes the need for larger, well-controlled trials to determine their clinical efficacy.
1 Introduction
The gut microbiota is a complex and diverse community of microorganisms that live in the digestive tract of humans and animals (Gomaa, 2020). The human intestinal microbiome consists of over 1,000 species of bacteria and other microorganisms (Martyniak et al., 2021). The total number of commensal microorganisms is approximately equal to the number of human eukaryotic cells, with an estimated ratio of 1:1 (Sender et al., 2016). The commensal bacteria of the gut play a role in fermenting complex fibers and carbohydrates (Jandhyala et al., 2015), producing vitamins (LeBlanc et al., 2013), and providing protection against possible invading pathogens (Panwar et al., 2021). These commensal bacteria respond to host hormones and chemicals; in response, they produce metabolites that maintain homeostasis in the gut, influence immune response maturation and host energy metabolism, and preserve the mucosal integrity (Zheng et al., 2020; Nieuwdorp et al., 2014; Schreiber et al., 2024).
The gut microbiota is dominated mainly by 6 different phyla that include Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia and Fusobacteria (Ross et al., 2024; Hasan and Yang, 2019). In particular, the phylum Proteobacteria is characterized by the prevalence of opportunistic pathogens including Escherichia coli, Salmonella, Campylobacter, Klebsiella, and Shigella which have been implicated in both metabolic and inflammatory disorders (Rizzatti et al., 2017). Conversely, beneficial bacteria include those of the genera Lactobacillus, Faecalibacterium, and Roseburia belonging to the Firmicutes phylum (Lindstad et al., 2021; Linares et al., 2016; Nie et al., 2021; Martín et al., 2023). Additionally, the genus Bifidobacterium, classified under the Actinobacteria phylum, is also recognized as a beneficial member of the gut microbiota (Linares et al., 2016). These microbes play several essential roles including but not limited to the production of key metabolites such as butyrate (Valdes et al., 2018).
The intestinal microbiome is easily altered by internal or external factors such as diet, aging, or antibiotic usage (Ross et al., 2024; Hasan and Yang, 2019). A change in the normal composition of a healthy microbiome is known as dysbiosis (Weiss and Hennet, 2017). Most of these dysbiotic states are transient leading to temporary symptoms (Blumstein et al., 2014).
However, in a small portion of cases, these dysbiotic changes can be permanent and lead to the emergence of chronic diseases or symptoms (Blumstein et al., 2014). The illnesses that can arise from a prolonged or permanent state of dysbiosis include gastrointestinal illnesses such as colorectal cancer (Rebersek, 2021) and inflammatory bowel diseases (IBD) (Yu, 2018). Dysbiosis may also exacerbate previous existing intestinal or extra-intestinal diseases such as cardiovascular diseases (Nesci et al., 2023).
Dysbiosis can be reversed or modulated through a plethora of interventions that include antibiotics, probiotics, prebiotics, postbiotics, and even fecal microbiota transplantation (Fong et al., 2020). The most notable of these interventions are probiotics which have gained traction over the past decades (Kim et al., 2019). According to the expert panel from the International Scientific Association for Probiotics and Prebiotics (ISAPP), probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (Hill et al., 2014). There is an abundance of literature illustrating the role of probiotics in the treatment of numerous disorders such as ulcerative colitis (UC), Crohn’s disease (CD), diarrhea, irritable bowel syndrome (IBS), obesity, cancer, and insulin resistance (Petrariu et al., 2024; Cerdó et al., 2019; Kijmanawat et al., 2019; Whelan and Quigley, 2013).
Prebiotics are a substrate that is selectively utilized by the beneficial host organisms to confer a health benefit (Yadav et al., 2022). The combination between a probiotic and prebiotic is referred to as a synbiotic (Yadav et al., 2022). Synbiotics, like probiotics, have been shown to restore the normal gut flora and treat inflammation along with a wide array of morbidities (Singh et al., 2023; Jadhav et al., 2023). In certain instances, synbiotics have been shown to have a greater efficacy than either probiotics or prebiotics used in isolation (Mohanty et al., 2018). Figure 1 represents the health benefits of probiotics, prebiotics, and synbiotics on the gut microbiome.
Figure 1. An illustration demonstrating the health benefits of probiotics, prebiotics, and synbiotics on the gut microbiome. This illustration was created using BioRender.
This narrative review aims to provide an overview on probiotics, prebiotics, synbiotics and their potential to treat IBDs. It will summarize clinical data and trials on probiotics, prebiotics, and synbiotics on IBD patients.
2 Methods
The literature review was conducted in October 2024, providing an overview on probiotics, prebiotics, synbiotics as well as relevant clinical trials. The utilized databases include PubMed, Scopus, and Google Scholar. The results were retrieved using the following key words: “Probiotics,” “Prebiotics,” “Synbiotics,” “Inflammatory bowel disease,” “VSL#3,” “Bifidobacterium,” “Lactobacillus,” as well as their relevant MeSH terms.
This strategy aimed to explore articles that used probiotics, prebiotics, or synbiotics as an intervention or adjunct therapy for the treatment of IBDs while providing an overview for the relevant mechanisms. Articles were selected based on their relevance and findings pertinent to this review. The inclusion criteria encompassed studies without restrictions on their type or country of origin, provided they were written in or translated into English.
In addition to primary research articles, relevant reviews were added to provide a comprehensive overview of the topic. References from selected articles were also examined to identify significant studies. This narrative review involved a thorough examination of each article’s relevance and validity to ensure a rigid and extensive synthesis regarding probiotics, prebiotics, synbiotics, and their potential role in treatments.
3 The immune and microbial factors in inflammatory bowel diseases
IBDs are of two types: CD and UC (Qiu et al., 2022). Over the past few decades, the incidence of these morbidities in Europe and North America has remained relatively constant, while their occurrence in newly industrialized countries has continued to increase rapidly (Roda et al., 2020; Kaplan, 2015; Ng et al., 2017). Despite the etiology of these diseases remaining largely unknown, a variety of factors are thought to be involved including genetic susceptibility, immune factors, and the gut microbiota (Qiu et al., 2022).
The inflammatory nature of both CD and UC is caused by an overly aggressive immune response to a subset of commensal enteric gut microbes (Sartor, 2006). On one hand, the immune response in CD is thought to be driven by Type-1 helper cell response (Zhang and Li, 2014). On the other hand, Type 2 helper cells are thought to instigate the immune response in UC (Zhang and Li, 2014). In terms of microbial composition, both CD and UC patients experience a decrease in microbial abundance, diversity, and stability (Carding et al., 2015). Specifically, there is a reduction in Firmicutes and Bacteroidetes accompanied by an increase in Proteobacteria and Actinobacteria (Alshehri et al., 2021). Additional studies also noted a decrease in anaerobic bacteria such as Bifidobacterium and Lactobacillus alongside an increase in Escherichia and Enterococci (Martyniak et al., 2021). These dysbiotic alterations can lead to a weakened intestinal epithelial barrier resulting in increased luminal antigen uptake and continuous immune activation (Alshehri et al., 2021).
In CD, the entire gastrointestinal tract can be affected with the most common segments affected being the terminal ileum and the colon (Torres et al., 2017). Inflammation in CD is typically segmental, asymmetrical, and transmural (Torres et al., 2017). This differs from UC, where inflammation is typically restricted to the mucosal surface (Ordás et al., 2012). Additionally, the primary organ affected by UC is the colon (Meier and Sturm, 2011). Treatments for IBDs typically include aminosalicylates, corticosteroids, immunomodulators, biologics, and probiotics (Cai et al., 2021). In addition to these conventional therapies, a recent advancement in the treatment of IBD involves the application of bio-nanomaterials which enable targeted drug delivery, control the release of the pharmacological compounds, and provide stimuli-responsive therapy while reducing side effects (Stojanov and Berlec, 2024).
4 Role of probiotics, prebiotics, and synbiotics in gut health
4.1 Role of probiotics
Historically, the consumption of probiotics began when early civilization humans began to consume fermented foods (Yadav et al., 2022). The term probiotics was coined to reflect the work of Elie Metchnikoff (Anukam and Reid, 2007). Metchnikoff made the ground-breaking observation that the regular consumption of lactic acid bacteria in fermented dairy products such as yogurt was associated with enhanced health and longevity in the Bulgarian Peasant populations (Anukam and Reid, 2007).
The latest definition of probiotics considers them to contain living organisms that must be ingested in a sufficient amount to have a positive effect on health that is not limited to the nutritional effects (Kechagia et al., 2013). The first available probiotics contained only one species of microorganisms, mainly those belonging to the Saccharomyces or Lactobacillus genera (Wieërs et al., 2020).
Numerous studies have shown that probiotics can modulate the microbial flora composition of the gut, inhibit pathogenic bacteria from colonizing the gut, and assist the host in building a healthy intestinal mucosa (Chandrasekaran et al., 2024). There are many mechanisms by which probiotics exert their beneficial effects (Chandrasekaran et al., 2024). Probiotics have been shown to increase the number of beneficial bacteria in the intestine by promoting the growth of endogenous desirable microbial populations as well as their own growth (Fassarella et al., 2021). Another mechanism by which probiotics favourably affect the gut is through competitive exclusion (Monteagudo-Mera et al., 2019). This process promotes the growth of beneficial bacteria and inhibits the growth of pathogenic ones (Monteagudo-Mera et al., 2019).
Additionally, probiotics have been shown to enhance or restore the gut barrier function (Abraham and Quigley, 2017). This happens by inhibiting the apoptosis of intestinal epithelial cells and promoting the synthesis of proteins that are critical components of tight junctions (Yan and Polk, 2002; Rose et al., 2021). Probiotics have also been shown to exhibit anti-inflammatory effects modulating local as well as mucosal inflammation (Cristofori et al., 2021). Studies have unveiled the ability of probiotics to reduce inflammation by increasing the production of certain anti-inflammatory cytokines such as interleukin 10 (IL-10) and transforming growth factor-beta (TGF-ß) while reducing the expression of pro-inflammatory cytokines such as interferon gamma (IFN-γ) and IL-1 (Cristofori et al., 2021). A potential mechanism for the modulation of inflammatory markers may involve the inhibition of the nuclear factor-kappa B (NF-κB) pathway (Mahapatro et al., 2023).
Probiotics have been shown to induce the expression of Immunoglobulin A (IgA) and stimulate the maturation of the humoral immune system (Maldonado Galdeano et al., 2019). They can stimulate the macrophages and dendritic cells which are immune cells that can aid in the identification and elimination of pathogens (Shi et al., 2017).
Another way through which probiotics exert their beneficial effects is through the creation of an acidic milieu that is inimical to proinflammatory bacteria but supportive to the growth of beneficial species of bacteria such as Lactobacilli and Bifidobacteria (Abraham and Quigley, 2017). Their ability to colonize the gastrointestinal tract is further aided by their production of bacteriocins which are molecules that inhibit the growth of pathogenic bacteria (Gillor et al., 2008).
4.2 Role of prebiotics
Based on a December 2016 microbiology expert panel, the term “prebiotics” refers to molecules that can be manipulated by the host microbiota to achieve health benefits like preventing disease or improving outcomes (Gibson et al., 2017). While more inclusive than the initial description of prebiotics which had narrowed them down to non-digestible oligosaccharides, even initial views stated that these molecules must preferentially enhance the development of certain beneficial gut bacteria over their non-beneficial counterparts (Gibson and Roberfroid, 1995). As opposed to the live organisms forming probiotics, prebiotics consist of substances that can supplement bacterial growth most commonly stemming from natural sources with plant-based oligosaccharides being the most common (Oniszczuk et al., 2021). Several everyday foods have been shown to exhibit prebiotic properties such as oats, soybeans, and honey (Olas, 2020) given their biochemical composition, but it must be noted that these are only some of the vast possible sources of the prebiotic compounds to be mentioned. Several forms of carbohydrates have demonstrated prebiotic potential such as lactulose, galactooligosaccharides, fructooligosaccharides, maltooligosaccharides, cyclodextrins, and lactosaccharose as well as fructans such as inulin and oligofructose (Markowiak and Śliżewska, 2017). In most cases, the main bacterial agents of the attempts to modify the gut microbiome are of the Bifidobacterium and Lactobacillus strains which metabolize the carbohydrate-based prebiotics to short-chained fatty acids (SCFAs) which decreases the gut pH (Gibson and Wang, 1994). This decrease in pH which the aforementioned bacteria tolerate helps inhibit the proliferation of pathogenic strains. Another relevant example is the water extract from silver fir (Abies alba) wood which represents a source of lignans and polyphenols. Its rich carbohydrate content explains its potential to act as a prebiotic for multiple strains of Lactobacillus (Stojanov et al., 2021).
Dietary habits have been shown to modulate the gut microbiome composition which can be divided into enterotypes. In a study by Wu et al., fecal analysis after controlled feeding of 10 subjects revealed two main enterotypes based on the type of dietary intake (Wu et al., 2011). Changes occurred within 24 h of the diet before reverting to a composition resembling the baseline. High fat/low fiber diets led to the prevalence of the Bacteroides-dominant enterotype, while the low fat/high fiber diets instead promoted a Prevotella-dominant enterotype (Wu et al., 2011). This proves that dietary fibers, many of which are considered prebiotics based on their promotion of certain bacterial strains’ growth, could modulate gut microbiome composition.
Prebiotics can reach the lower GI tract intact due to their resistance to digestion by gastric acid or mammalian hydrolases and to absorption in the upper GI tract (Ashaolu et al., 2021). Once in the colon, their selective fermentation by beneficial bacteria will promote those strains’ growth in favor of a more balanced microbiota composition (Ashaolu et al., 2021). Furthermore, a direct result of microbiome metabolism of prebiotics is the production of SCFAs which have been shown to regulate gut microbiome homeostasis and contribute to the pathogenesis of multiple disease and inflammatory processes when deficient (Fusco et al., 2023). SCFAs produced in the gut include acetate, propionate, and butyrate which are the most abundant anions in the human body (Portincasa et al., 2022).
Patients with UC have been shown to have a lower prevalence of butyrate-producing bacteria in their gut such as Roseburia hominis and Faecalibacterium prausnitzii with their abundance being inversely related to disease activity (Machiels et al., 2014). The SCFAs produced by species selected-for by prebiotics can relieve the chronic inflammation seen in multiple inflammatory disorders (Liu et al., 2023). Butyrate and propionate have been shown to minimize the recruitment of monocytes and neutrophils through the inhibition of the inducible expression of adhesion molecules and chemokine production as part of an anti-inflammatory mechanism that could benefit patients with autoimmune diseases (Vinolo et al., 2011). Butyrate in particular suppresses LPS- and cytokine-mediated production of pro-inflammatory mediators such as TNF-α, IL-6, and nitric oxide (NO) while upregulating the release of anti-inflammatory cytokines such as IL-10 (Vinolo et al., 2011). In addition, the SCFAs synthesized through prebiotic administration have been shown to decrease gut pH due to protons being a byproduct of the reaction (den Besten et al., 2013). This pH change could be gut-protective given that some beneficial bacteria are selected for under weakly acidic pH, and most pathogenic bacteria would be selected against in this acidic environment as they thrive in more pH-neutral conditions (Yamamura et al., 2023). Butyric acid-producing bacteria like Faecalibacterium and Roseburia have been shown to grow better in weakly acidic environments rather than at neutral pH (Walker et al., 2005).
Prebiotics also help preserve intestinal barrier integrity through upregulating tight junction proteins involved in the assembly of the zonula occludens ZO-1 (Wongkrasant et al., 2020). This was proven to be through the AMP-activated protein kinase promotion of tight junction assembly through a calcium sensing receptor (CaSR)-phospholipase C (PLC)- Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKKβ) pathway in an intestinal epithelial cell model (Wongkrasant et al., 2020).
4.3 Role of synbiotics
The most recent definition of “Synbiotics” by the International Scientific Association for Probiotics and Prebiotics (ISAPP) described them as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” where the host microbes can either be the resident microflora of the host ingesting the synbiotic or the exogenous “host” microbes within the synbiotic mixture (Wu et al., 2011). This definition combines both concepts of probiotics as the live microbes and prebiotics as the substrates selectively promoting those microbes as well as the resident flora into one holistic treatment (Swanson et al., 2020). This implies that consuming a combination of both confers an increased microbial survival of the probiotic formulation as well as a better chance for the established healthy microflora to thrive (Takahashi et al., 2018). Figure 2 highlights the relationship between probiotics and prebiotics and how they combine to form synbiotics.
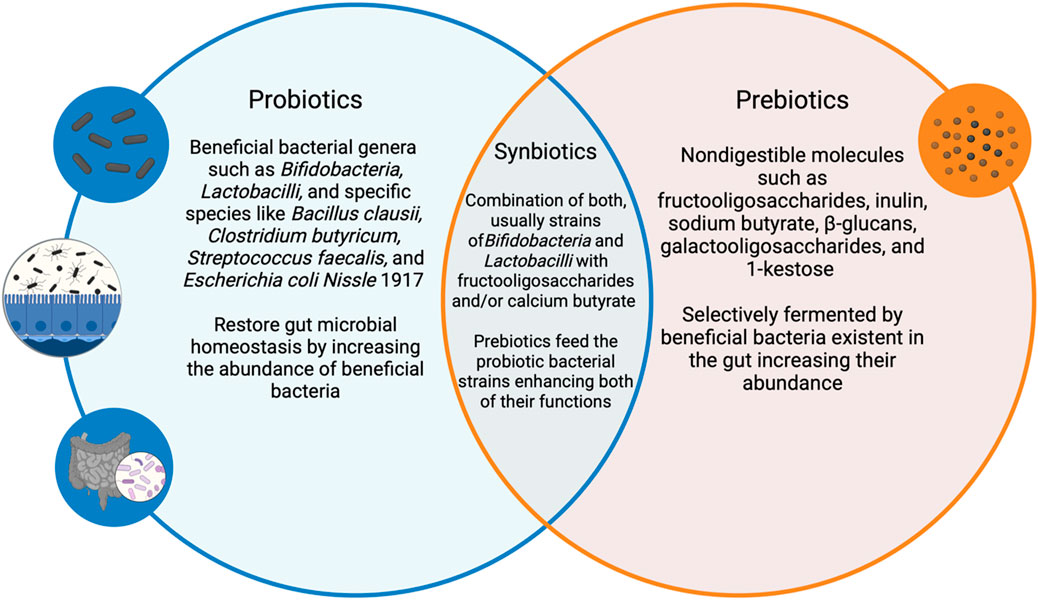
Figure 2. An illustration demonstrating the relationship between probiotics, prebiotics and synbiotics along with relevant examples. This illustration was created using BioRender.
5 Intervention of probiotics, prebiotics, and synbiotics in IBD: evidence from clinical trials and observational studies
5.1 Intervention of probiotics
Numerous clinical trials and observational studies have assessed the effectiveness of probiotics in inducing and maintaining remission in both UC and CD patients. The selected studies were grouped into different categories based on similar outcome measures.
5.1.1 Clinical activity and endoscopic improvement in UC
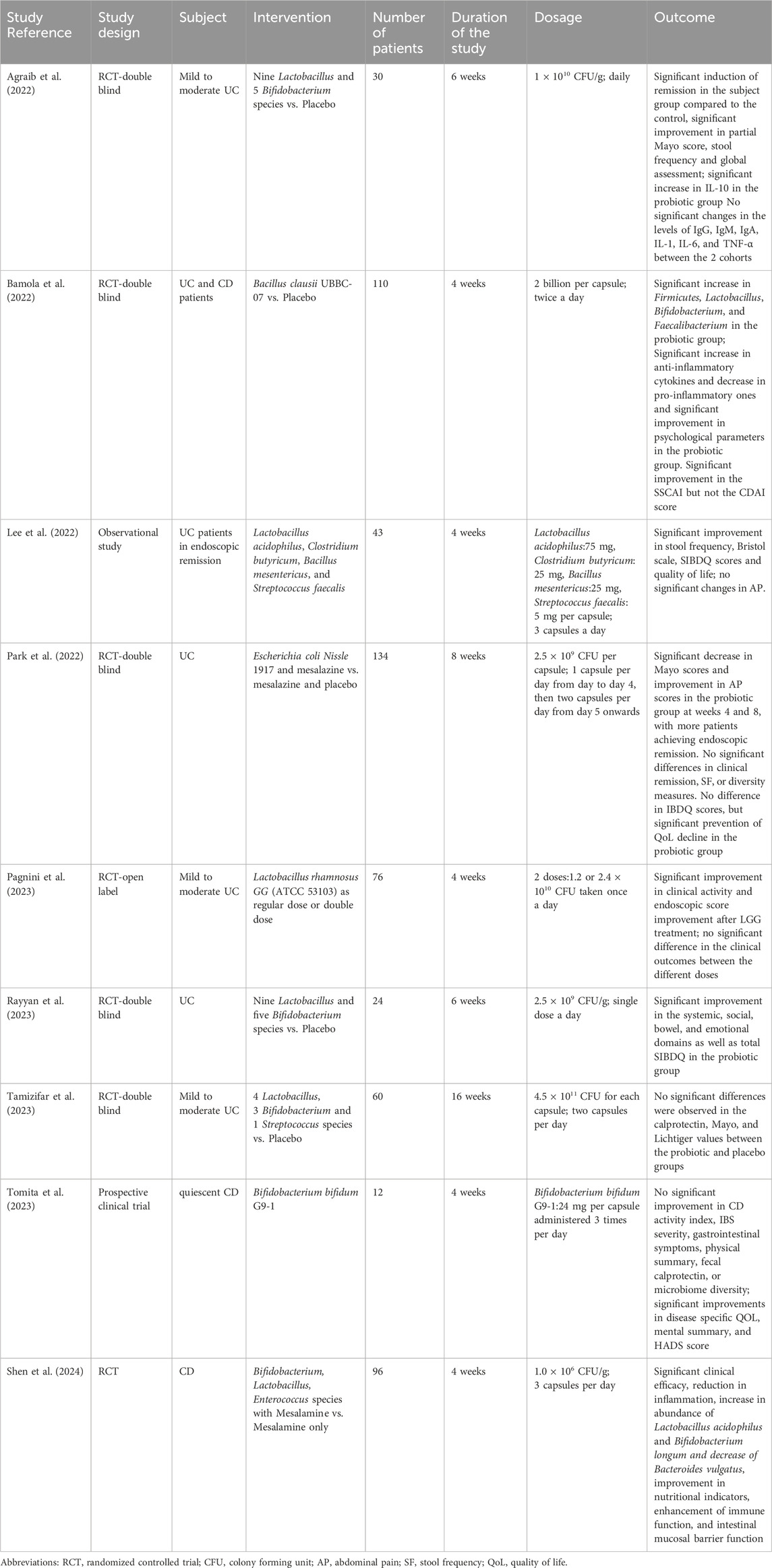
Several of the selected studies demonstrated significant improvements in clinical indices and endoscopic outcomes in UC patients. A study conducted by Agraib et al. tested the efficacy of a probiotic blend containing nine Lactobacillus and five Bifidobacterium species on UC patients for 6 weeks (Agraib et al., 2022). The study reported a significant increase in the partial Mayo score, stool frequency, and global assessment (Agraib et al., 2022). Another randomized double blind clinical trial tested the efficacy of Escherichia coli Nissle 1917 and mesalazine against mesalazine and placebo only (Park et al., 2022). The patients that were treated with the probiotic and mesalazine observed a significant decrease in their partial Mayo score and improved abdominal pain score as well as higher rates of endoscopic remission compared to those receiving mesalazine with the placebo (Park et al., 2022). However, this study also reported no significant difference in clinical remission rates, and stool frequency (Park et al., 2022). An additional randomized open label clinical trial tested the efficacy of Lactobacillus rhamnosus GG as a regular dose or double dose (Pagnini et al., 2023). The study observed significant improvement in the clinical activity and endoscopic scores in the UC patients treated with the probiotic over 4 weeks irrespective of the dosage administered (Pagnini et al., 2023). Conversely, a study testing the efficacy of a probiotic mixture containing eight strains (four Lactobacillus, three Bifidobacterium, and one Streptococcus) did not observe significant changes in the clinical indices (Tamizifar et al., 2023). After 16 weeks of treatment, the calprotectin levels, Mayo, and Lichtiger scores did not significantly change demonstrating variability in clinical outcomes (Tamizifar et al., 2023). Additionally, another study reported no significant improvement in the CD activity index, IBS severity, gastrointestinal symptoms, and fecal calprotectin despite probiotic administration for 4 weeks (Tomita et al., 2023). A different study reported an improved Simple Clinical Colitis Activity Index (SCCAI) but no significant difference in the Crohn’s disease activity index (CDAI) score between the 2 cohorts (Bamola et al., 2022).
5.1.2 Modulation of inflammatory markers and gut microbiota
Five of the selected studies focused on the biochemical and microbiological effects of probiotic therapy. Agraib et al. noted that despite the pro-inflammatory cytokines IL-1, IL-6, tumor necrosis factor- α (TNF- α) remaining unchanged, there was a significant increase in the anti-inflammatory cytokine IL-10 in the probiotic group compared to the placebo (Agraib et al., 2022). Another double blind randomized controlled trial (RCT) study studied the effects of Bacillus clausii UBBC-07 in both UC and CD patients for 4 weeks (Bamola et al., 2022). The study also reported an increased abundance of the Firmicutes, Lactobacillus, Bifidobacterium, and Faecalibacterium in the gut microbiota composition (Bamola et al., 2022). Additionally, the study reported a significant increase in anti-inflammatory cytokines, alongside a marked decrease in pro-inflammatory cytokines (Bamola et al., 2022). A third study conducted by Shen et al. demonstrated the ability of the probiotic to regulate the intestinal microbiota (Shen et al., 2024). In particular, there was significant increase in the abundance of Lactobacillus acidophilus and Bifidobacterium longum along with a decrease of Bacteroides vulgatus (Shen et al., 2024). This study also reported a significant reduction in inflammation alongside an improvement in immune and intestinal barrier function (Shen et al., 2024). Conversely, another study demonstrated a lack of gut microbial alterations despite probiotic administration in the CD population (Tomita et al., 2023). A fifth study also demonstrated the lack of gut microbiome alteration despite probiotic intervention (Park et al., 2022).
5.1.3 Quality of life and symptom relief
Lee et al. conducted an observational study composed of 43 UC patients in endoscopic remission (Lee et al., 2022). The patients were given a probiotic blend consisting of Lactobacillus acidophilus, Clostridium butyricum, Bacillus mesentericus, and Streptococcus faecalis (Lee et al., 2022). The intervention was shown to significantly improve the stool frequency, Bristol scale, and short inflammatory bowel disease questionnaire scores in UC patients (Lee et al., 2022). However, there was no alteration in the abdominal pain (Lee et al., 2022). Another double blind RCT studied the effects of a probiotic mixture consisting of nine Lactobacillus and five Bifidobacterium strains in the UC population (Rayyan et al., 2023). The study demonstrated that over 6 weeks, the UC patients receiving the probiotic exhibited significant enhancement in the systemic, social, bowel, and emotional domains in addition to a significant improvement in the total SIBDQ score (Rayyan et al., 2023). A study conducted on the CD population reported significant improvements in the disease specific quality of life, mental summary, and Hospital Anxiety and Depression Scale (HADS) score (Tomita et al., 2023). Another study conducted by Bamola et al. also reported a significant improvement in the psychological parameters in the probiotic group (Bamola et al., 2022). However, one study reported no differences in the inflammatory bowel disease questionnaire scores (IBDQ) between the two groups (Park et al., 2022).
Overall, the use of probiotics in IBD shows promise, but results are mixed. Several studies reported improvements in clinical activity, endoscopic outcomes, inflammatory markets, and gut microbiota composition, especially in UC patients. However, other trials found no significant changes in these indices and criteria highlighting the variability in patient response and in the efficacy of different probiotic formulations.
The probiotic studies in IBD are presented in Table 1.
5.2 Intervention of prebiotics
5.2.1 Clinical remission and disease activity
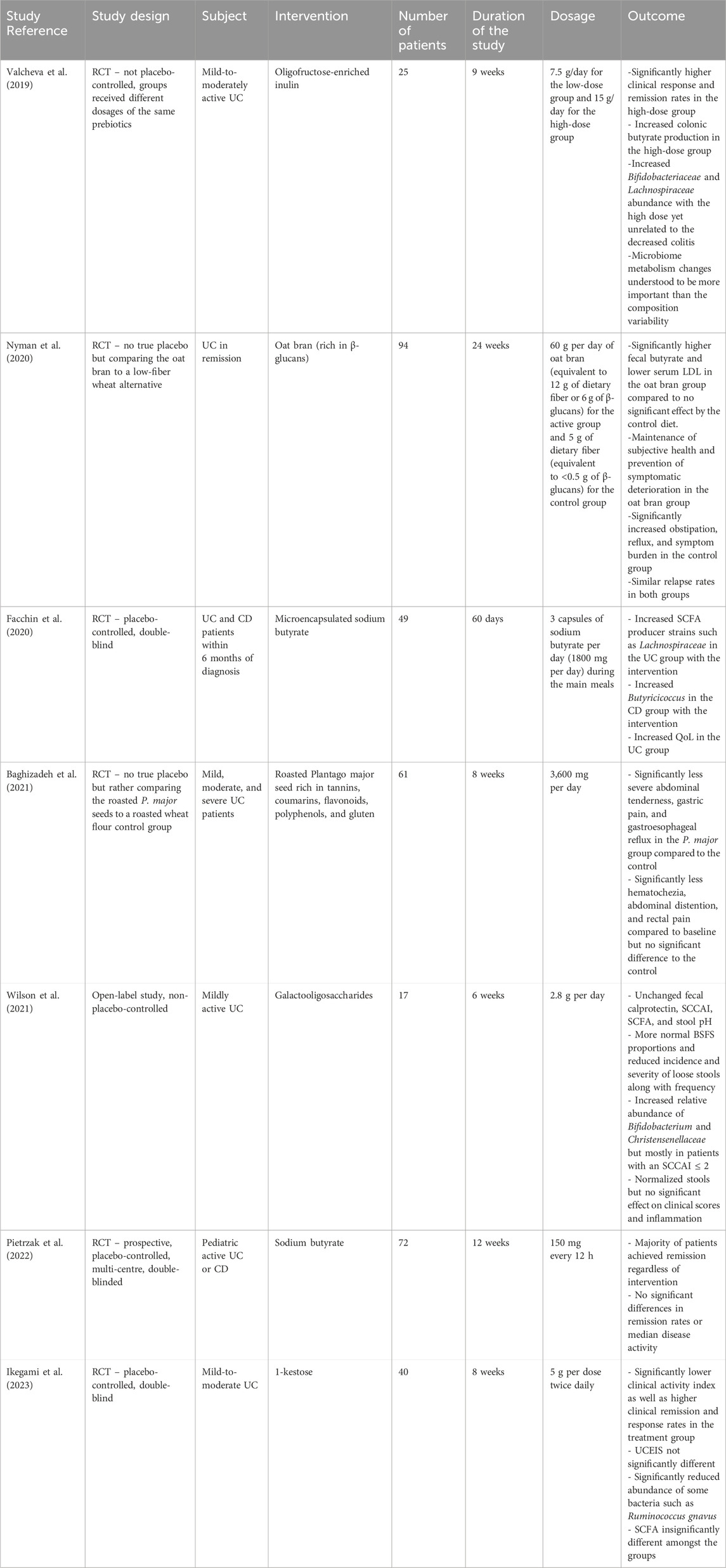
An RCT conducted by Valcheva et al. assessed the effectiveness of administering different dosages of oligofructose-enriched inulin on 25 patients with mild-to-moderately active UC for 9 weeks (Valcheva et al., 2019). The high-dose group reported significantly higher clinical response and remission rates compared to the low-dose group. Pietrzak et al. conducted a double-blind RCT comparing the efficacy of sodium butyrate compared to a placebo to treat 72 pediatric patients with either UC or CD over 12 weeks (Pietrzak et al., 2022). The study revealed that most patients achieved clinical remission regardless of receiving the intervention or not. Both groups showed no significant differences in terms of their remission rates and median disease activities. Another study by Ikegami et al. studied the use of 1-kestose over 8 weeks compared to a placebo in a double-blind RCT on 40 mild-to-moderately active UC patients (Ikegami et al., 2023). The treatment group exhibited a significantly lower clinical activity index along with a significant improvement in clinical remission and response rates. However, the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) scores were not significantly different amongst the treatment and control groups.
5.2.2 Symptom relief and quality of life
In an RCT conducted by Nyman et al., the researchers compared the use of oat bran rich in β-glucans to a low-fiber alternative in treating 94 patients with UC in remission (Nyman et al., 2020). Over 24 weeks, the oat bran group achieved subjective health maintenance along with the prevention of symptomatic deterioration more effectively than the low-fiber group. While the relapse rates were similar in both groups, the control group did exhibit significantly more obstipation, reflux, and symptom burden. A different study by Facchin et al. addressed the quality-of-life changes of 49 patients with either UC or CD within 6 months of their diagnosis through an RCT that compared microencapsulated sodium butyrate treatments to a placebo (Facchin et al., 2020). After the 60-day period of the trial, the UC patients receiving the treatment experienced improvements in quality of life based on changes in their IBD Questionnaire (IBDQ) answers.
A paper published by Baghizadeh et al. presented an RCT that investigated the use of Plantago major (P. major) seeds compared to a roasted wheat flour control group to treat 61 patients with mild, moderate, and severe UC over 8 weeks (Baghizadeh et al., 2021). The P. major seeds were selected for their richness in prebiotic compounds such as tannins, coumarins, flavonoids, polyphenols, and gluten. The treatment group experienced significantly less severe abdominal tenderness, gastric pain, and gastroesophageal reflux compared to the control. While they did also exhibit a significant decrease of hematochezia, abdominal distention, and rectal pain compared to their own baseline, that difference was not significant compared to the changes in those values in the control group. Finally, in a non-placebo-controlled open label trial by Wilson et al., 17 patients with mildly active UC were given a galactooligosaccharide supplement daily over 6 weeks (Wilson et al., 2021). The patients experienced more normalized stools but no significant effects on clinical scores and inflammation. Their stools showed more normalized Bristol Stool Form Scale (BSFS) values along with the reduced incidence and severity of loose stools. However, they showed no significant changes in fecal calprotectin, SCCAI scores, and stool pH.
5.2.3 Microbiota composition and SCFA production
Multiple of the selected studies investigated the effects of the interventions in affecting gut microbiota composition and changes in metabolism including SCFA production as an important indicator. In the trial using oligofructose-enriched inulin (Valcheva et al., 2019), the high-dose group experienced more colonic butyrate production which could be explained by the increase in abundance of Bifidobacteriaceae and Lachnospiraceae, yet this change was found to be unrelated to the decreased colitis. The researchers identified that microbiome metabolism changes such as butyrate production are more important than the microbiota composition itself. With regards to the oat bran supplement trial (Nyman et al., 2020), the treatment group had significantly higher fecal butyrate levels and lower serum low-density lipoprotein (LDL) compared to the control group who did not experience significant changes.
In the microencapsulated sodium butyrate study by Facchin et al. (2020), the UC group receiving treatment had more SCFA-producing strains in their stools such as Lachnospiraceae compared to their control. Moreover, the CD group receiving treatment exhibited an increase in stool Butyricicoccus compared to their respective control group. In terms of the galactooligosaccharide trial by Wilson et al. (2021), the treatment group’s stools showed no significant changes in stool SCFA levels even though patients with SCCAI ≤ 2 had an increased relative abundance of Bifidobacterium and Christensenellaceae. Finally, in the Ikegami et al. (2023) 1-kestose trial, SCFA values were unaffected regardless of the significantly reduced abundance of Ruminococcus gnavus.
The prebiotic studies in IBD are presented in Table 2.
5.3 Intervention of synbiotics
5.3.1 Clinical activity measures and laboratory markers
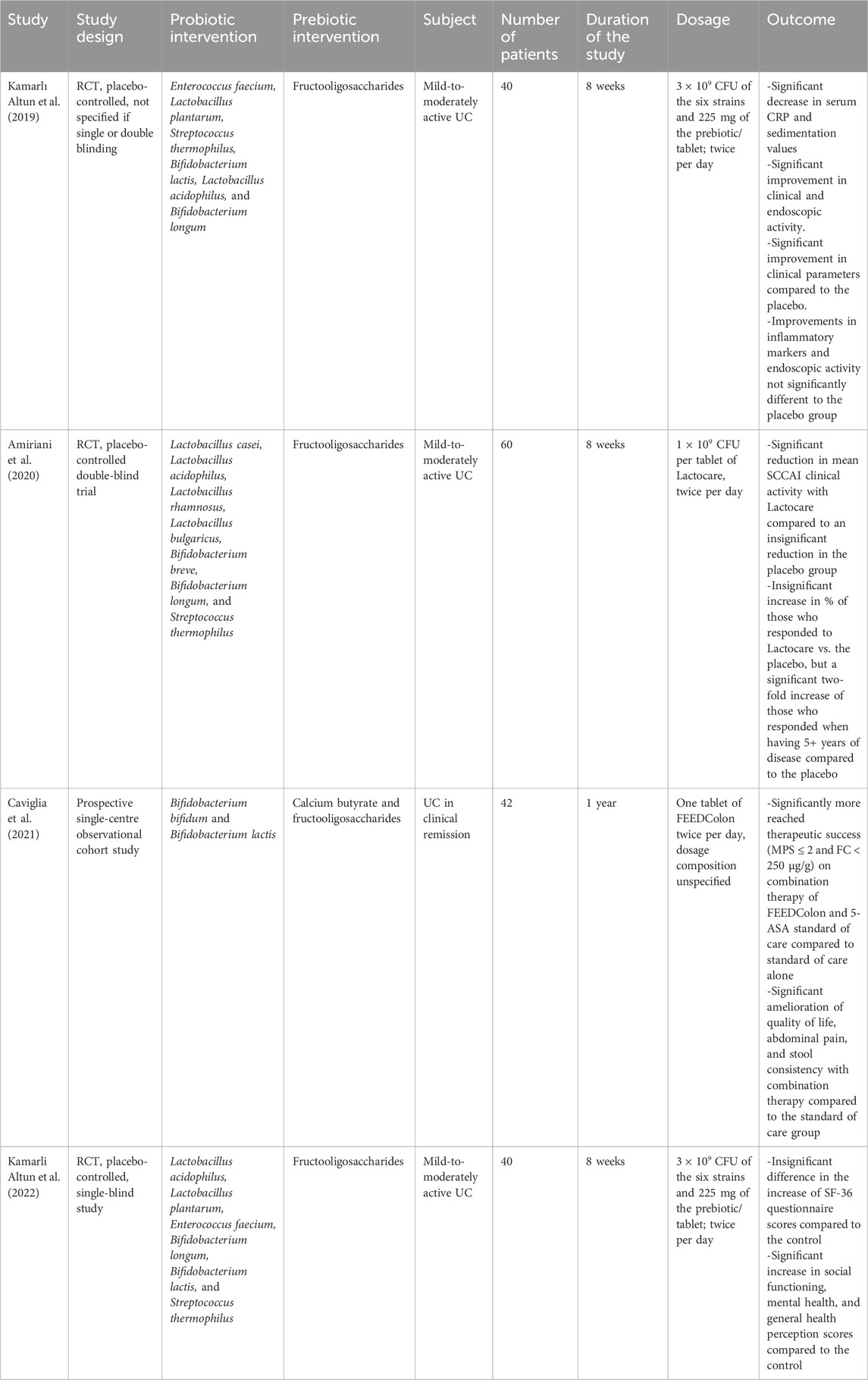
In a placebo-controlled RCT conducted by Kamarlı Altun et al. (2019), researchers studied the effects of combining six bacterial strains (two Lactobacillus, two Bifidobacterium, one Enterococcus, and one Streptococcus) along with fructooligosaccharides against a placebo to treat 40 patients with mild-to-moderately active UC. After the 8-week duration of the trial, the treatment group showed a significant decrease in serum C-reactive protein (CRP) and sedimentation values compared to baseline indicating a decrease in systemic inflammation. Moreover, they experienced significant improvement in their clinical parameters compared to the placebo group. While they showed significant improvement in clinical inflammatory markers and endoscopic disease activity compared to baseline, this was not significantly different to that of the placebo group.
Another study by Amiriani et al. (2020) investigated the use of seven bacterial strains (four Lactobacillus, two Bifidobacterium, and one Streptococcus) with fructooligosaccharides in a placebo-controlled, double-blinded RCT to treat 60 patients with mild-to-moderately active UC over 8 weeks. The group receiving the treatment labelled as Lactocare showed a significant reduction in their mean SCCAI compared to an insignificant reduction in the control group. While the percentage of patients who responded to the Lactocare treatment was not significantly more than those who responded to the placebo, there was a two-fold increase in the proportion of those responding to treatment when they had at least 5 years of disease activity compared to the placebo. In a prospective observational cohort study by Caviglia et al. (2021), two strains of Bifidobacterium were combined with calcium butyrate and fructooligosaccharides to treat 42 patients with UC in clinical remission over a year. The group receiving the treatment labelled FEEDColon were given the pill twice per day along with their standard of care. A significantly higher proportion of those receiving the combination therapy reached therapeutic success defined as Mayo partial score (MPS) ≤ 2 and fecal calprotectin (FC) < 250 μg/g compared to receiving standard of care alone.
5.3.2 Symptomatic relief and quality of life improvement
The FEEDColon study (Caviglia et al., 2021) showed that those receiving the combination therapy with standard of care treatment experienced significant amelioration of quality of life, abdominal pain, and stool consistency compared to the group receiving standard of care alone. Moreover, following the same study by Kamarli Altun et al., the treatment’s impact on the patients’ quality of life was investigated (Kamarli Altun et al., 2022). Using the short-form 36 (SF-36) questionnaire to assess the quality of life, the treatment group had an insignificant difference in the increase of SF-36 values compared to the control group. However, the treatment group showed a significant increase in the social functioning, mental health, and general health perception scores compared to the control.
The synbiotic studies in IBD patients are presented in Table 3.
6 Conclusion
Based on most of the studies included, there does not appear to be a straightforward answer when it comes to the effectiveness of microbiome modulation in the induction and maintenance of remission of IBD. The selected clinical trials highlight a gap in the IBD research field when it comes to the availability of uniform research protocols with large sample sizes. Most of the studies were limited by having small sample sizes which may not provide the most solid evidence concerning the possibility of commercializing the use of any studied compound. Moreover, most studies did not address the long-term side effects of the combinations used which could provide the basis for future research questions. Another possible limitation is the presence of confounding factors such as variations in dietary habits among IBD patients and differences in medication use which may influence study outcomes.
When it comes to probiotics, the formulations that provided the most favorable results included single strains such as Lactobacillus rhamnosus GG and Bacillus clausii UBBC-07 as well as multi-strain formulations combining Lactobacillus and Bifidobacterium species. The aforementioned probiotic formulations were shown to modulate the gut microbiota, reduce pro-inflammatory cytokines, and improve psychological parameters. They also demonstrated clinical and endoscopic benefits. As for the prebiotics, the oligofructose-enriched inulin showed the most promising potential of significantly impacting remission rates with galactooligosaccharides and roasted P. major seeds also helping reduce symptomatic manifestations. In terms of the synbiotic studies, most trials included a probiotic core of strains of Lactobacillus and Bifidobacterium species along with fructooligosaccharides as their corresponding prebiotic with results being fairly promising despite the limited recent studies published.
However, despite these promising effects, the results remained inconclusive due to contradictory findings. While some studies confirmed the ability of probiotics to alleviate clinical symptoms and maintain remission, these benefits were more pronounced in the UC patients. This discrepancy arose due to the unavailability of extensive trials on CD patients, and this population requires further investigation. As for prebiotics, results were also contradictory where some studies showed a significant improvement when using prebiotics compared to controls, yet other studies suggested that while certain improvements were seen, those improvements would also be seen when adhering to standard of care treatment alone. The same applies for the synbiotic studies which pose an even bigger challenge to interpret given the many variables in research protocol, yet the findings are promising and require further investigation as well. It must be noted that while the compounds and organisms mentioned earlier were the most effective, this could also simply be due to most studies happening to cover them over other interventions. The reviews mostly targeted adult patients so the pediatric population could benefit from further investigation. Moreover, more research should be done studying the possibility of utilizing these interventions as a form of primary prevention rather than as an aid to treatment.
A potential area for future research is the personalized formulation of probiotics and prebiotics, or the personalized combination of probiotics and prebiotics to form synbiotics for IBD patients. Such personalized holistic therapy, biotics in combination with other traditional pharmacological therapies, can potentially increase the effectiveness of treatment while also reducing the side effects.
Author contributions
FY: Writing – original draft, Writing – review and editing. AN: Writing – original draft. MB: Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. AI was used to correct English linguistic mistakes.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IBD, Inflammatory bowel disease; UC, Ulcerative Colitis; CD, Crohn’s Disease; IgA, Immunoglobulin A; SCFA, Short-Chain Fatty Acids; QoL, Quality of Life; BSFS, Bristol Stool Form Scale; SCCAI, Simple Clinical Colitis Activity Index; CDAI, Crohn’s disease activity index; SIBDQ, Short Inflammatory bowel disease questionnaire; IBDQ, Inflammatory bowel disease questionnaire; IBS, Inflammatory Bowel syndrome; HADS, Hospital anxiety and depression scale; LDL, Low-density lipoprotein; FC, Fecal calprotectin; IFN-γ, Interferon gamma; NF-κB, Nuclear factor-kappa B; NO, Nitric Oxide; Il-10, Interleukin 10; TGF-ß, Transforming growth factor-beta; UCEIS, Ulcerative Colitis Endoscopic Index of Severity; AP, Abdominal Pain; SF, Stool frequency; RCT, Randomized controlled trial; CFU, Colony forming units.
References
Abraham, B. P., and Quigley, E. M. M. (2017). Probiotics in Inflammatory bowel disease. Gastroenterol. Clin. North Am. 46 (4), 769–782. doi:10.1016/j.gtc.2017.08.003
Agraib, L. M., Yamani, M. I., Tayyem, R., Abu-Sneineh, A. T., and Rayyan, Y. M. (2022). Probiotic supplementation induces remission and changes in the immunoglobulins and inflammatory response in active ulcerative colitis patients: a pilot, randomized, double-blind, placebo-controlled study. Clin. Nutr. ESPEN 51, 83–91. doi:10.1016/j.clnesp.2022.08.020
Alshehri, D., Saadah, O., Mosli, M., Edris, S., Alhindi, R., and Bahieldin, A. (2021). Dysbiosis of gut microbiota in inflammatory bowel disease: current therapies and potential for microbiota-modulating therapeutic approaches. Bosn. J. Basic Med. Sci. 21 (3), 270–283. doi:10.17305/bjbms.2020.5016
Amiriani, T., Rajabli, N., Faghani, M., Besharat, S., Roshandel, G., Akhavan Tabib, A., et al. (2020). Effect of Lactocare® synbiotic on disease severity in ulcerative colitis: a randomized placebo-controlled double-blind clinical trial. Middle East J. Dig. Dis. 12 (1), 27–33. doi:10.15171/mejdd.2020.160
Anukam, K. C., and Reid, G. (2007). Probiotics: 100 years (1907–2007) after Elie Metchnikoff's observation. Commun. Curr. Res. Educ. Top. trends Appl. Microbiol. 1, 466–474.
Ashaolu, T. J., Ashaolu, J. O., and Adeyeye, S. A. O. (2021). Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: a critical review. J. Appl. Microbiol. 130 (3), 677–687. doi:10.1111/jam.14843
Baghizadeh, A., Davati, A., Heidarloo, A. J., Emadi, F., and Aliasl, J. (2021). Efficacy of Plantago major seed in management of ulcerative colitis symptoms: a randomized, placebo controlled, clinical trial. Complement. Ther. Clin. Pract. 44, 101444. doi:10.1016/j.ctcp.2021.101444
Bamola, V. D., Dubey, D., Samanta, P., Kedia, S., Ahuja, V., Madempudi, R. S., et al. (2022). Role of a probiotic strain in the modulation of gut microbiota and cytokines in inflammatory bowel disease. Anaerobe 78, 102652. doi:10.1016/j.anaerobe.2022.102652
Blumstein, D. T., Levy, K., Mayer, E., and Harte, J. (2014). Gastrointestinal dysbiosis. Evol. Med. Public Health 2014 (1), 163. doi:10.1093/emph/eou029
Cai, Z., Wang, S., and Li, J. (2021). Treatment of Inflammatory bowel disease: a comprehensive review. Front. Med. 8, 765474. doi:10.3389/fmed.2021.765474
Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M., and Owen, L. J. (2015). Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 26, 26191. doi:10.3402/mehd.v26.26191
Caviglia, G. P., De Blasio, F., Vernero, M., Armandi, A., Rosso, C., Saracco, G. M., et al. (2021). Efficacy of a preparation based on calcium butyrate, Bifidobacterium bifidum, Bifidobacterium lactis, and Fructooligosaccharides in the prevention of relapse in ulcerative colitis: a prospective observational study. J. Clin. Med. 10 (21), 4961. doi:10.3390/jcm10214961
Cerdó, T., García-Santos, J. A., G Bermúdez, M., and Campoy, C. (2019). The role of Probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 11 (3), 635. doi:10.3390/nu11030635
Chandrasekaran, P., Weiskirchen, S., and Weiskirchen, R. (2024). Effects of Probiotics on gut microbiota: an overview. Int. J. Mol. Sci. 25 (11), 6022. doi:10.3390/ijms25116022
Cristofori, F., Dargenio, V. N., Dargenio, C., Miniello, V. L., Barone, M., and Francavilla, R. (2021). Anti-Inflammatory and immunomodulatory effects of Probiotics in gut inflammation: a door to the body. Front. Immunol. 12, 578386. doi:10.3389/fimmu.2021.578386
den Besten, G., van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D. J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54 (9), 2325–2340. doi:10.1194/jlr.R036012
Facchin, S., Vitulo, N., Calgaro, M., Buda, A., Romualdi, C., Pohl, D., et al. (2020). Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 32 (10), e13914. doi:10.1111/nmo.13914
Fassarella, M., Blaak, E. E., Penders, J., Nauta, A., Smidt, H., and Zoetendal, E. G. (2021). Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut 70 (3), 595–605. doi:10.1136/gutjnl-2020-321747
Fong, W., Li, Q., and Yu, J. (2020). Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene 39 (26), 4925–4943. doi:10.1038/s41388-020-1341-1
Fusco, W., Lorenzo, M. B., Cintoni, M., Porcari, S., Rinninella, E., Kaitsas, F., et al. (2023). Short-Chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients 15 (9), 2211. doi:10.3390/nu15092211
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14 (8), 491–502. doi:10.1038/nrgastro.2017.75
Gibson, G. R., and Roberfroid, M. B. (1995). Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125 (6), 1401–1412. doi:10.1093/jn/125.6.1401
Gibson, G. R., and Wang, X. (1994). Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 77 (4), 412–420. doi:10.1111/j.1365-2672.1994.tb03443.x
Gillor, O., Etzion, A., and Riley, M. A. (2008). The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 81 (4), 591–606. doi:10.1007/s00253-008-1726-5
Gomaa, E. Z. (2020). Human gut microbiota/microbiome in health and diseases: a review. Ant. Van Leeuwenhoek 113 (12), 2019–2040. doi:10.1007/s10482-020-01474-7
Hasan, N., and Yang, H. (2019). Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 7, e7502. doi:10.7717/peerj.7502
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterology & Hepatology 11 (8), 506–514. doi:10.1038/nrgastro.2014.66
Ikegami, S., Nakamura, M., Honda, T., Yamamura, T., Maeda, K., Sawada, T., et al. (2023). Efficacy of 1-kestose supplementation in patients with mild to moderate ulcerative colitis: a randomised, double-blind, placebo-controlled pilot study. Aliment. Pharmacol. Ther. 57 (11), 1249–1257. doi:10.1111/apt.17387
Jadhav, A., Jagtap, S., Vyavahare, S., Sharbidre, A., and Kunchiraman, B. (2023). Reviewing the potential of probiotics, prebiotics and synbiotics: advancements in treatment of ulcerative colitis. Front. Cell. Infect. Microbiol. 13, 1268041. doi:10.3389/fcimb.2023.1268041
Jandhyala, S. M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., and Nageshwar Reddy, D. (2015). Role of the normal gut microbiota. World J. Gastroenterol. 21 (29), 8787–8803. doi:10.3748/wjg.v21.i29.8787
Kamarlı Altun, H., Akal Yıldız, E., and Akın, M. (2019). Effects of synbiotic therapy in mild-to-moderately active ulcerative colitis: a randomized placebo-controlled study. Turk J. Gastroenterol. 30 (4), 313–320. doi:10.5152/tjg.2019.18356
Kamarli Altun, H., Akal Yildiz, E., and Akin, M. (2022). Impact of synbiotic therapy on the quality of life in patients with mild-to-moderately active ulcerative colitis. J. Gastrointestin Liver Dis. 31 (4), 417–423. doi:10.15403/jgld-4345
Kaplan, G. G. (2015). The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterology & Hepatology 12 (12), 720–727. doi:10.1038/nrgastro.2015.150
Kechagia, M., Basoulis, D., Konstantopoulou, S., Dimitriadi, D., Gyftopoulou, K., Skarmoutsou, N., et al. (2013). Health benefits of probiotics: a review. ISRN Nutr. 2013, 481651, doi:10.5402/2013/481651
Kijmanawat, A., Panburana, P., Reutrakul, S., and Tangshewinsirikul, C. (2019). Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: a double-blind randomized controlled trial. J. Diabetes Investig. 10 (1), 163–170. doi:10.1111/jdi.12863
Kim, S. K., Guevarra, R. B., Kim, Y. T., Kwon, J., Kim, H., Cho, J. H., et al. (2019). Role of Probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 29 (9), 1335–1340. doi:10.4014/jmb.1906.06064
LeBlanc, J. G., Milani, C., de Giori, G. S., Sesma, F., van Sinderen, D., and Ventura, M. (2013). Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24 (2), 160–168. doi:10.1016/j.copbio.2012.08.005
Lee, J., Park, S. B., Kim, H. W., Lee, H. S., Jee, S. R., et al. (2022). Clinical efficacy of probiotic therapy on bowel-related symptoms in patients with ulcerative colitis during Endoscopic remission: an observational study. Gastroenterol. Res. Pract. 2022, 9872230. doi:10.1155/2022/9872230
Linares, D. M., Ross, P., and Stanton, C. (2016). Beneficial Microbes: the pharmacy in the gut. Bioengineered 7 (1), 11–20. doi:10.1080/21655979.2015.1126015
Lindstad, L. J., Lo, G., Leivers, S., Lu, Z., Michalak, L., Pereira, G. V., et al. (2021). Human gut Faecalibacterium prausnitzii deploys a highly efficient conserved system to cross-feed on β-Mannan-Derived oligosaccharides. mBio 12 (3), e0362820. doi:10.1128/mBio.03628-20
Liu, X. F., Shao, J. H., Liao, Y. T., Wang, L. N., Jia, Y., Dong, P. J., et al. (2023). Regulation of short-chain fatty acids in the immune system. Front. Immunol. 14, 1186892. doi:10.3389/fimmu.2023.1186892
Machiels, K., Joossens, M., Sabino, J., De Preter, V., Arijs, I., Eeckhaut, V., et al. (2014). A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63 (8), 1275–1283. doi:10.1136/gutjnl-2013-304833
Mahapatro, A., Bawna, F., Kumar, V., Daryagasht, A. A., Gupta, S., Raghuma, N., et al. (2023). Anti-inflammatory effects of probiotics and synbiotics on patients with non-alcoholic fatty liver disease: an umbrella study on meta-analyses. Clin. Nutr. ESPEN 57, 475–486. doi:10.1016/j.clnesp.2023.07.087
Maldonado Galdeano, C., Cazorla, S. I., Lemme Dumit, J. M., Vélez, E., and Perdigón, G. (2019). Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metabolism 74 (2), 115–124. doi:10.1159/000496426
Markowiak, P., and Śliżewska, K. (2017). Effects of probiotics, prebiotics, and Synbiotics on human health. Nutrients 9 (9), 1021. doi:10.3390/nu9091021
Martín, R., Rios-Covian, D., Huillet, E., Auger, S., Khazaal, S., Bermúdez-Humarán, L. G., et al. (2023). Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol. Rev. 47 (4), fuad039. doi:10.1093/femsre/fuad039
Martyniak, A. P., Synbiotics, P., Skoczeń, S., and Tomasik, P. J. (2021). Prebiotics, Probiotics, Synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules 11 (12), 1903. doi:10.3390/biom11121903
Meier, J., and Sturm, A. (2011). Current treatment of ulcerative colitis. World J. Gastroenterol. 17 (27), 3204–3212. doi:10.3748/wjg.v17.i27.3204
Mohanty, D., Misra, S., Mohapatra, S., and Sahu, P. S. (2018). Prebiotics and synbiotics: recent concepts in nutrition. Food Biosci. 26, 152–160. doi:10.1016/j.fbio.2018.10.008
Monteagudo-Mera, A., Rastall, R. A., Gibson, G. R., Charalampopoulos, D., and Chatzifragkou, A. (2019). Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 103 (16), 6463–6472. doi:10.1007/s00253-019-09978-7
Nesci, A., Carnuccio, C., Ruggieri, V., D'Alessandro, A., Di Giorgio, A., Santoro, L., et al. (2023). Gut microbiota and cardiovascular disease: evidence on the metabolic and Inflammatory background of a complex relationship. Int. J. Mol. Sci. 24 (10), 9087. doi:10.3390/ijms24109087
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390 (10114), 2769–2778. doi:10.1016/S0140-6736(17)32448-0
Nie, K., Ma, K., Luo, W., Shen, Z., Yang, Z., Xiao, M., et al. (2021). Roseburia intestinalis: a beneficial gut organism from the discoveries in genus and species. Front. Cell Infect. Microbiol. 11, 757718. doi:10.3389/fcimb.2021.757718
Nieuwdorp, M., Gilijamse, P. W., Pai, N., and Kaplan, L. M. (2014). Role of the microbiome in energy regulation and metabolism. Gastroenterology 146 (6), 1525–1533. doi:10.1053/j.gastro.2014.02.008
Nyman, M., Nguyen, T. D., Wikman, O., Hjortswang, H., and Hallert, C. (2020). Oat bran increased fecal butyrate and prevented gastrointestinal symptoms in patients with quiescent ulcerative colitis-randomized controlled trial. Crohns Colitis 360 2 (1), otaa005. doi:10.1093/crocol/otaa005
Olas, B. (2020). Probiotics, prebiotics and Synbiotics-A promising strategy in prevention and treatment of cardiovascular diseases? Int. J. Mol. Sci. 21 (24), 9737. doi:10.3390/ijms21249737
Oniszczuk, A., Oniszczuk, T., Gancarz, M., and Szymańska, J. (2021). Role of gut microbiota, Probiotics and prebiotics in the cardiovascular diseases. Molecules 26 (4), 1172. doi:10.3390/molecules26041172
Ordás, I., Eckmann, L., Talamini, M., Baumgart, D. C., and Sandborn, W. J. (2012). Ulcerative colitis. Lancet 380 (9853), 1606–1619. doi:10.1016/s0140-6736(12)60150-0
Pagnini, C., Di Paolo, M. C., Urgesi, R., Pallotta, L., Fanello, G., Graziani, M. G., et al. (2023). Safety and potential role of Lactobacillus rhamnosus GG administration as monotherapy in ulcerative colitis patients with mild–moderate clinical activity. Microorganisms 11 (6), 1381. doi:10.3390/microorganisms11061381
Panwar, R. B., Sequeira, R. P., and Clarke, T. B. (2021). Microbiota-mediated protection against antibiotic-resistant pathogens. Genes & Immun. 22 (5), 255–267. doi:10.1038/s41435-021-00129-5
Park, S. K., Kang, S. B., Kim, S., Kim, T. O., Cha, J. M., Im, J. P., et al. (2022). Additive effect of probiotics (Mutaflor) on 5-aminosalicylic acid therapy in patients with ulcerative colitis. Korean J. Intern Med. 37 (5), 949–957. doi:10.3904/kjim.2021.458
Petrariu, O.-A., Barbu, I. C., Niculescu, A. G., Constantin, M., Grigore, G. A., Cristian, R. E., et al. (2024). Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front. Microbiol. 14, 1296447. doi:10.3389/fmicb.2023.1296447
Pietrzak, A., Banasiuk, M., Szczepanik, M., Borys-Iwanicka, A., Pytrus, T., Walkowiak, J., et al. (2022). Sodium butyrate effectiveness in children and adolescents with newly diagnosed Inflammatory bowel diseases-randomized placebo-controlled multicenter trial. Nutrients 14 (16), 3283. doi:10.3390/nu14163283
Portincasa, P., Bonfrate, L., Vacca, M., De Angelis, M., Farella, I., Lanza, E., et al. (2022). Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int. J. Mol. Sci. 23 (3), 1105. doi:10.3390/ijms23031105
Qiu, P., Ishimoto, T., Fu, L., Zhang, J., Zhang, Z., and Liu, Y. (2022). The gut microbiota in Inflammatory bowel disease. Front. Cell. Infect. Microbiol. 12, 733992. doi:10.3389/fcimb.2022.733992
Rayyan, Y. M., Agraib, L. M., Alkhatib, B., Yamani, M. I., Abu-Sneineh, A. T., and Tayyem, R. F. (2023). Does probiotic supplementation improve quality of life in mild-to-moderately active ulcerative colitis patients in Jordan? A secondary outcome of the randomized, double-blind, placebo-controlled study. Eur. J. Nutr. 62 (7), 3069–3077. doi:10.1007/s00394-023-03207-8
Rebersek, M. (2021). Gut microbiome and its role in colorectal cancer. BMC Cancer 21 (1), 1325. doi:10.1186/s12885-021-09054-2
Rizzatti, G., Lopetuso, L. R., Gibiino, G., Binda, C., and Gasbarrini, A. (2017). Proteobacteria: a common factor in human diseases. Biomed. Res. Int. 2017, 9351507. doi:10.1155/2017/9351507
Roda, G., Chien Ng, S., Kotze, P. G., Argollo, M., Panaccione, R., Spinelli, A., et al. (2020). Crohn’s disease. Nat. Rev. Dis. Prim. 6 (1), 22. doi:10.1038/s41572-020-0156-2
Rose, E. C., Odle, J., Blikslager, A. T., and Ziegler, A. L. (2021). Probiotics, prebiotics and epithelial tight junctions: a promising approach to modulate intestinal barrier function. Int. J. Mol. Sci. 22 (13), 6729. doi:10.3390/ijms22136729
Ross, F. C., Patangia, D., Grimaud, G., Lavelle, A., Dempsey, E. M., Ross, R. P., et al. (2024). The interplay between diet and the gut microbiome: implications for health and disease. Nat. Rev. Microbiol. 22 (11), 671–686. doi:10.1038/s41579-024-01068-4
Sartor, R. B. (2006). Mechanisms of Disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterology & Hepatology 3 (7), 390–407. doi:10.1038/ncpgasthep0528
Schreiber, F., Balas, I., Robinson, M. J., and Bakdash, G. (2024). Border control: the role of the microbiome in regulating epithelial barrier function. Cells 13 (6), 477. doi:10.3390/cells13060477
Sender, R., Fuchs, S., and Milo, R. (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14 (8), e1002533. doi:10.1371/journal.pbio.1002533
Shen, M., Shi, Y., Ge, Z., and Qian, J. (2024). Effects of mesalamine combined with live combined Bifidobacterium, Lactobacillus and Enterococcus capsules on intestinal mucosa barrier function and intestinal microbiota in mildly active crohn's disease patients. J. Invest Surg. 37 (1), 2297565. doi:10.1080/08941939.2023.2297565
Shi, N., Duan, X., and Niu, H. (2017). Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 4, 14. doi:10.1186/s40779-017-0122-9
Singh, N. K., Beckett, J. M., Kalpurath, K., Ishaq, M., Ahmad, T., and Eri, R. D. (2023). Synbiotics as supplemental therapy for the alleviation of chemotherapy-associated symptoms in patients with solid tumours. Nutrients 15 (7), 1759. doi:10.3390/nu15071759
Stojanov, S., and Berlec, A. (2024). Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease. Nanotechnol. Rev. 13 (1). doi:10.1515/ntrev-2024-0057
Stojanov, S., Ravnikar, M., Berlec, A., and Kreft, S. (2021). Interaction between silver fir (Abies alba) wood water extract and lactobacilli. Pharmazie 76 (12), 614–617. doi:10.1691/ph.2021.1794
Swanson, K. S., Gibson, G. R., Hutkins, R., Reimer, R. A., Reid, G., Verbeke, K., et al. (2020). The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterology & Hepatology 17 (11), 687–701. doi:10.1038/s41575-020-0344-2
Takahashi, J., and Rindfleisch, J. A. (2018). “Chapter 105 - prescribing probiotics,” in Integrative medicine. 4th Edition, Editor D. Rakel (Elsevier), 986–995.e4.
Tamizifar, B., Feizi, A., Rahim khorasani, M., Kassaian, N., Zamanimoghadam, A., Arbabnia, S., et al. (2023). The effects of probiotics in ulcerative colitis patients: a randomized controlled double blind clinical trial. Funct. foods health Dis. 13 (11), 605–615. doi:10.31989/ffhd.v13i11.1098
Tomita, T., Fukui, H., Okugawa, T., Nakanishi, T., Mieno, M., Nakai, K., et al. (2023). Effect of Bifidobacterium bifidum G9-1 on the intestinal environment and diarrhea-predominant irritable bowel syndrome (IBS-D)-like symptoms in patients with quiescent crohn's disease: a prospective pilot study. J. Clin. Med. 12 (10), 3368. doi:10.3390/jcm12103368
Torres, J., Mehandru, S., Colombel, J. F., and Peyrin-Biroulet, L. (2017). Crohn's disease. Lancet 389 (10080), 1741–1755. doi:10.1016/S0140-6736(16)31711-1
Valcheva, R., Koleva, P., Martínez, I., Walter, J., Gänzle, M. G., and Dieleman, L. A. (2019). Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes 10 (3), 334–357. doi:10.1080/19490976.2018.1526583
Valdes, A. M., Walter, J., Segal, E., and Spector, T. D. (2018). Role of the gut microbiota in nutrition and health. Bmj 361, k2179. doi:10.1136/bmj.k2179
Vinolo, M. A., Rodrigues, H. G., Nachbar, R. T., and Curi, R. (2011). Regulation of inflammation by short chain fatty acids. Nutrients 3 (10), 858–876. doi:10.3390/nu3100858
Walker, A. W., Duncan, S. H., McWilliam Leitch, E. C., Child, M. W., and Flint, H. J. (2005). pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 71 (7), 3692–3700. doi:10.1128/AEM.71.7.3692-3700.2005
Weiss, G. A., and Hennet, T. (2017). Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 74 (16), 2959–2977. doi:10.1007/s00018-017-2509-x
Whelan, K., and Quigley, E. M. M. (2013). Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr. Opin. Gastroenterology 29 (2), 184–189. doi:10.1097/MOG.0b013e32835d7bba
Wieërs, G., Belkhir, L., Enaud, R., Leclercq, S., Philippart de Foy, J. M., Dequenne, I., et al. (2020). How Probiotics affect the microbiota. Front. Cell. Infect. Microbiol. 9. doi:10.3389/fcimb.2019.00454
Wilson, B., Eyice, Ö., Koumoutsos, I., Lomer, M. C., Irving, P. M., Lindsay, J. O., et al. (2021). Prebiotic galactooligosaccharide supplementation in adults with ulcerative colitis: exploring the impact on peripheral blood gene expression, gut microbiota, and clinical symptoms. Nutrients 13 (10), 3598. doi:10.3390/nu13103598
Wongkrasant, P., Pongkorpsakol, P., Ariyadamrongkwan, J., Meesomboon, R., Satitsri, S., Pichyangkura, R., et al. (2020). A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed. Pharmacother. 129, 110415. doi:10.1016/j.biopha.2020.110415
Wu, G. D., Chen, J., Hoffmann, C., Bittinger, K., Chen, Y. Y., Keilbaugh, S. A., et al. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334 (6052), 105–108. doi:10.1126/science.1208344
Yadav, M. K., Kumari, I., Singh, B., Sharma, K. K., and Tiwari, S. K. (2022). Probiotics, prebiotics and synbiotics: safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 106 (2), 505–521. doi:10.1007/s00253-021-11646-8
Yamamura, R., Inoue, K.Y., Nishino, K., and Yamasaki, S. (2023). Intestinal and fecal pH in human health, Fron. Microbiomes.2.doi:10.3389/frmbi.2023.1192316
Yan, F., and Polk, D. B. (2002). Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 277 (52), 50959–50965. doi:10.1074/jbc.M207050200
Yu, L.C.-H. (2018). Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J. Biomed. Sci. 25 (1), 79. doi:10.1186/s12929-018-0483-8
Zhang, Y. Z., and Li, Y. Y. (2014). Inflammatory bowel disease: pathogenesis. World J. Gastroenterol. 20 (1), 91–99. doi:10.3748/wjg.v20.i1.91
Keywords: probiotics, prebiotics, synbiotics, IBD, Bifidobacterium, Lactobacillus, fructooligosaccharides
Citation: Yassine F, Najm A and Bilen M (2025) The role of probiotics, prebiotics, and synbiotics in the treatment of inflammatory bowel diseases: an overview of recent clinical trials. Front. Syst. Biol. 5:1561047. doi: 10.3389/fsysb.2025.1561047
Received: 15 January 2025; Accepted: 02 April 2025;
Published: 16 April 2025.
Edited by:
Carlo Vittorio Cannistraci, Tsinghua University, ChinaReviewed by:
Spase Stojanov, Institut Jožef Stefan (IJS), SloveniaHonghua Hu, Macquarie University, Australia
Manoj Kumar Yadav, Dankook University, Republic of Korea
Jing-Dong Han, Peking University, China
Copyright © 2025 Yassine, Najm and Bilen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melhem Bilen, bWIyMjhAYXViLmVkdS5sYg==
 Fayez Yassine
Fayez Yassine Adam Najm
Adam Najm Melhem Bilen
Melhem Bilen