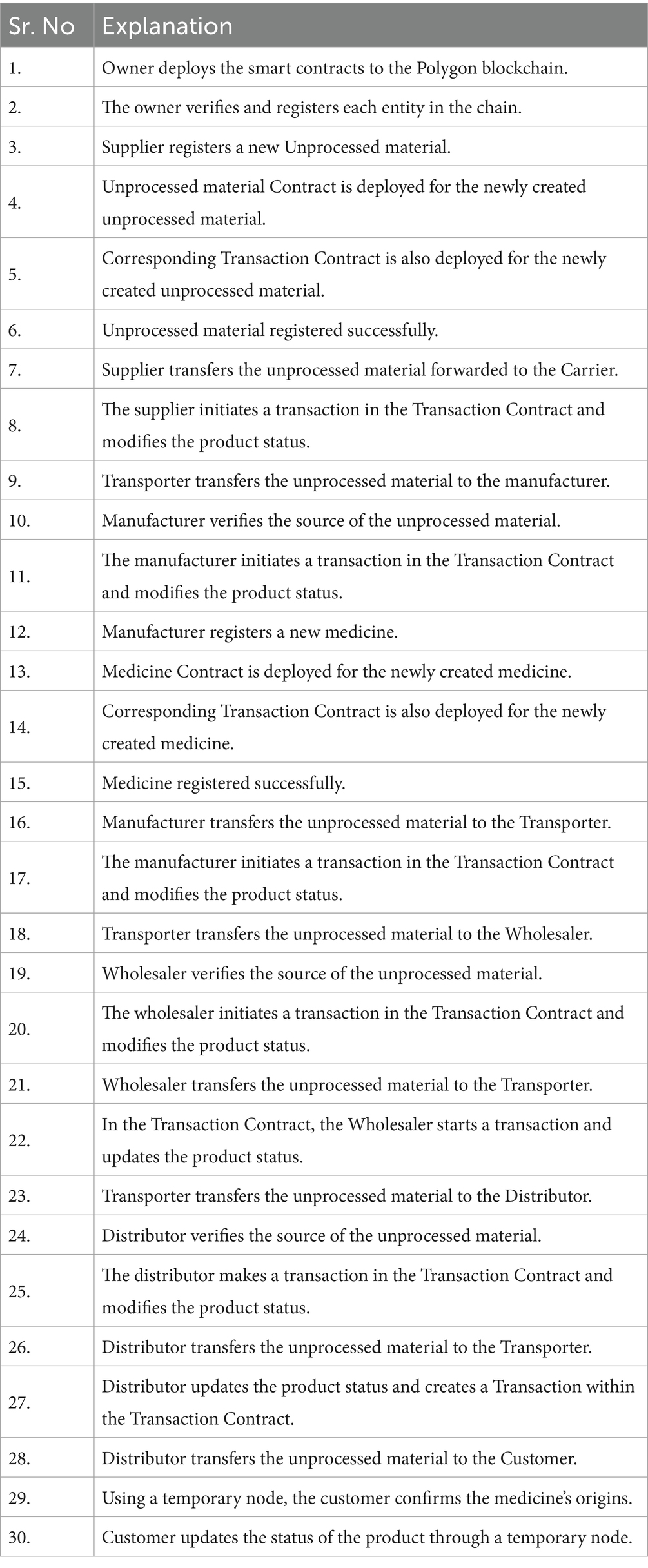
- 1CSE Department, Maharishi Markandeshwar (Deemed To Be University), Mullana-Ambala, Haryana, India
- 2Department of Management Information Systems, College of Business Administration, King Faisal University, Al-Ahsa, Saudi Arabia
- 3Chitkara University Institute of Engineering and Technology, Chitkara University, Rajpura, Punjab, India
- 4Department of CSE, Chandigarh University, Ludhiana, Punjab, India
Counterfeit drugs pose significant health risks due to their variable efficacy and potential harmful ingredients. To combat this issue, a reliable and secure track-and-trace system is essential for pharmaceutical supply chains. This paper proposes an Immutable and Decentralized Pharma (IDP) model, leveraging blockchain technology to ensure the safe and efficient distribution of medications. The IDP model utilizes smart contracts to record transactions between entities onto a blockchain, enabling end-to-end product tracking and provenance. Experimental results on a polygon blockchain test network demonstrate the feasibility and enhanced security of the IDP model in a collaborative environment. Our solution addresses the challenges of data privacy, openness, and authenticity inherent in centralized track-and-trace systems, providing a promising approach to eliminate counterfeits and guarantee product safety in pharmaceutical supply chains.
1 Introduction
The network of the pharmaceutical supply chain (Bocek et al., 2017) is the system via which patients receive produced prescription drugs. However, this supply chain is quite complex and consist of multiple stockholders at different geographical location. Some of the stockholders are suppliers, manufacturers, transporters, wholesalers, distributors, retailers, etc (Ouchetto et al., 2018). This makes it difficult to track every prescription medication along the supply chain and identify where it came from. Drug fraud is a major issue around the world. As stated by the Health Research Funding agency (Goldacre et al., 2016) between 9 and 30 percent of the pharmaceuticals fake counterfeit goods in developing countries goods are a serious issue since they may have a variety of negative consequences on human health. The World Health Organization (WHO) estimates that roughly 32% of all pharmaceuticals sold in Latin America, Asia, and Africa are wrong. Contrary to popular belief, the biggest issue with illegal substances is that they differ from authentic medications in terms of their negative effects on human health.
The numbers show that every year, United States pharmaceutical companies lose money of revenue of almost $250 billion as a result of these fake medications. These medications may not aid in the patients’ recovery from the illness yet do possess a variety of additional harmful side effects. In underdeveloped nations, consumers utilize low-quality, counterfeit drugs every 10th time, according to a survey report from the World Health Organization (WHO). Therefore, a system that can trace and monitor drug delivery at each stage is required to address the counterfeiting issue (WHO, 2020).
The supply chain process may be handled and tracked on the blockchain extremely well. Because of the current system’s lack of transparency, it is quite challenging for clients or purchasers to comprehend the worth of the products. When there is a suspicion of unlawful or unethical acts, it is also quite challenging to look into the tampering inside the supply chain (Tripathi and Kumar, 2019). As a result, such supply chains are very inefficient as vendors, suppliers, etc. attempt to link the various entities and determine who, when, and how needs what. Customers and purchasers can no longer determine the true value of items because of the system’s significant lack of clarity. When evidence of unethical or immoral behavior exists, it is exceedingly difficult to investigate chain tampering. Retailers and producers may be dishonest when they attempt to ascertain who, when, why, and how wants what. A revolutionary technology is blockchain (Argiyantari et al., 2020). Blockchain offers a distributed ledger with decentralized management of the system. Private information, such as customer or drug information cannot be changed because every blockchain transaction is irreversible. Manufacturers, intermediaries like distributors and suppliers, and end-users like customers, retailers, and hospitals are just a few of the many key Supply Chain participants. The complete transparency of blockchains promotes trust. Each product in the chain may be moved across the various authenticated chain entities using event request-response architecture. Smart contracts are used on the blockchain to record every transaction between all of the entities (Monrat et al., 2019). The proposed Immutable and Decentralized Pharma (IDP) model is designed to integrate with existing pharmaceutical supply chain systems by utilizing blockchain technology and smart contracts. The IDP model can be integrated with existing systems through the use of APIs and adapters, which allow for communication between the blockchain network and existing systems.
1.1 Motivation and contribution
An essential part of the healthcare environment is the pharmaceutical supply chain. Traditional supply chain is facing various issues like tracking counterfeited drugs, monitoring orders, receipts, invoicing, payments, data integrity, immutability, Drug traceability, identification of fake drug seller, data privacy, and so on. These issues need to be tackled carefully because it is directly connected with the life of human beings. Emerging technologies can help to overcome mentioned challenges. Blockchain technology is used in the pharmaceutical supply chain to create an ecosystem that can address the above-mentioned issues. The following are the article’s main research questions?
• How to ensure data integrity and immutability in the pharmaceutical supply chain??
• What measures are implemented to ensure data privacy and security in blockchain-based pharmaceutical supply chains? How can sensitive information be protected while maintaining transparency and accountability?
• How the costs effectiveness achieved with implementing blockchain technology in the pharmaceutical supply chain, and what are the potential benefits?
The remaining portion of the paper has been split into the following sections. The literature study is presented in Section II, and the Conventional model and its limitations are covered in Section III. The suggested model is explained in depth in Section IV. The suggested model is implemented and the outcomes are reviewed in Section V. In Section VI results are compared with the existing one. Section VII is where the paper is conclude & finished.
2 Literature review
Over the years, a wide range of academics and businesses have engaged in substantial study and discussions about utilizing blockchain technology to improve and manage the supply chain’s current state.
According to a recent report by Evaluate Pharma, the global pharmaceutical industry is projected to grow at a CAGR of 6.3% through 2022, reaching a market value of $1.2 trillion. Counterfeit products pose a significant challenge in this sector, with potentially harmful consequences. Unlike other industries, counterfeit drugs not only impact the economy but also endanger public health by providing ineffective or dangerous treatments. These products range from ineffective to dangerously adulterated versions of branded, generic, or over-the-counter drugs.
KyungSup et al. (2015) studied a few recent blockchain-based applications for gathering, sharing, and preserving data. Data independence and diverse semantic models for the same issue of information delivery were two major issues with current blockchain architectures for supply chain and accounting transactions that are examined in this article. Database, application, and presentation levels are frequently combined into one ledger in applications leveraging blockchain technology.
Kumari (2020) the authors address issues with traditional data handling methods and drug traceability. They propose a blockchain-based system capable of tracing drugs in the supply chain to reduce counterfeiting. The process involves manufacturing drugs, assigning a unique hash code, and storing manufacturing details on the blockchain, followed by distribution.
Bodkhe et al. (2020) pay attention to for the diagnosis and treatment of patients, pharmaceutical drugs are essential. However, in the decade preceding, an issue of drug misuse and fraud in the supply chain of pharmaceuticals has been brought to light more and more frequently. To solve these issues, the present pharmaceutical supply chain needs to be modified by integrating technology for monitoring from the chemical source to the customer. In the present study, we look at the modern blockchain and IoT-based pharmaceutical governance.
Ahmadi et al. (2022) this paper suggest using blockchain to enhance the pharmaceutical supply chain’s traceability, security, and visibility in addition to preventing drug fraud. A permission blockchain is created specifically for this purpose, allowing only vetted parties to join the network and push transactions to the blockchain.
Raj et al. (2019) in this work, we provide a new methodology, utilizing Polygon blockchain technology to enhance the traceability of healthcare supply chain goods. Our method leverages smart contracts and decentralized off-chain storage to create a secure and immutable transaction history, eliminating the need for intermediaries. We provide an in-depth explanation of the intricate system architecture and algorithms that support our proposed strategy. To evaluate the system’s performance in enhancing pharmaceutical supply chains’ traceability, we conducted testing and evaluation, including cost and safety assessments.
Musamih et al. (2021) focus on incorporating Blockchain technology into the selection of products for deletion, which means eliminating a product and its related data from its inventory. Four fundamental processes make up the process of product deletion: identification, analysis and renewal, evaluation and decision-making, and implementation. Blockchain technology, which also boosts information efficiency, enables the numerous entities that make up the supply chain to interact and collaborate more successfully.
Pettit et al. (2019) the blockchain concept can be used in the pharmaceutical supply chain to spot fake medicines while yet maintaining sufficient oversight over the supply and demand for medicines. Pharmaceutical firms will be able to regulate phoney and unregistered medications thanks to this. Another key way through which blockchain innovation could assist in achieving enhancement of the execution of the supply chain is the capability to employ clever contracts to automate forms and reduce expenses. The blockchain acceptance model of the pharmaceutical supply chain network provided in this study may be useful for integrating technology with related processes in supply chains.
Badhotiya et al. (2021) the problem of the illegal drug trade has claimed lives all around the world. The growth of unlawful sales persists despite the efforts of several international entities to halt this problem. The essay that follows suggests using Blockchain technology to address the issues with traceability and lack of regulation in the drug trade, as well as how various parties would engage in it.
Jangirala et al. (2020) emphasizes the need of adopting Blockchain technology to track and spot fake items in the supply chain. The risk of counterfeit goods decreased due to the ease with which the blockchain’s data might be utilized to trace a medicinal product as it passes from one entity to another. The primary problems are addressed by blockchain technology since it permits companies to track their goods across the supply chain and time-stamps every new transaction. By pinpointing the precise location of the medicine, stakeholders are given the ability to take action in the event of any problems.
Jamil et al. (2019) to concentrate operations management (OM) research on the development of supply chain tracking systems by defining the essential operational concerns. Before arguing that two utterly unrelated industries–cobalt miners and pharmaceuticals–need to build traceability processes. We initially write out the business needs as well as critical implementation success standards.
Hastig and Sodhi (2020) the blockchain technology is thoroughly discussed in this paper, along with how it might be applied to create a flawless method for eradicating fake goods in the pharmaceutical sector. One of the biggest problems facing the pharmaceutical sectors, according to studies and publications, is the entry of counterfeit goods into the primary supply.
Azaria et al. (2016) looked into the use of blockchain technology for management of digital assets for collaborative environment. It was found that integrating the blockchain technology with conventional models can provides multiple benefits such as privacy, integrity, authentication etc.
Soundarya et al. (2018) in order to investigate how blockchain adoption may affect the pharmaceutical supply chain’s long-term sustainability and efficacy, this study develops a theoretical model. The researchers demonstrated how blockchain technology may be used to tackle and enhance supply chain sustainability but highlighting issues with modern pharmaceutical supply chain management. Blockchain-based technologies is still in its infancy, but experts have already seen advancements in its application to everyday life, notably in the banking and pharmaceutical industries.
Rana and Rana (2020) pharmaceutical goods may be tracked wherever they are at all-time using Internet of Things (IoT) technologies including RFID, locator sensors, and QR codes. These technologies also help to validate the accuracy of data sources. By utilizing distributed ledger technology from blockchain, the system has also enabled data sharing and storing possible at every stage of the supply chain. This guarantees the data’s traceability, transparency, and integrity.
Liang et al. (2017) investigated the use of distributed database technology in the ecosystem of the digital food supply chain. It helped in recording all the transactions occurred between different stakeholders of the supply chain system. This model was implemented and tested using Ethereum blockchain framework.
Lingayat et al. (2021) outlines the typical process for creating, validating, and structuring a strategy to incorporate blockchain technology into business strategy. In addition, they highlight how blockchain technology may help with Internet of Things security concerns include monitoring data, authenticating users, recognizing different devices and upholding trust, regulating access, and ensuring accountability in IoT-based applications.
Alharthi et al. (2020) a design for a safe blockchain-based infrastructure for legitimate parties in medical supply chains has been described. The manufacturer’s authenticity and drug security can be secured by this framework. To protect from man-in-the-middle and replay attacks, it utilizes the use of PKI and digital signatures.
Feng et al. (2019) studied about the evolution of industrial revolution 5.0 and role of emerging technology in this evolution. They also explored the role of distributed ledger technology in the evolution of industry 5.0 generation. Various advantages of this integration was also discussed.
Rana et al. (2021) focus on integrating Blockchain technology into the process of eliminating a product from the company’s portfolio, which involves erasing the product and any associated data. The four main phases in the product deletion process have been determined to include acknowledgment, research and renewal, review and making choices, and execution. Blockchain technology helps the several players in the supply chain collaborate and communicate more successfully while also increasing information efficiency and effectiveness and reducing informational disputes.
Shi et al. (2019) worked on the use of blockchain technology and artificial intelligence to enhance the quality of healthcare ecosystem. Healthcare ecosystem has multiple stakeholders like doctor, patient, hospital, insurance company etc. all the communication between different stakeholders was secured using distributed ledger technology. It provides various benefits that improved the overall ecosystem.
Kumar et al. (2020) established anti-counterfeit techniques that work even when an RFID tag’s data is copied later in the supply chain. In the article, they therefore suggested the concept of product possession If any entity is unable to demonstrate that it has the real thing, counterfeit items can be identified. Because Bitcoin enables users to verify their ownership without the need for any determining or centralized authority, they chose to adopt blockchain.
Khanna et al. (2020) explain the significance of Drugledger, a scenario-driven blockchain system designed for the oversight and control of pharmaceuticals. Drugledger manages the entire process, encompassing pharmaceutical packaging, repackaging, unpackaging, and the cancelation of drug transactions. It also handles the entry and exit points in the drug supply chain, employing a UTXO-based transaction model alongside the supply chain. This system effectively safeguards data privacy and ensures the authenticity of drug monitoring services, effectively differentiating between data breaches and legitimate tracking of pharmaceuticals.
Rana and Rana (2021) investigated the possibilities of integration of distributed ledger technology with the Internet of Medical Things. IoMT includes the various medical devices that collect the data and forward the data to the blockchain. It contributed to enhancing the security and privacy of the data obtained from medical devices.
Dutta et al. (2020) a double-chain public Blockchain has been created to boost the efficiency of food supply chain networks. They provided instances of how their approach equips public service platforms with flexible mechanisms for rent-seeking and matching. It ensures the security and transparency of transaction information in addition to the confidentiality of firm information. The scale of the underlying distributed ledger and related performance challenges are the main constraints.
Rana et al. (2022) they have created a credit rating system for the food supply chain, leveraging blockchain technology for enhanced management and monitoring capabilities. The system utilizes blockchain smart contracts for collecting trader credit assessment narratives, and it employs an approach to deep learning called Long Short-Term Memory (LSTM) to analyze this textual data. However, a notable shortcoming of this system is its failure to comprehensively consider both the advantages and disadvantages of its strategy, despite showcasing its effectiveness.
Kukreja et al. (2021) have described four use cases of blockchain in logistics, and SCM explored both theoretically and practically. Identifying fake goods, streamlining the origin documentation process, handling straightforward paperwork for marine freight, and managing the Internet of Things are some of the use scenarios.
3 Conventional pharmaceutical supply chain management
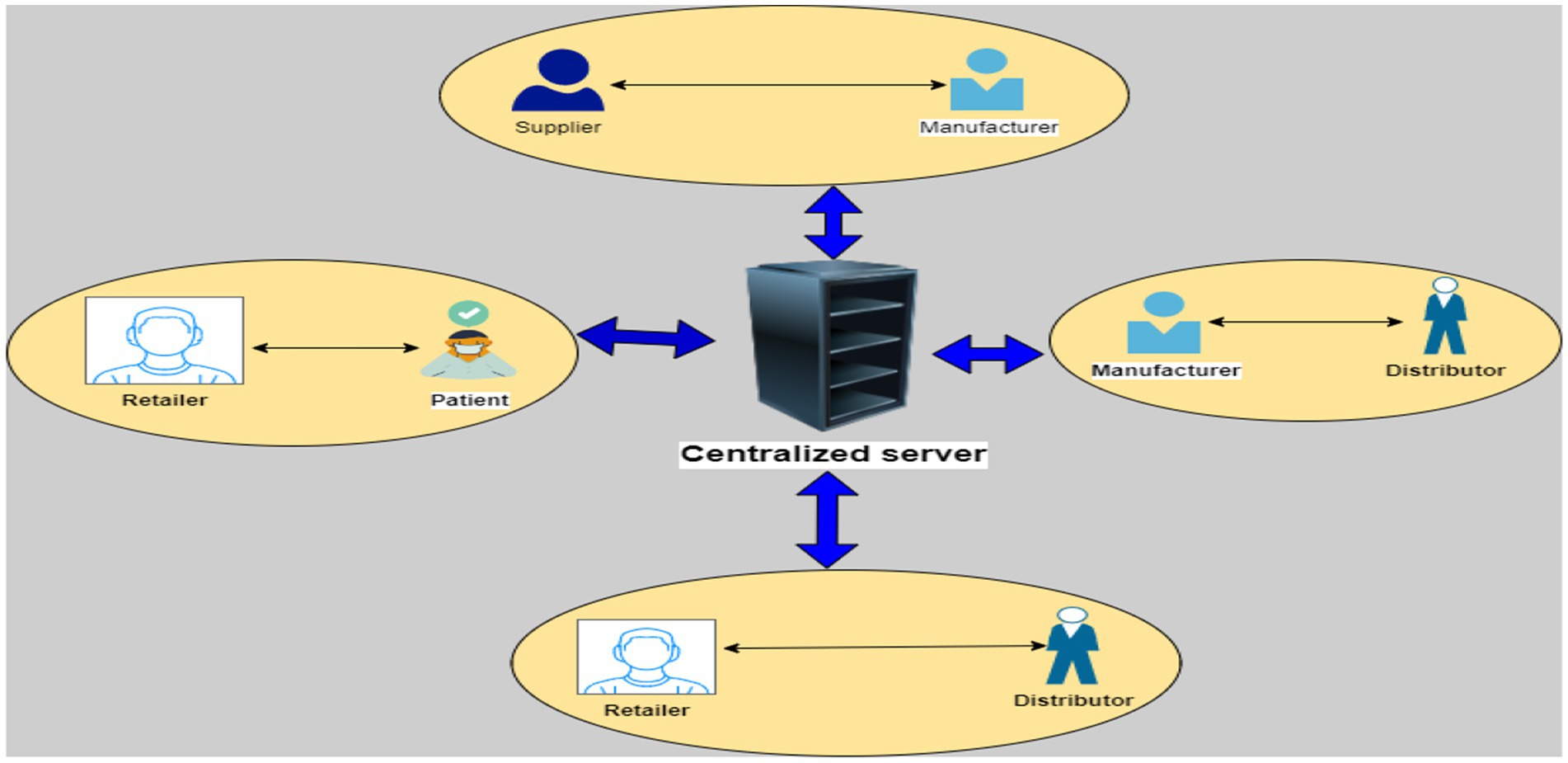
Since product distribution is subject to a wide range of intricate rules and criteria, the pharmaceutical supply chain (PSC) operates substantially distinct from other networks of supply. To ensure on-time and perfect delivery of the goods, the regulations must be meticulously complied too. In addition, the numerous supply chain participants must maintain adequate levels of trust and transparency. Suppliers of raw materials, producers, warehouse owners, sellers, pharmacists, and customers or customers make up nearly all of the PSC network’s stakeholders (Rahman et al., 2021). The Conventional pharmaceutical supply chain management (CPSM) system’s general operating procedures are as shown in Figure 1.
• To create a product, the large pharmaceutical producers gather their raw ingredients from several sources in the first phase. The correct synthesis of medicines requires a lot of effort and research.
• The manufacturer transfers the finished product to the warehouse in the second step after it has been developed. When employing a single manufacturing unit, a corporation only needs one warehouse, however when using numerous manufacturing units, the product is stored in various warehouses (either central or regional warehouses), depending on the geographic conditions.
• In the third phase, the merchants receive the goods and distribute from it to the subsequent chain participants, such as pharmacists or other equivalent organizations like hospitals, clinics, and health care facilities. Typically, they place product orders based on needs and demand.
Pharmacists sell the drugs directly to customers or end users in the last phase.
3.1 Limitations with conventional supply chain management for pharmaceuticals
The CPSM system that is now in place has several shortcomings. Below is a list of a few of them.
• Lack of Transparency: The biggest problem in the sector is the lack of visibility in CPSM. Millions of dollars have been invested to find solutions to issues like the need for a little more transparent supply chain, for each drug to be identified specifically, and for the issue of fake medications. However, there has not been much success in this field (Singla and Rana, 2023).
• Traceability of Products: Each stakeholder as in the conventional CPSM system is in charge of a database that contains data concerning a particular product (Rana et al., 2022). Therefore, it is very difficult to predict when a product will be in consumer demand and manually tracking each product adds time and delay. By utilizing smart contracts and a CPSM system that is based on the blockchain, this issue may be resolved quickly.
• Lack of Trust: Before the final customer receives the goods, the CPSM system involves a lot of participants. In an SCM this large and intricate, it might be difficult to keep players’ trust (Zhu et al., 2022). As a result, it may also have an impact on how smoothly the supply chain operates.
• Delivery of Expiring Items: Customers anticipate items that are not out-of-date, but occasionally the supply chain procedure takes a long time and results in the refusal of the medications at the very end, creating the entire process pointless (D’souza et al., 2021).
• Temperature-Controlled Shipping: Most supply chain companies lack the necessary tools to transport pharmaceuticals that must be transported at specific temperatures (Kumar et al., 2020). And the current pharmaceutical sector is dealing with an increase in this type of medication, which results in significant losses of both money and drug items.
• Products that are fake: Because of the supply chain system’s insufficient openness and antiquated methods of exchanging information, fake goods are supplied to customers, which have an impact on the economy and people’s quality of life (Huang et al., 2018). Research from the Economic Cooperation and Development Organization estimates that the SCM process wastes half a billion dollars annually from the global economy.
• Compliance with Regulations and Documentation: In the present supply chain management system, complex payment agreements, letters of credit, and ownership changes are tracked via paper-based trails (Alamri et al., 2021).
As a result, conventional supply chain contracts are intricate and out of date. Blockchain technology can help to overcome the above-mentioned issues. The supply chain system built on the blockchain uses smart contracts to offer automation for the aforementioned problems (Mahboob et al., 2021). IDP framework supported by blockchain technology should therefore be considered and covered in the next part.
4 Proposed model
4.1 System model
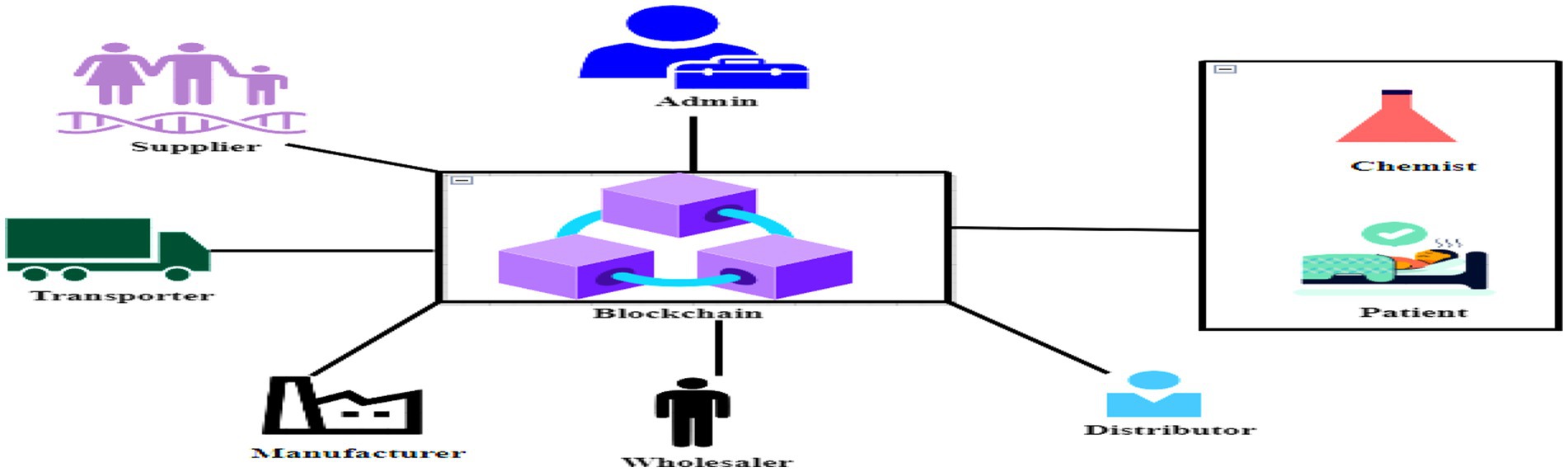
This section explains the IDP model for product traceability, a system made possible by the decentralization and data immutability of blockchain technology. Every node participating in the process might exhibit elements of supply and demand within this framework. An event response system is incorporated throughout the whole process of creating a product transaction in in order to guarantee everyone’s agreement transaction participants about the receipt and distribution of medicines. The blockchain’s transaction data will be permanently preserved via smart contracts.
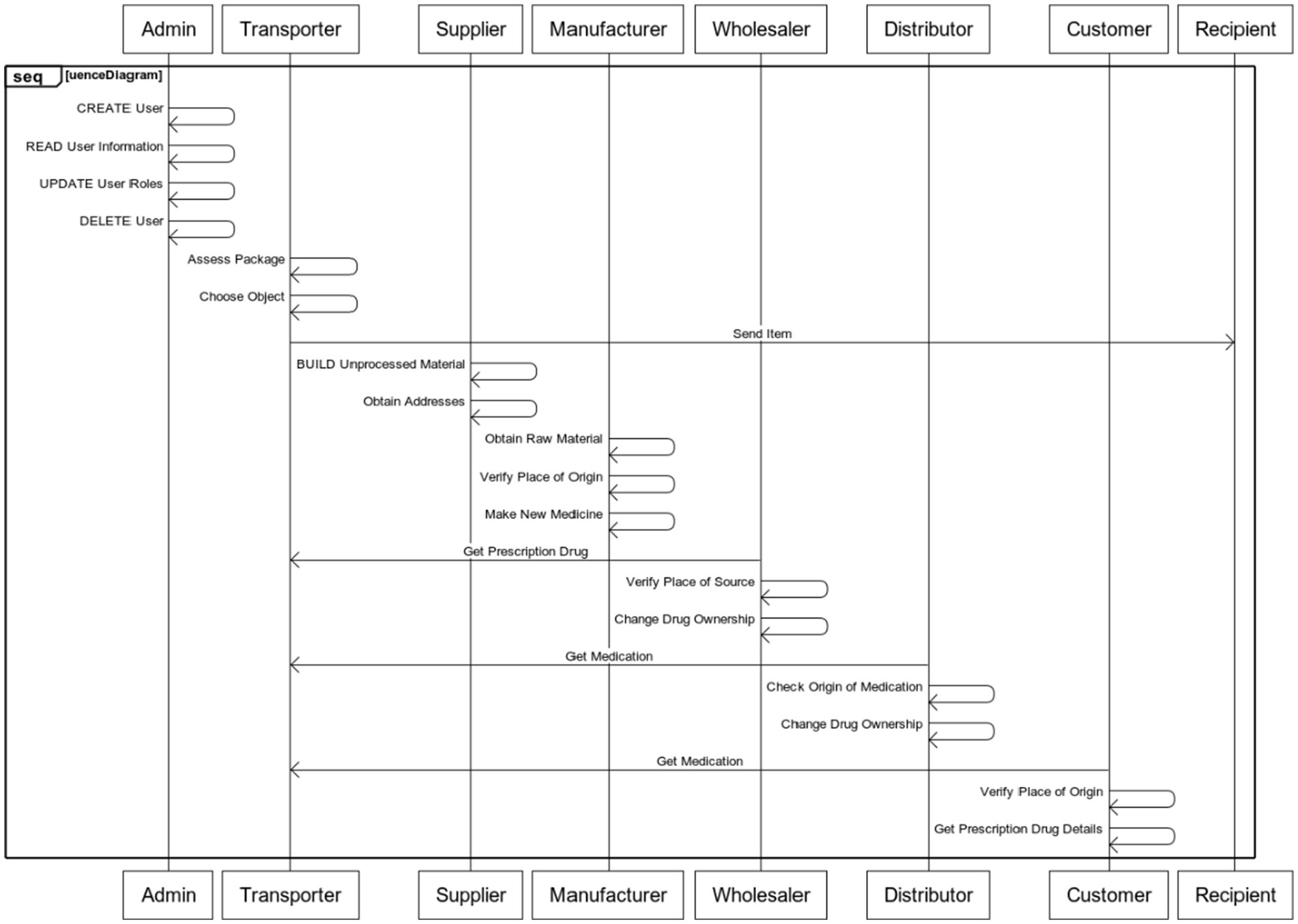
As shown in Figure 2, the recommended system is made up of many different organizations, including admin, vendors, transporters, producers, distributors, wholesalers, and customers/retailers. The system is connected by a decentralized network. The aforementioned participants in the supply chain are all nodes on the open blockchain. These nodes all have a distinct Polygon account that may be used to identify it. The explanation of each of their unique roles and duties by using a sequence diagram as shown in Figure 3:
1. Admin
• To add a new user to the chain, CREATE them.
• READ any user’s information.
• UPDATE a user’s roles.
• A user in the chain is DELETED.
2. Transporter
• Assess the package (Unprocessed material or Medicine).
• Choose the object from a being (based on transporter type).
• Send the item to the recipient.
3. Supplier
• BUILD an unprocessed material.
• Obtain the addresses for the newly generated unprocessed material
4. Manufacturer
• Obtain the raw material from the source via the medium of transport.
• Verify the product’s place of origin.
• Make a new medicine with the unprocessed materials received.
5. Wholesaler
• Using the transporter, get the prescription drug from the supplier.
• Verify the medication’s place of source.
• The drug’s ownership is changed.
6. Distributor
• Utilize the transporter to take the medication from the Wholesaler.
• Check the origin of the medication.
• The drug’s ownership is changed.
7. Customer
• Utilize the transporter to take the medication from the distributor.
• Verify the medication’s place of origin.
• Gets details on prescription drugs
The proposed IDP model delineates specific responsibilities and tasks for each participant within the pharmaceutical supply chain. Administrators oversee user management, including creating, accessing, updating, and removing user profiles. Transporters are tasked with evaluating and delivering packages, while suppliers are responsible for generating unprocessed materials and providing associated addresses. Manufacturers receive raw materials, confirm their sources, and produce medicines. Wholesalers and distributors facilitate drug movement and adjust ownership accordingly. Customers receive medications, verify their origins, and access prescription details. Each role executes distinct functions vital to supply chain operations, ensuring transparency and accountability. Leveraging blockchain technology, transactional data is securely recorded, guaranteeing an immutable and transparent log of actions performed by all participants. This system enhances traceability, mitigates counterfeit risks, and fosters overall efficiency and integrity within the supply chain.
4.1.1 Blockchain
Blockchain is a distributed and decentralized digital ledger technology for recording and authenticating transactions throughout a computer network. It operates on the principle of transparency and immutability, ensuring After the content gets added to a “block,” It is not possible to remove or change it. Each new block contains a set of transactions and is linked to the previous one, forming a chain (Sakshi and Ahuja, 2021). This design provides security and trust in various applications, including cryptocurrencies like Bitcoin, supply chain management, and smart contracts. Various blockchain frameworks can be used as shown in Table 1.
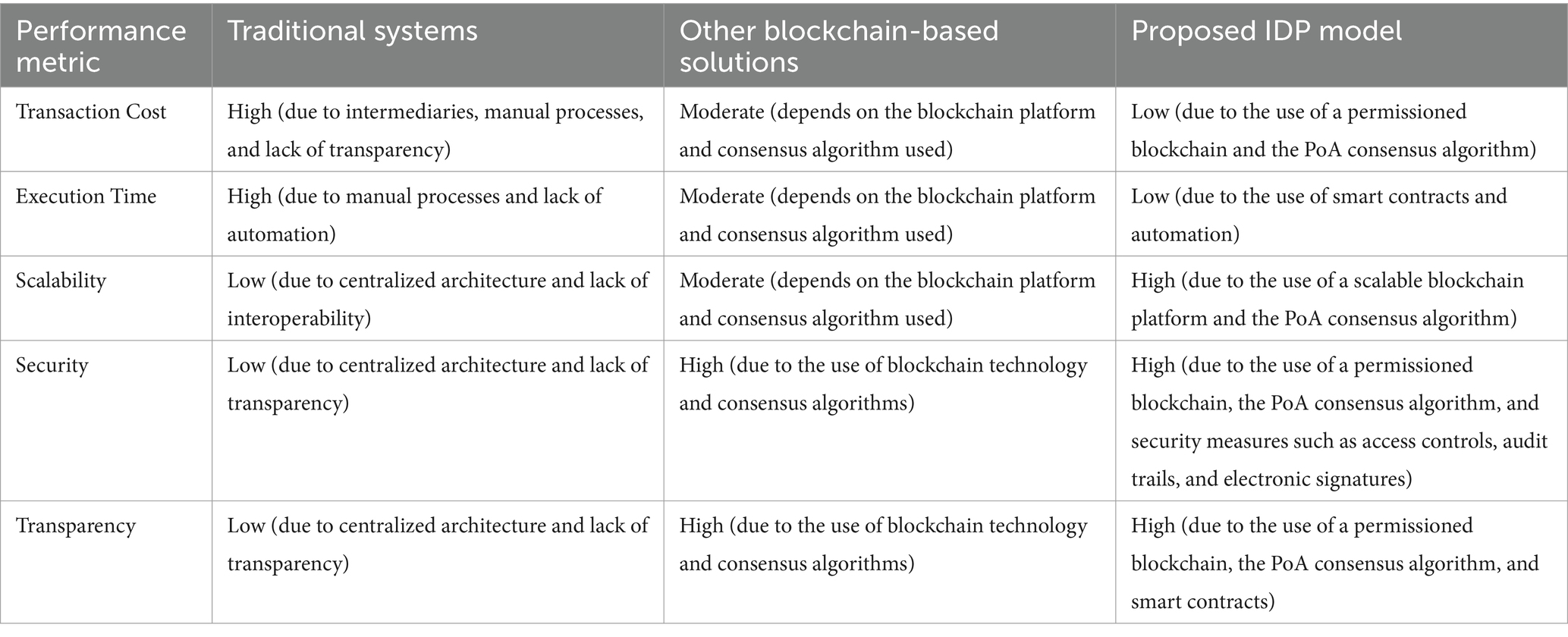
Table 1. Comparison of the proposed model’s performance metrics with traditional systems and other blockchain-based solutions.
4.1.2 Polygon
Bitcoin has a low degree of universality because it is not Turing-complete and was created only for scenarios involving virtual currencies. This led to the invention of more blockchain-based innovations, and as a result, a variety of software types will now be replicated on the blockchain through smart contracts (Rana et al., 2022). In Polygon, the complete integration of blockchain and smart contracts was first noted. A blockchain-based smart contract will adhere to preset rules and cannot be altered by anyone. The Polygon Virtual Machine uses the EVM code, a stack-based low-level byte code language, to carry out the smart contract in Polygon.
4.1.3 Smart contracts
Self-executing code, or smart contracts, is programs that operate on blockchain technology, facilitating, executing, and enforcing agreements between parties that are not reliable independently, without the need for a neutral third party (Miyachi and Mackey, 2021). Smart contracts automate networks and make it possible to convert paper contracts into digital ones. Smart contracts, as opposed to conventional contracts, allowed for automatic transactions without requiring for central oversight, as well as allowing individuals to officially document their agreements and trust-based connections. To avoid contract manipulation, duplicates of smart contracts are sent to all nodes in the blockchain network. By allowing processes to be carried out by machines and utilizing the features provided by blockchain platforms, human error might be avoided in order to prevent disputes with regard to such contracts. A smart contract is a piece of software that runs on the blockchain system and uses the method of consensus to carry out a number of tasks (Hackius and Petersen, 2020). A smart contract can be utilized in many different industries to automate the system and eliminate the use of third-party transactions. In this paper, we present 7 different use cases for blockchain- and smart contract-based systems. Smart contracts can be developed and utilized on different blockchain systems, including NXT, Polygon, and Hyper Ledger Fabric.
4.1.4 Solidity
High-level Turing-complete programming language Solidity has a syntax like JavaScript, is statically typed, supports polymorphism and inheritance, as well as libraries and sophisticated user-defined types (Singla and Rana, 2022). Contracts developed using Solidity has a structure akin to classes in object-oriented programming languages. Like traditional imperative programming, contract code consists of variables and functions that read and alter these. In our model solidity constructs and libraries used are:
4.1.4.1 Solidity constructs
• Contract: The Admin contract is used to manage user roles and permissions in the drug supply chain.
• Enum: The roles enum is used to define different roles in the supply chain, such as supplier, transporter, manufacturer, distributor, retailer, and revoke.
• Struct: The User Info struct is used to store user information, including name, location, Ethereum address, and role.
• Mapping: The Users Details mapping is used to store user information and retrieve it by Ethereum address.
• Modifiers: The only Owner modifier is used to restrict access to certain functions to only the contract owner.
The libraries such as OpenZeppelin’s SafeMath and Chainlink’s VRF are used in the implementation of the smart contract.
4.2 Smart contract design
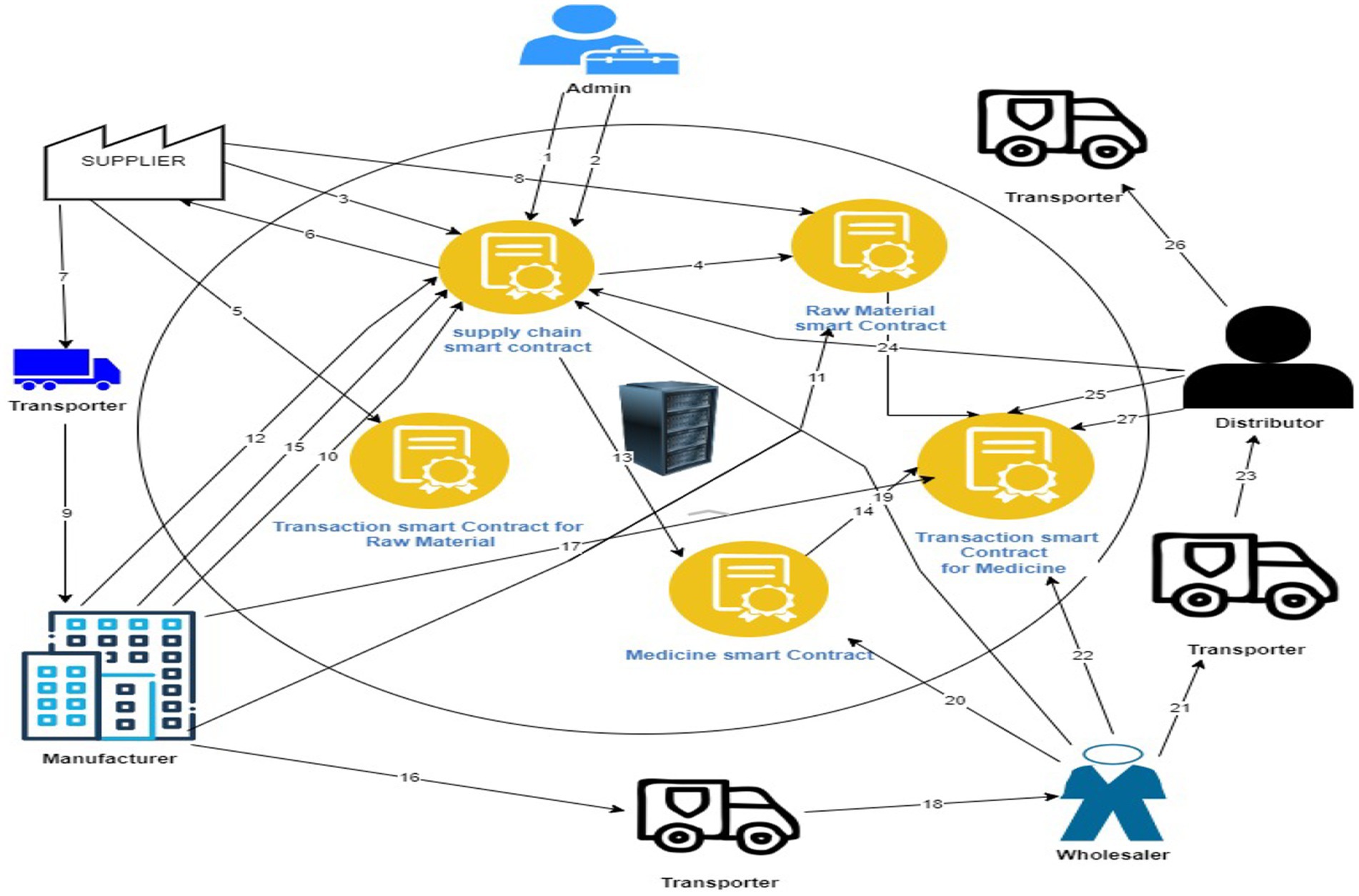
Supply Chain Contract: The chain’s Owner is the one who deploys this contract. It is made up of a variety of supply chain participants, including the owner, supplier, transporter, manufacturer, wholesaler, distributor, and customer. To have real-time communication with the user interface, it also includes a large number of Solidity events. It is only accessible to the roles that the contract has designated for each function. This is performed using Solidity’s “modifiers. No entity therefore has access to a particular intent without performing a specific role. Thus, data kept on or obtained from the blockchain becomes more trustworthy and valuable.
Unprocessed material Contract: The pertinent Provider starts the Unprocessed Material Contract, and the supplier in charge of producing the unprocessed material includes it in the chain of supply after that. Relevant details must be submitted by the Supplier, like the Supplier’s EA (Polygon Address), DateTime, Transporter’s EA, Transaction Contract Address, and more, to produce a raw material that will be used in the chain. Additionally, this system has actions that quickly pinpoint the location of the delivery. The event request-response mechanism is used to update the recipient’s (the manufacturer’s) EA. It also maintains records of the drugs’ present state, including who is in charge of the raw materials at the moment.
Medicine Agreement: The right manufacturer implements the Medicine Contract. Once a drug is physically created, the producer adds it to the supply chain. When the drug is being made, the producer is asked to provide details such as EA (Polygon Address) of the substances that will be used to make it, DateTime, the transporter’s EA, Transaction Contract Address, etc. to be place to the chain. Events within it can also rapidly pinpoint the location of the item. The EAs for Wholesaler, Distributor, and Customer are changed later using the event request-response approach. It also records the current location of the medication or the owner of the package.
Transaction Agreement: When they are created, the unprocessed data and smart contracts for drugs automatically trigger the Transaction Contract. The contract takes into account data including DateTime, SenderEA, ReceiverEA, Location, TransactionHash, and the Previous Transaction Hash. The transaction hash is 32 bytes long. The preceding transaction hash, which is maintained for entities to utilize to verify the source of goods in the chain, is a representation of the transaction information in the smart Transactionn contract.
In the orchestrated dance of a pharmaceutical supply chain, various roles play integral parts as shown in Figure 4. The Admin serves as the architect, creating and managing user profiles while overseeing the interconnected web of actions. Transporters assess packages, select items based on type, and ensure safe delivery to recipients. Suppliers contribute by crafting unprocessed materials and obtaining destination addresses. Manufacturers receive raw materials via transporters, verify product origins, and transform materials into medicines. Wholesalers utilize transporters to acquire prescription drugs from suppliers, verifying their origins and facilitating ownership transfers. Distributors extend the distribution, taking medications from wholesalers, confirming origins, and managing ownership transitions. Finally, the Customer, the ultimate endpoint, employs transporters to receive medications from distributors, verifies origins, and gains details on prescribed drugs. This seamless collaboration demonstrates the synergy between roles, from the creation of materials to the delivery of medicines, illustrating the intricate steps involved in ensuring the safe and efficient flow of pharmaceuticals through the supply chain. The Admin’s role in user management harmonizes with the specialized functions of each entity, collectively forming a comprehensive and interconnected system that navigates the complexities of pharmaceutical production and distribution. The process flow of IDP model is explained in Table 2.
The various Algorithms are used to run the IDP Model. Some of the algorithm is listed below:
ALGORITHM 1: Add new users
Creator Admin deployed the contract.
If ((msg.sender = Admincreator))
Then “new user U can be added withparticular role”
Enter U name, U location, U role and hexadecimal address U addr for new user
If (U name, U location, U role and U addr values are of desired type)
Then “user U is registered with entered role”
Else “error returned”
Else “Operation refused and new users cannot be added.”
In Algorithm 1 for adding new users, the Admin Creator deploys a smart contract and verifies their identity. If authenticated, the algorithm prompts input for a new user’s name, location, role, and hexadecimal address. Verification checks ensure the entered data is of the desired type. Upon successful validation, the new user is registered with the specified role, while any type mismatch triggers an error message. If the deploying entity is not the Admin Creator, the operation is refused, emphasizing the exclusive authority of the Admin Creator to initiate contract deployment and user additions. This algorithm establishes a secure and controlled process for managing user registrations within the smart contract.
ALGORITHM 2: Creation of medicine by manufacturer
If (msg.sender =U manufacturer)
Then “CreateMedicine function can be executed”
Enter “enter Address of M manufacturer,M desc,M rawAddr, M quantity, M transporterAddr,M receiverAddr”
If (M manufacturer,M desc,M rawAddr, M quantity, M transporterAddr,M receiverAddr values are of desired type)
Then “Medicine M is registered and details are stored”
Else “error returned”
Else “Function cannot be executed and operation declined”
In Algorithm 2, it allows the execution of the “CreateMedicine” function if the sender is identified as the Manufacturer. Upon triggering, the algorithm prompts the user for details like manufacturer address, medicine description, and more. It validates the input, registers the medicine if the values are of the desired type, and stores the details. In case of input errors, it triggers an error message. If the sender is not the Manufacturer, the algorithm rejects the operation. This structured process ensures secure and controlled creation of medicines, restricting access to authorized entities while validating and recording essential information.
4.3 Tools used
The model that is suggested is suitable for collaborative environment and developed using several tools such as Solidity, truffle framework, Ganache, Metamask etc. as given in Table 3. Remix online IDE, is a no-setup tool with a GUI for developing smart contracts using solidity. Solidity Language is used to develop smart contracts. Smart contracts are the program that contains the logic of the proposed model. Truffle Framework provides a development Environment for decentralized application and provides a test framework. Ganache is used to create the local blockchain test environment for IDP model. By using a mobile app or browser plugin, MetaMask (Rana et al., 2022) is a digital wallet that connects to the Polygon blockchain and may be used to communicate with decentralized applications. With the help of the decentralized Ethereum scaling platform Polygon, programmers can create user-friendly, scalable decentralized applications with minimal transaction costs and never compromise on security.
5 Implementation and Result
To implement the IDP model, Ethereum scaling solution polygon layer2 blockchain environment is used. Firstly testing network of polygon is added in our metamask wallet as shown in Figure 5.
After adding this testing network, Matic test token are required for testing purposes. These Matic test tokens are requested from polygon faucet and added into the meta mask wallet as shown in Figure 6.
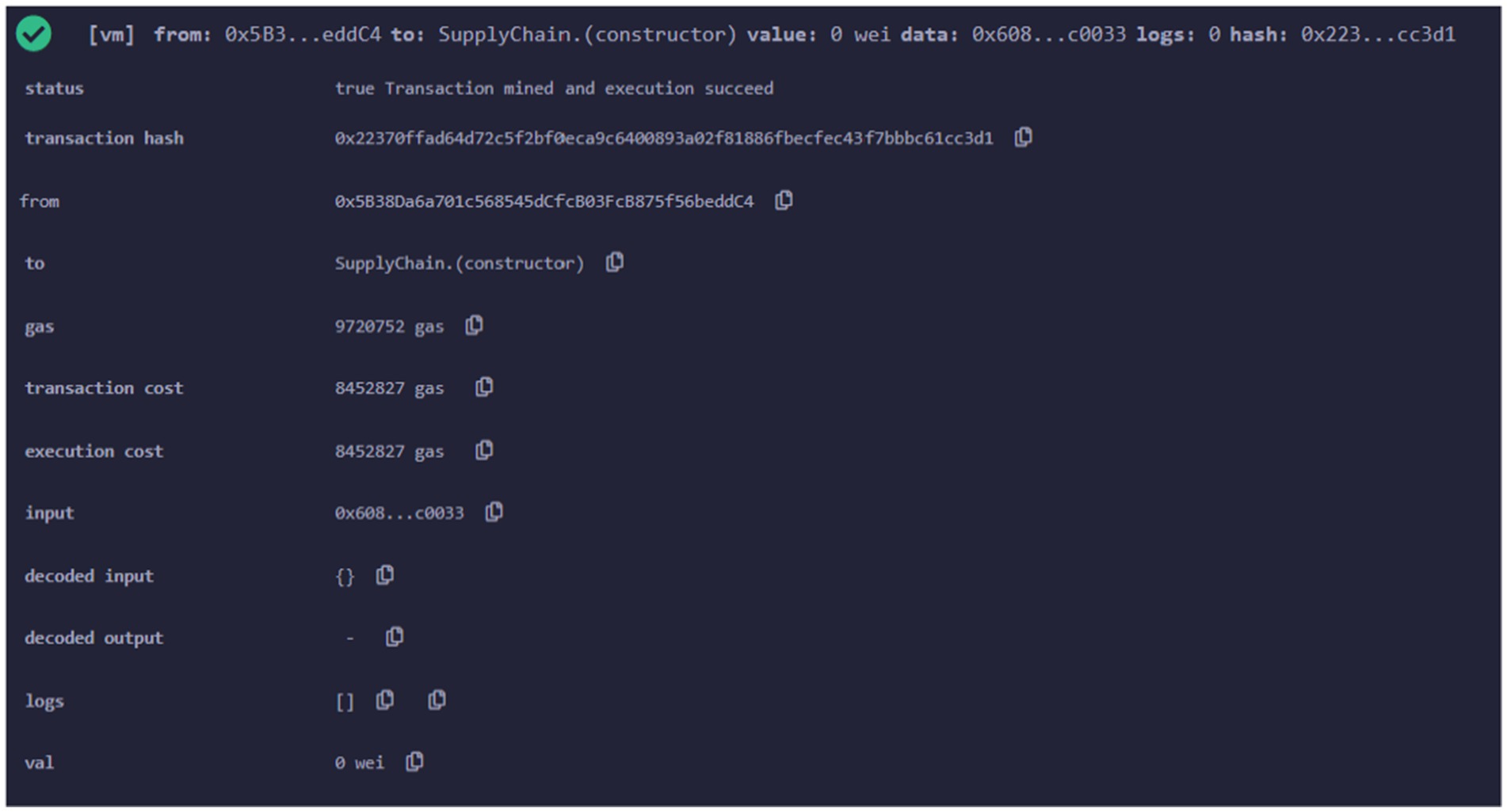
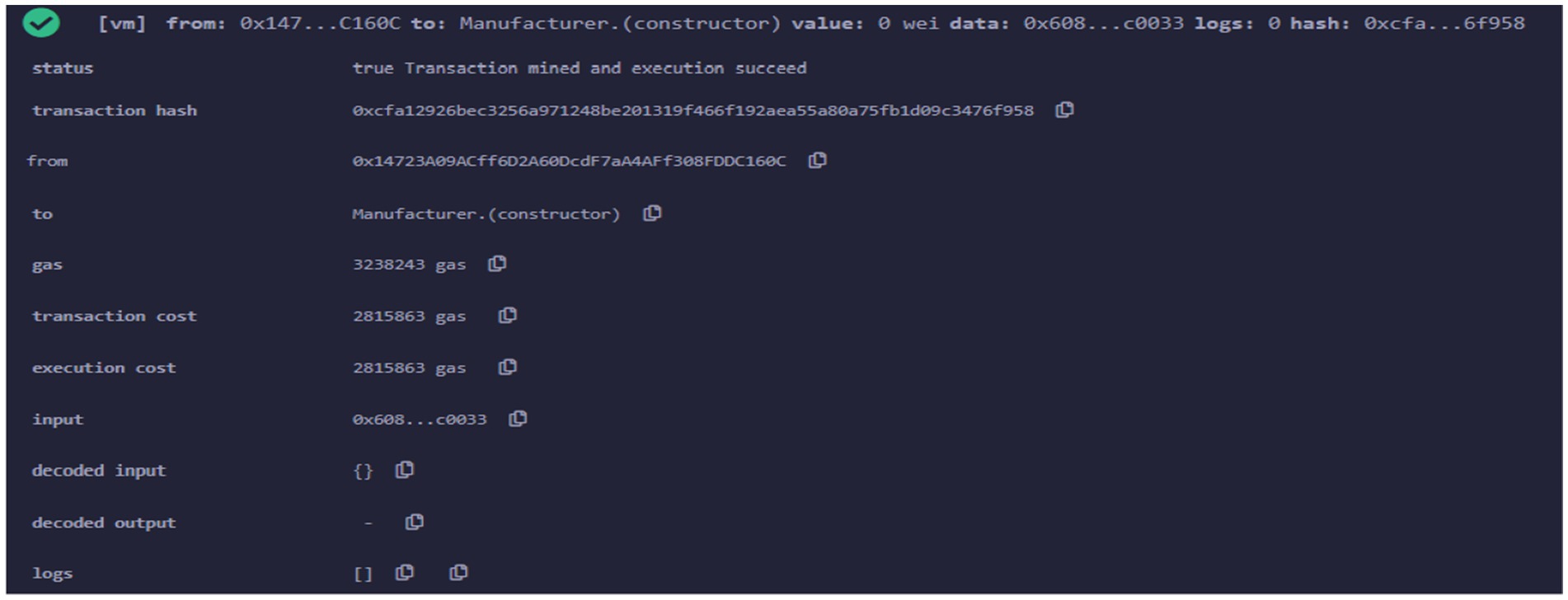
After that different smart contracts are deployed using Remix online IDE. The metamask wallet receives this deployment transaction for verification. Firstly, the supply chain smart contract is implemented on the polygon test network. The transaction details after contract deployment are shown in Figure 7. It gives information such as transaction hash address of the contract deployer, amount of gas consumed etc.
Second smart contract gives the overview of various function of supplier entity. Deployed Supplier smart contract details are shown in Figure 8.
Next, smart contract is related to Manufacturer entity that provides the various function of Manufacturer in supply chain ecosystem. The transaction details after Manufacturer smart contract deployment are shown in Figure 9.
Next smart contract provides the information about the Distributor and its functionality. The transaction details after Distributor smart contract deployment are shown in Figure 10.
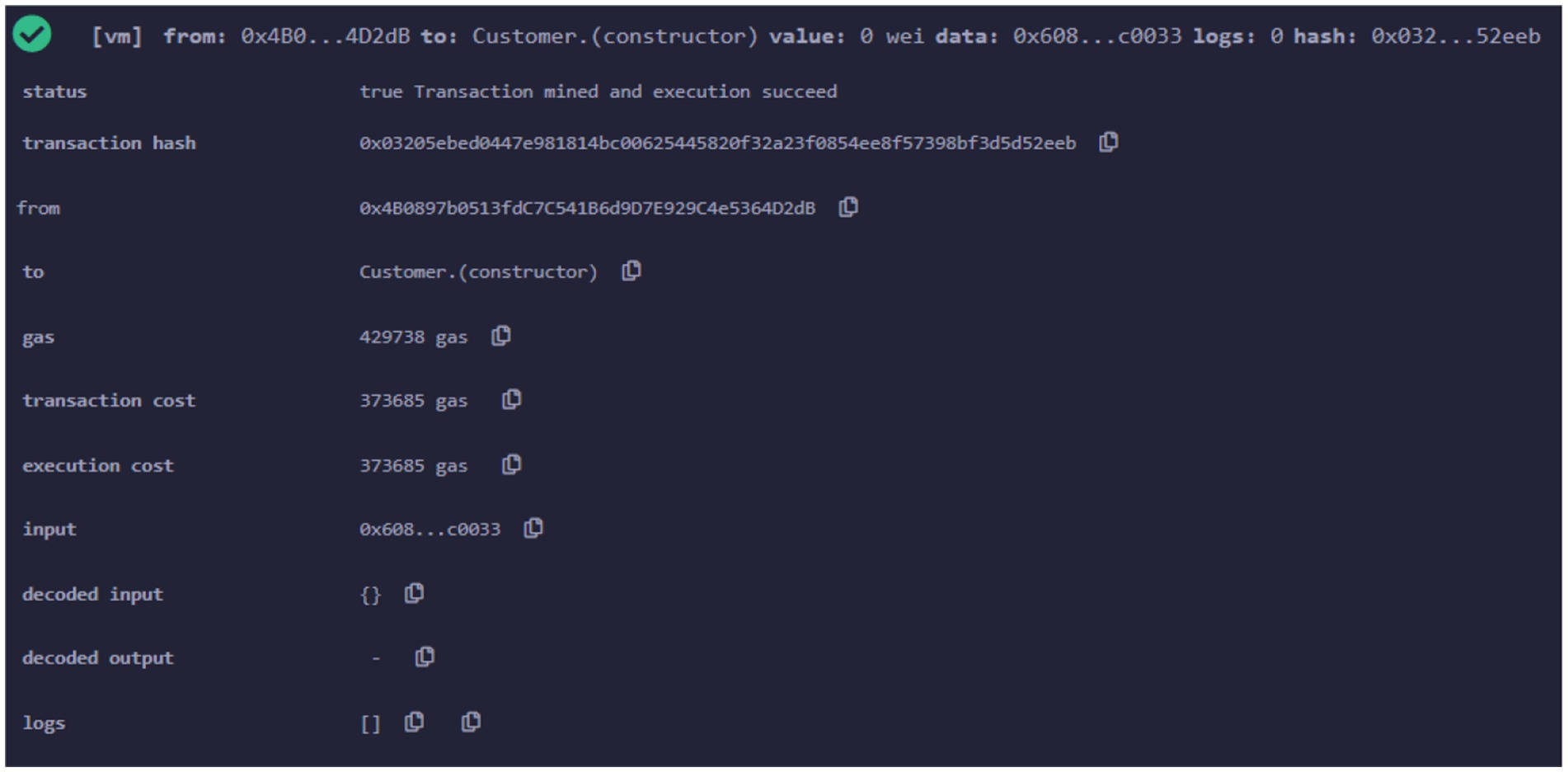
The information regarding the Customer and its functionality is provided through the Customer smart contract. Figure 11 displays the transaction specifics following the implementation of the customer’s smart contract.
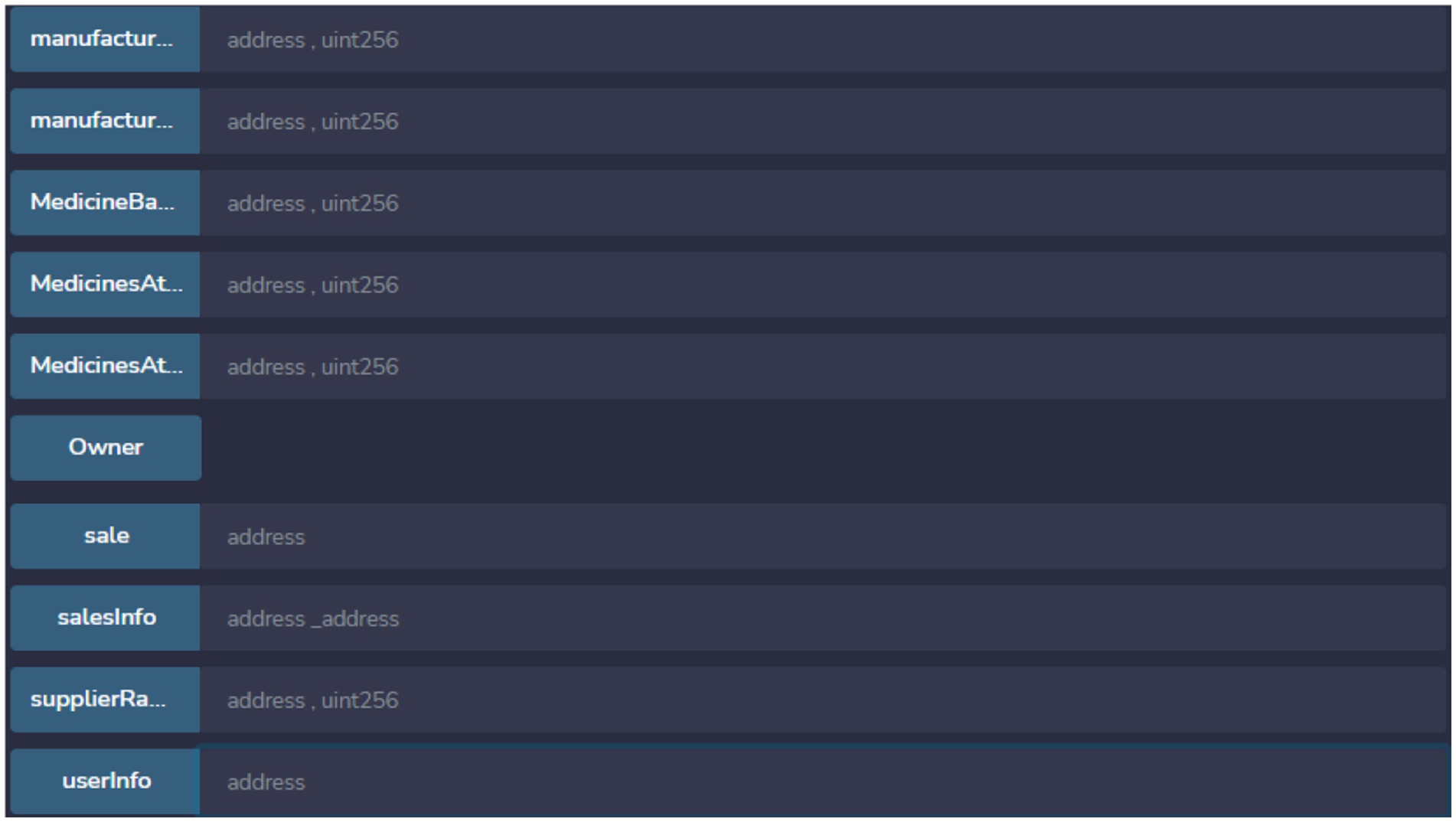
View after deployment of supply chain smart contract in shown Figures 12, 13. Function shown with orange color have the ability to update information related to various stockholder in IDP model.
Functions with blue color are read only so these are not able to modify any data. These function can be used by different stack holders to view specific information.
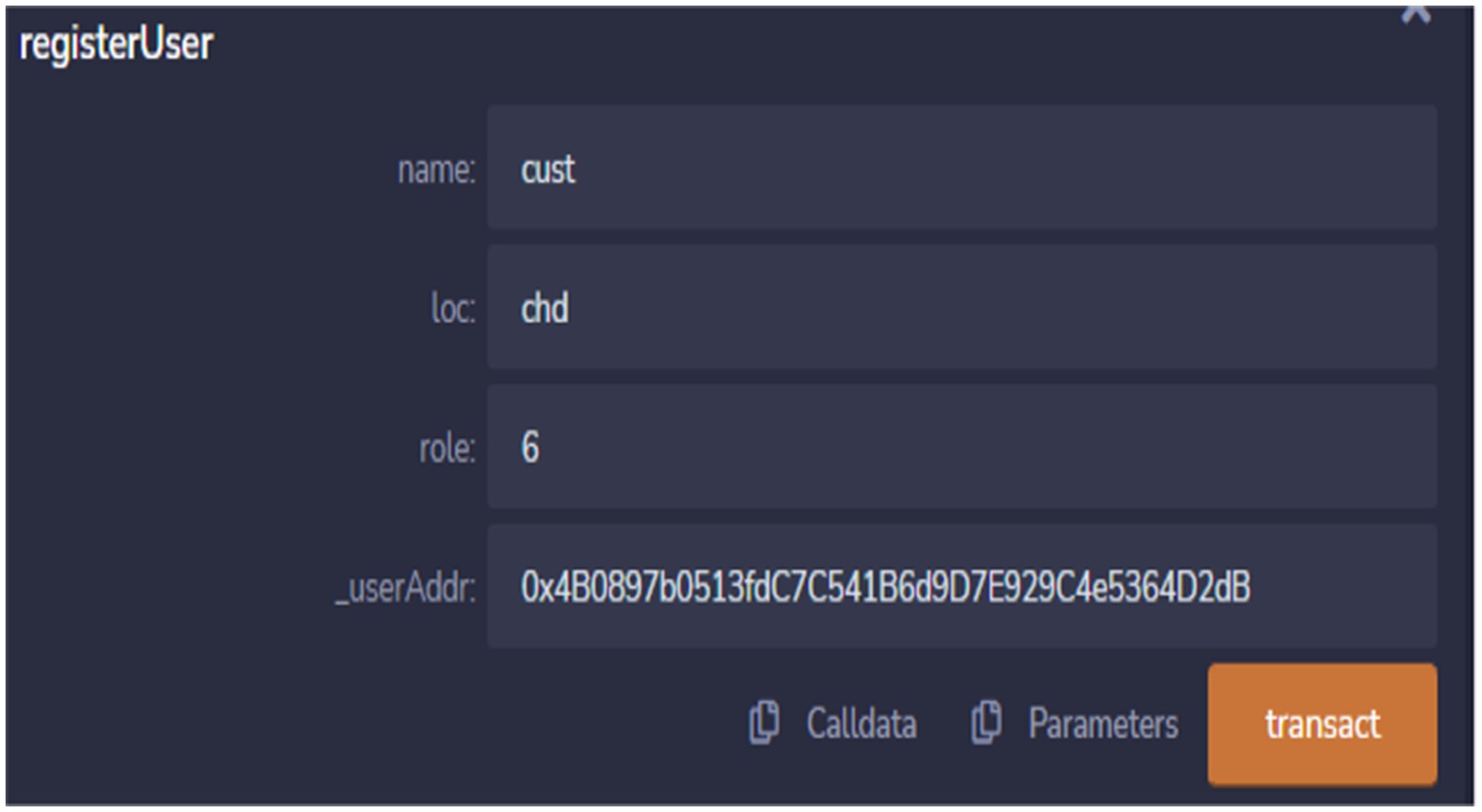
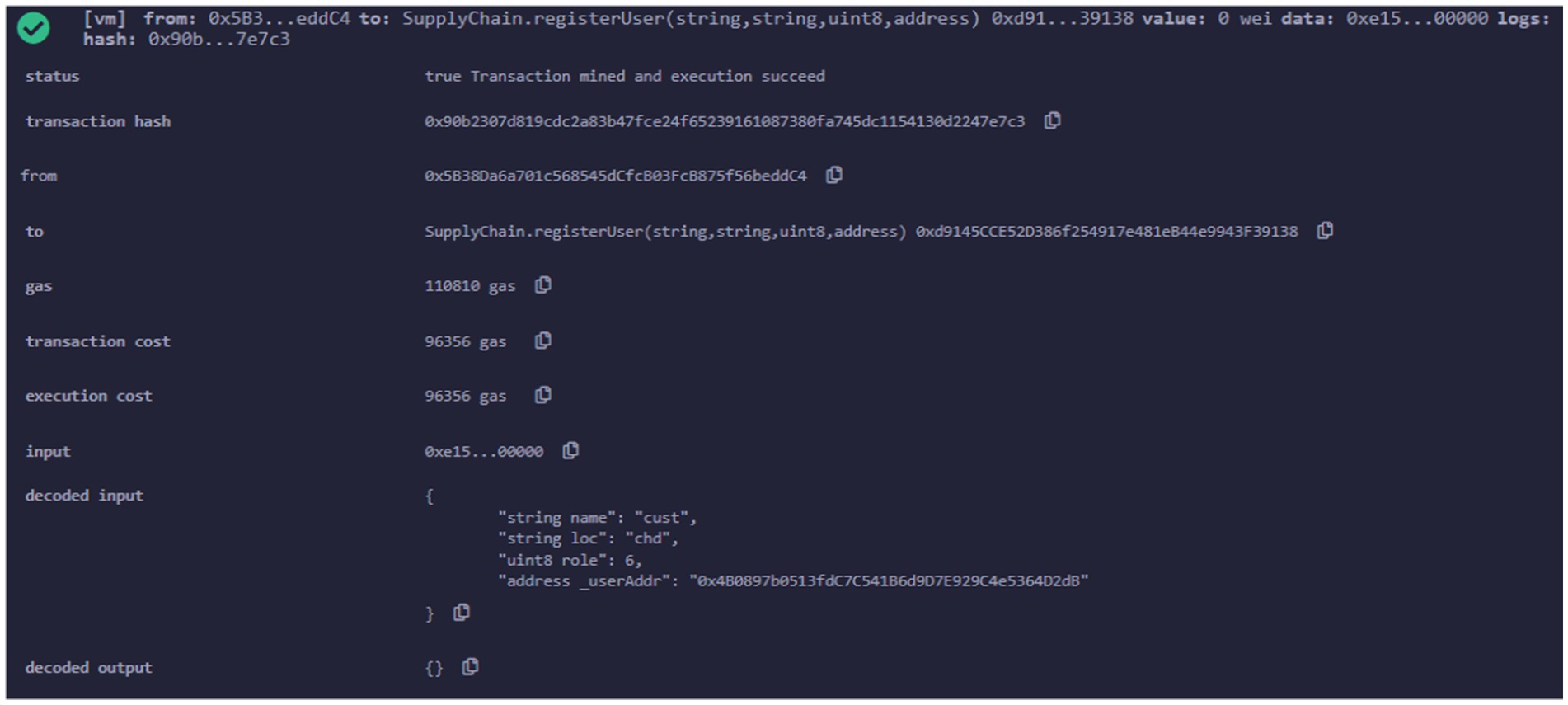
There are various functions in smart contract for different purposes.one of the function is register user as shown in Figure 14. This function is used to register the different entitles of pharmaceutical supply chain ecosystem. Values such as name, location, role and address are provided to register a particular entity.
Transaction details after execution of register user is shown in Figure 15. Inputs values given at run time is available in the decoded input.
5.1 Experimental results of a smart contract deployment
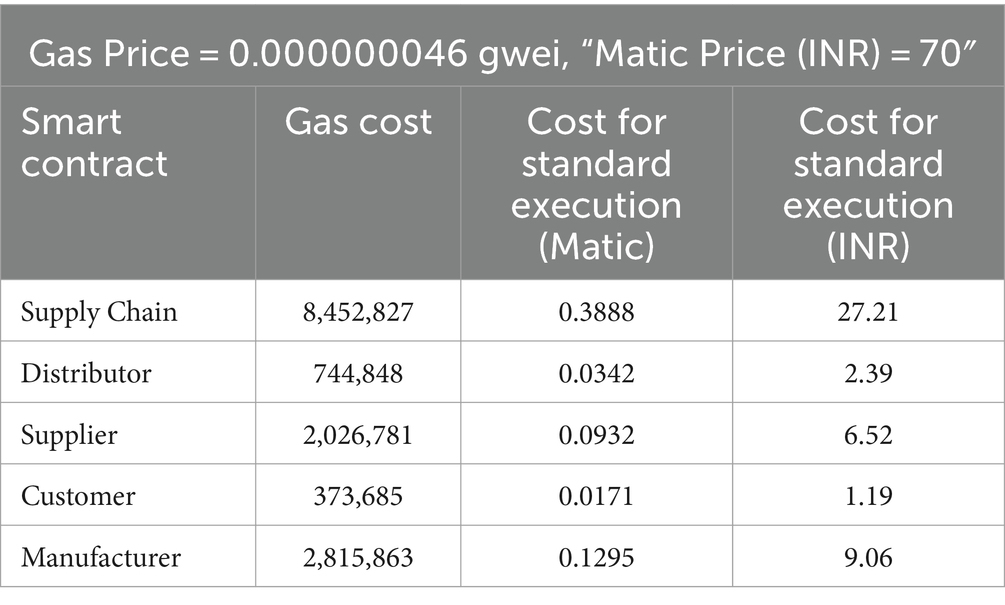
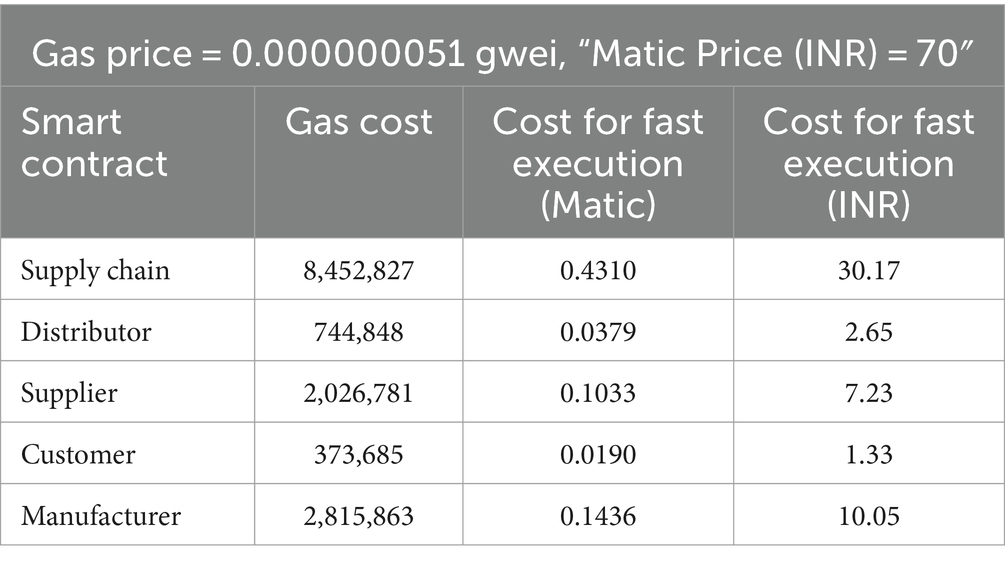
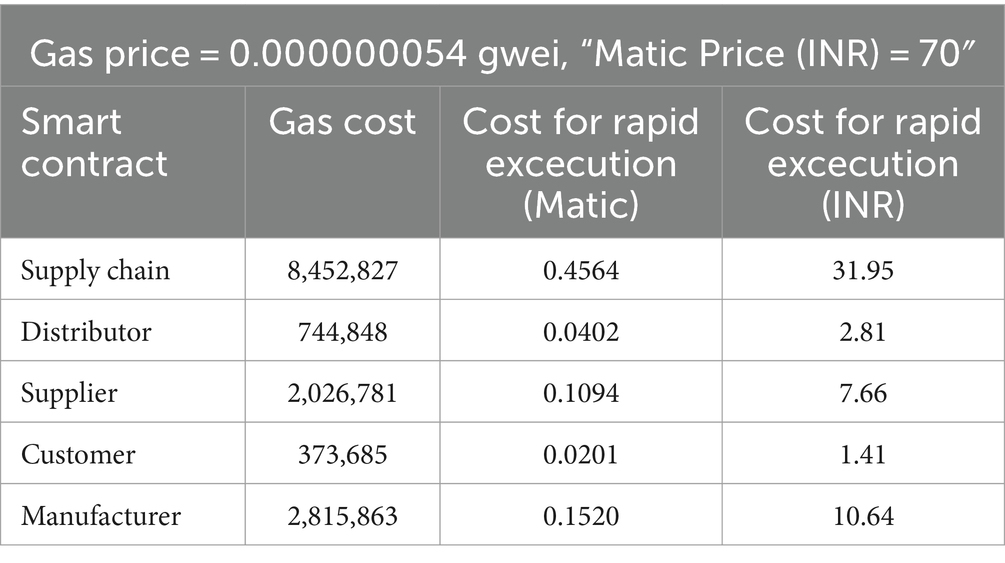
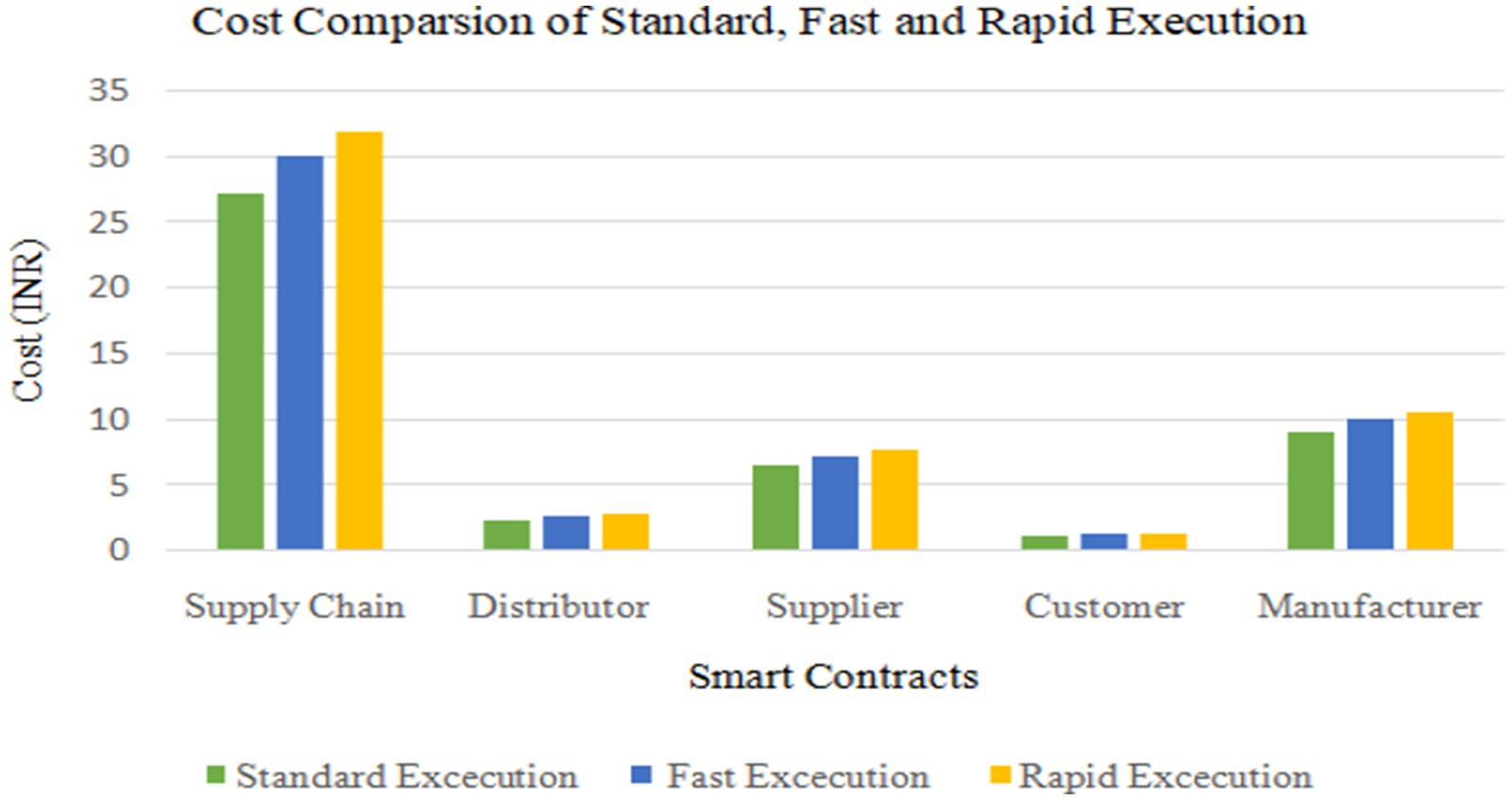
The smart contracts were deployed on a polygon blockchain. There are three different ways for execution such as Standard, Fast and Rapid. Cost analysis of different smart contracts in Standard, Fast and Rapid execution are shown in Tables 4–6 respectively.
The Table 4 details the costs associated with standard executions in various smart contracts operating within a supply chain on the Matic network. The “Gas Price” denotes the computational cost in gwei, while “Matic Price (INR)” indicates the equivalent value of Matic in Indian Rupees. In the Supply Chain contract, with a gas cost of 8,452,827, the standard execution incurs a cost of 0.3888 Matic (approximately 27.21 INR). Similarly, the Distributor, Supplier, Customer, and Manufacturer contracts each exhibit varying gas costs and associated execution expenses. This data provides a comprehensive overview of the computational and financial implications of executing standard operations within distinct components of the Matic-based supply chain.
The Table 5 summarizes costs for executing operations in Matic network smart contracts, based on a gas price of 0.000000051 gwei and Matic priced at 70 INR. Supply Chain incurs a gas cost of 8,452,827, with a Matic cost of 0.4310 (30.17 INR) for fast execution. Distributor, Supplier, Customer, and Manufacturer contracts have varying gas costs and Matic costs ranging from 0.0190 to 0.1436, equivalent to 1.33 to 10.05 INR. These values illustrate the computational expenses associated with different contract executions, indicating potential intricacies and resource needs within the Matic network’s supply chain processes.
The Table 6 outlines the costs associated with executing smart contracts in a blockchain system, denoted in Gwei. With a Gas Price of 0.000000054 Gwei and Matic Price at 70 INR, the Supply Chain incurs a gas cost of 8,452,827, translating to 0.4564 Matic (31.95 INR). Similarly, the Distributor and Supplier contracts result in gas costs of 744,848 (0.0402 Matic, 2.81 INR) and 2,026,781 (0.1094 Matic, 7.66 INR) respectively. The Manufacturer’s contract bears a gas cost of 3,736,853 (0.0201 Matic, 1.41 INR). These figures shed light on the computational expenses and tokenized values associated with smart contract execution at different stages in the outlined blockchain supply chain.
Cost Comparison of various smart contracts for Standard, Fast, and Rapid Execution is show in Figure 16.
6 Discussion
Here, we’ll discuss how the suggested strategy assures performance for Fast Execution and scalability. We additionally address the main security objectives our strategy helps achieve, compared it with different methods.
6.1 Execution time and scalability
The choice of consensus method and data storage approach significantly impacts execution speed and scalability. An algorithm’s scalability is measured by its transaction throughput. By leveraging the Proof of Authority (PoA) consensus method, we can achieve high throughput due to its reliance on a small number of validators. Additionally, storing data hashes on the blockchain instead of actual data ensures data scalability. The use of PoA also resolves the issue of low execution time. Compared to networks utilizing Proof of Work (PoW) consensus, PoA networks exhibit faster transaction times.
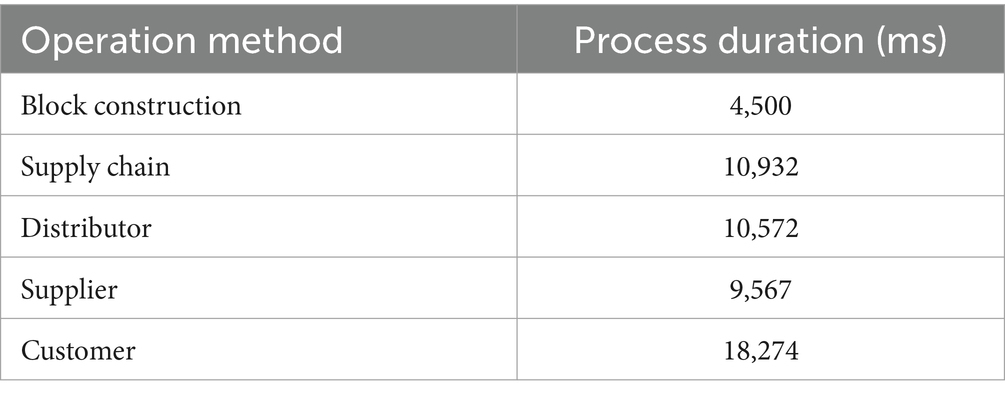
In our scenario, we have set the gas limit to 4,500,000 and the block formation period to 4 s. With this configuration, we can process 43 transactions per second. Table 7 illustrates the processing times for various operations in the IDP model.
6.2 Transaction performance and latency comparison
To further evaluate the performance of our proposed model, we compared its transaction performance and latency to conventional systems and other blockchain solutions.
6.2.1 Transaction throughput
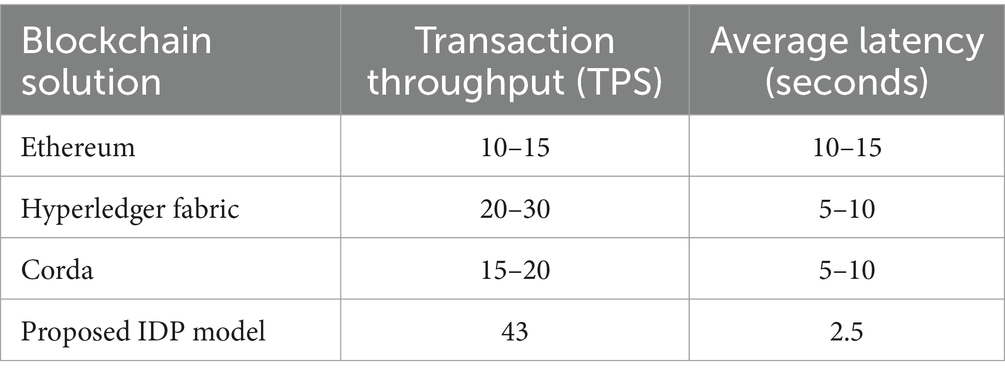
Our proposed model achieves a transaction throughput of 43 transactions per second (TPS), outperforming traditional supply chain management systems which typically process around 10–20 TPS. In comparison, other blockchain-based solutions such as Ethereum and Hyperledger Fabric achieve around 10–15 TPS and 20–30 TPS, respectively.
6.2.2 Latency
The average latency of our proposed model is 2.5 s, significantly lower than traditional supply chain management systems which can take up to 30 min to process a transaction as shown in the Table 8. Other blockchain-based solutions such as Ethereum and Hyperledger Fabric have average latencies of around 10–15 s and 5–10 s, respectively shown in Table 9.
The results demonstrate that our proposed model outperforms both conventional systems and other blockchain-based solutions in terms of transaction throughput and latency, making it a viable solution for supply chain management.
6.3 Cost analysis for day-to-day operations
To estimate the potential costs of running the IDP model on the Matic blockchain, we calculated the gas costs for each operation. Assuming a gas price of 20 Gwei, the estimated costs are as follows:
• Block Construction: 0.09 USD
• Supply Chain Creation: 0.22 USD
• Distributor On boarding: 0.21 USD
• Supplier On boarding: 0.19 USD
• Customer Order Processing: 0.37 USD
Based on these estimates, the daily operating costs for the IDP model can be calculated as follows:
• Assuming 100 supply chains are created per day, the daily cost would be approximately 22 USD
• Assuming 500 distributors are onboarded per day, the daily cost would be approximately 105 USD
• Assuming 1,000 suppliers are onboarded per day, the daily cost would be approximately 190 USD
• Assuming 5,000 customer orders are processed per day, the daily cost would be approximately 185 USD
The total daily operating cost for the IDP model would be approximately 502 USD.
6.4 Security evaluation
6.4.1 Security model insurance
• Accessibility and security: To make our IDP model more secure, we have added a blockchain security layer and data encryption. Decentralization and data replication are two of the most significant characteristics of blockchain. By doing so, a single point of failure is prevented and data availability is guaranteed.
• Privacy: Through encryption, permissioned networks, identity verification, private transactions, data minimization, immutability, access control, safe data sharing, transparency with privacy, and audit trails, blockchain technology improves privacy in the pharmaceutical drug supply chain. Our model permits selective data sharing, guarantees the security of sensitive information, and limits access to those who are permitted. The pharmaceutical business will ultimately benefit from improved trust and responsibility as a result of maintaining transparency while protecting patient information and regulatory compliance.
• Confidentiality: Blockchain-powered confidentiality in the pharmaceutical medicine supply chain protects private data using encryption, permissioned access, smart contracts, and audit trails. It promotes confidence and transparency among approved supply chain actors while ensuring data protection, traceability, and compliance.
• Integrity: By utilizing Blockchain technology, data integrity is improved in our system. On the one hand, hash data is stored on the Blockchain. The immutability feature, which guards against data manipulation, prevented this hash from being altered once it had been registered. On the other hand, the Blockchain’s smart contracts govern who has access to the hash data. Therefore, only parties with authorization can access and add data. By keeping track of all data manipulations and actions, blockchain also enables traceability.
6.4.2 Against attacks
• DDoS attacks: During a distributed denial of service assault, a service is bombarded with requests until it is unable to process anymore, so interfering with its normal traffic. The proposed remedy is entirely built on a decentralized and distributed system that makes use of Blockchain. By doing so, DDoS assaults may be prevented.
• Impersonation attacks: An attacker impersonates a reliable entity in this type of attack. Because the ECDSA is challenging to solve, the elliptic curve discrete logarithm problem is also challenging.
• Message forgery attack: It is used to falsify or alter communications, as the name suggests. Additionally, since every network transaction is signed and confirmed before it’s added to the Blockchain, this attack is not feasible.
• Man-in-the-middle attacks: This attack involves intercepting two entities’ communications without knowing for sure that the communications are secure. Our approach encrypts and signs the transaction using a private key that is known only to its owner. For the transaction to be deemed legitimate, it must to be backed up with an authentic signature from the emitting address. Therefore, without the associated private key, it would be extremely difficult for an attacker to produce a phony signature.
6.5 Compared with the earlier works
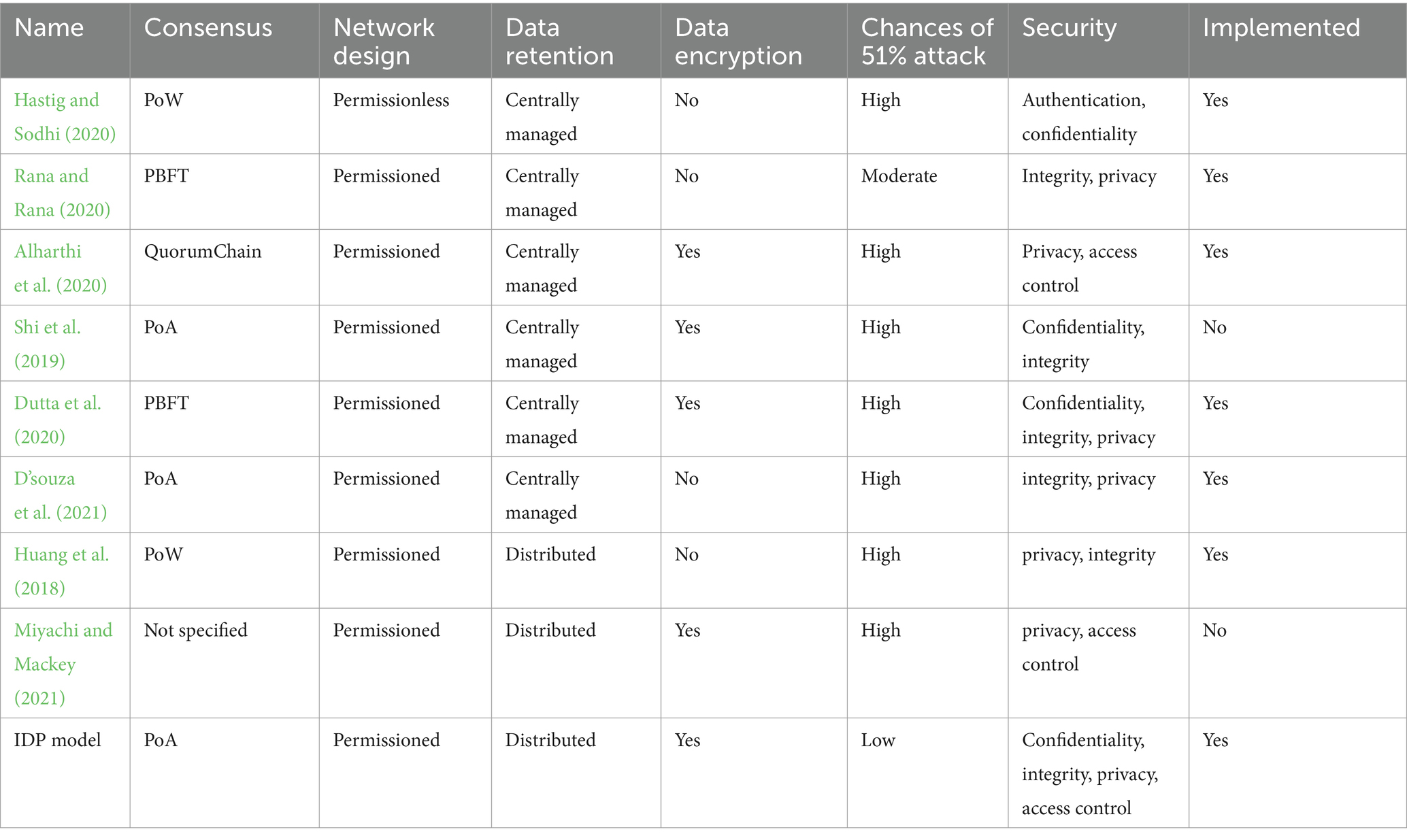
In this section, we contrast the proposed research with the most illustrious studies that have looked at using Blockchain technology to safeguard medical supply chain data in a collaborative setting. The basis for this comparison is the kind of Blockchain, the consensus algorithm, and the type of network that is being used (see Table 8). Additionally, it provides details on the data storage utilized by each job and whether or not it is taken into account by its system. It can also reveal the degree of security offered by each architecture and whether or not data is encrypted before being stored. Only two pieces of work (Jangirala et al., 2020; Alamri et al., 2021) employ the PoW consensus algorithm. The primary issue with this approach is how much energy it uses to validate a block. Regarding transaction speed, it can also be characterized as being slow. Furthermore, data encryption was not taken into account. In terms of data storage, each work referenced makes use of a single database. This type of storage is susceptible to hacking and DDoS attacks. While using smart contracts and product registration and transfer, the proposed method permanently registers product transferring records in the immutable ledger. Furthermore, unlike previous works that focuses only on a few of these needs, our approach takes into account the fundamental security requirements of confidentiality, integrity, privacy, and access control (Juneja et al., 2020; Wani et al., 2021; Mittal et al., 2022; Rai, 2023; Reegu et al., 2023, 2024). Additionally, the majority of the works mentioned have not yet been put into practice. Proposed IDP model is equated with some existing approaches for some attributes such as execution speed, efficiency, privacy etc. It is found that IDP model performs better than existing approaches as shown in Table 10.
In Table 11, we compare the suggested approach to the pertinent current methods for a traceable supply chain for pharmaceuticals.
7 Conclusion and future work
Blockchain technology-based model (IDP) is proposed as solution for a smart anti-counterfeit pharmacy supply chain. With the use of smart contracts and product registration and transfer, all product transferring records are permanently registered in the immutable ledger. The integration of smart contracts enables product tracking. Blockchain’s data immutability feature which means that once data is uploaded to the edger, it cannot be withdrawn or changed offers a great solution for data security and integrity. Data security is preserved because to its decentralized storage, which prevents any one party from manipulating data simultaneously. A crucial component of every supply chain is transaction transparency. All participants in our suggested solution will have access to and be able to examine the validated transactions in a secure setting. In order to verify the legitimacy of the event by validating the signature included in the event and the identities of all participants, an event request-response method was also created. Every occurrence can be documented and kept as a log in the blockchain, where it can be accessed in real time. Finally, IDP model is developed and tested on the polygon blockchain test network. The system exhibits data accessibility, tamper-resistance, and defense against man-in-the-middle assaults. The objectives of our proposed application are to improve the healthcare supply chain’s efficiency, foster transparency, and ensure economic stability. As future work it can be extended for implementation on various business models. We intend to build on this work in a subsequent effort by putting our solution into practice utilizing the Hyperledger Blockchain and contrasting it with the existing Ethereum-based solution. In order to make our system smarter and add more functions, we also intend to include artificial intelligence into it. We could offer data analysis, forecasts, and prevention by using artificial intelligence. The structure would support doctors in making better clinical judgments and offering a successful treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
DS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. SK: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. YG: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MM: Conceptualization, Methodology, Visualization, Writing – review & editing. DG: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. WJ: Conceptualization, Methodology, Visualization, Writing – review & editing. NS: Conceptualization, Methodology, Visualization, Writing – review & editing. SA: Conceptualization, Methodology, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Deanship of Scientific Research, the Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (GrantA023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmadi, V., Benjelloun, S., El Kik, M., Sharma, T., Chi, H., and Zhou, W. (2022). Drug governance: IoT-based blockchain implementation in the pharmaceutical supply chain. In: 2020 sixth international conference on Mobile and secure services (MobiSecServ), pp. 1–8.
Alamri, B., Javed, I. T., and Margaria, T. (2021). A GDPR-compliant framework for IoT-based personal health records using Blockchain. In: 2021 11th IFIP international conference on new technologies, mobility and security (NTMS). Paris, France: IEEE, pp. 1–5.
Alharthi, S., Cerotti, P. R., and Far, S. M. (2020). An exploration of the role of blockchain in the sustainability and effectiveness of the pharmaceutical supply chain. J. Suppl. Chain Customer Relat. Manage. 2020, 1–29. doi: 10.5171/2020.562376
Argiyantari, B., Simatupang, T. M., and Basri, M. H. (2020). Pharmaceutical supply chain transformation through application of the lean principle: a literature review. J. Ind. Eng. Manage. 13, 475–494. doi: 10.3926/jiem.3100
Azaria, A., Ekblaw, A., Vieira, T., and Lippman, A. (2016). Using Blockchain for medical data access and permission management. In: 2016 2nd international conference on open and big data (OBD). Vienna, Austria: IEEE, pp. 25–30.
Badhotiya, G. K., Sharma, V. P., Prakash, S., Kalluri, V., and Singh, R. (2021). Investigation and assessment of blockchain technology adoption in the pharmaceutical supply chain. Mater. Today 46, 10776–10780. doi: 10.1016/j.matpr.2021.01.673
Bocek, T., Rodrigues, B. B., Strasser, T., and Stiller, B. (2017). Blockchains everywhere-a use-case of blockchains in the pharma supply-chain. In: 2017 IFIP/IEEE symposium on integrated network and service management (IM), pp. 772–777.
Bodkhe, U., Tanwar, S., Parekh, K., Khanpara, P., Tyagi, S., Kumar, N., et al. (2020). Blockchain for industry 4.0: a comprehensive review. IEEE Access 8, 79764–79800. doi: 10.1109/access.2020.2988579
D’souza, S., Nazareth, D., Vaz, C., and Shetty, M. (2021). Blockchain and AI in pharmaceutical supply chain. In: Proceedings of the International Conference on Smart Data Intelligence (ICSMDI 2021).
Dutta, P., Choi, T. M., Somani, S., and Butala, R. (2020). Blockchain technology in supply chain operations: applications, challenges and research opportunities. Transp. Res. E Logist. Transp. Rev. 142:102067. doi: 10.1016/j.tre.2020.102067
Feng, Q., He, D., Zeadally, S., Khan, M. K., and Kumar, N. (2019). A survey on privacy protection in blockchain system. J. Netw. Comput. Appl. 126, 45–58. doi: 10.1016/j.jnca.2018.10.020
Goldacre, B., Hart, G., De Lusignan, S., Fottrell, E., Kostkova, P., Brewer, H., et al. (2016). Who is the data’s owner? Healthcare with open data. Public Health Frontiers, Vol. 4. p. 7.
Hackius, N., and Petersen, M. (2020). Translating high hopes into tangible benefits: how incumbents in supply chain and logistics approach blockchain. IEEE Access 8, 34993–35003. doi: 10.1109/ACCESS.2020.2974622
Hastig, G. M., and Sodhi, M. S. (2020). Blockchain for supply chain traceability: business requirements and critical success factors. Prod. Oper. Manag. 29, 935–954. doi: 10.1111/poms.13147
Huang, Y., Wu, J., and Long, C. (2018). Drugledger: a practical blockchain system for drug traceability and regulation. In: 2018 IEEE international conference on internet of things (iThings) and IEEE green computing and communications (GreenCom) and IEEE cyber, physical and social computing (CPSCom) and IEEE smart data (SmartData), IEEE, pp. 1137–1144.
Jamil, F., Ahmad, S., Whangbo, T. K., Muthanna, A., and Kim, D. H. (2022). Improving blockchain performance in clinical trials using intelligent optimal transaction traffic control mechanism in smart healthcare applications. Comput. Ind. Eng. 170:108327. doi: 10.1016/j.cie.2022.108327
Jamil, F., Lei, H., Kim, K., and Kim, D. (2019). A novel medical blockchain model for drug supply chain integrity management in a smart hospital. Electronics 8:505. doi: 10.3390/electronics8050505
Jangirala, S., Das, A. K., and Vasilakos, A. V. (2020). Designing secure lightweight Blockchain-enabled RFID-based authentication protocol for supply chains in 5G mobile edge computing environment. IEEE Trans. Industr. Inform. 16, 7081–7093. doi: 10.1109/tii.2019.2942389
Juneja, S., Juneja, A., and Anand, R. (2020). Healthcare 4.0-digitizing healthcare using big data for performance improvisation. J. Comput. Theor. Nanosci. 17, 4408–4410. doi: 10.1166/jctn.2020.9087
Khanna, T., Nand, P., and Bali, V. (2020). Permissioned Blockchain model for end-to-end Trackability in supply chain management. Int. J. E Collab. 16, 45–58. doi: 10.4018/ijec.2020010104
Kukreja, V., Kumar, D., and Kaur, A. (2021). Deep learning in human gait recognition: an overview. In: 2021 International Conference on Advance Computing and Innovative Technologies in Engineering, ICACITE 2021, pp. 9–13.
Kumar, R., Marchang, N., and Tripathi, R. (2020). Distributed off-chain storage of patient diagnostic reports in healthcare system using IPFS and Blockchain. In: 2020 international conference on COMmunication systems and NETworkS (COMSNETS). Bengaluru, India: IEEE, pp. 1–5.
Kumar, G., Saha, R., Buchanan, W. J., Geetha, G., Thomas, R., Kumar, M., et al. (2020). Decentralized accessibility of e-commerce products through blockchain technology. Sustain. Cities Soc. 62:102361. doi: 10.1016/j.scs.2020.102361
Kumari, K. (2020). Kavita Saini “Data Handling and Drug Traceability: Blockchain meets healthcare to combat counterfeit drugs”. Int. J. Sci. Technol. Res. 9, 728–731.
KyungSup, K., Daehan, K., Humaun Kabir, M., Hossain, M., and Riazul Islam, S. M. (2015). An extensive survey of the internet of things in healthcare. IEEE Access 3, 678–708. doi: 10.1109/ACCESS.2015.2437951
Liang, X., Zhao, J., Shetty, S., Liu, J., and Li, D. (2017). Integrating blockchain for data sharing and collaboration in mobile healthcare applications. In: IEEE 28th annual international symposium on personal, indoor, and Mobile radio communications (PIMRC). IEEE: Montreal, QC 2017, pp. 1–5.
Lingayat, V., Pardikar, I., Yewalekar, S., Khachane, S., and Pande, S. (2021). Securing pharmaceutical supply chain using Blockchain technology. In: ITM web of conferences, Vol. 37. EDP Sciences, p. 01013.
Mahboob, T., Zahid, M., and Ahmad, G. (2021). Adopting information security techniques for cloud computing–a survey. In: 2016 1st international conference on information technology, Information Systems and Electrical Engineering (ICITISEE).
Mittal, S., Bansal, A., Gupta, D., Juneja, S., Turabieh, H., Elarabawy, M. M., et al. (2022). Using identity-based cryptography as a foundation for an effective and secure cloud model for e-health. Comput. Intell. Neurosci. 2022, 1–8. doi: 10.1155/2022/7016554
Miyachi, K., and Mackey, T. K. (2021). hOCBS: a privacy-preserving blockchain framework for healthcare data leveraging an on-chain and off-chain system design. Inf process. Manage 58:102535. doi: 10.1016/j.ipm.2021.102535
Monrat, A. A., Schelén, O., and Andersson, K. (2019). A survey of blockchain from the perspectives of applications, challenges, and opportunities. IEEE Access 7, 117134–117151. doi: 10.1109/ACCESS.2019.2936094
Musamih, A., Salah, K., Jayaraman, R., Arshad, J., Debe, M., Al-Hammadi, Y., et al. (2021). A blockchain-based approach for drug traceability in healthcare supply chain. IEEE Access 9, 9728–9743. doi: 10.1109/ACCESS.2021.3049920
Ouchetto, O., Fetjah, L., Sekkaki, A., Andaloussi, S. J., and Azbeg, K. (2018). A platform for diabetes self-management using blockchain and IoT for security and privacy. In: 4th international conference on applications and Technologies of Cloud Computing, 2018 (Cloudtech). IEEE, Brussels, Belgium, pp. 1–5.
Pettit, T. J., Croxton, K. L., and Fiksel, J. (2019). The evolution of resilience in supply chain management: a retrospective on ensuring supply chain resilience. J. Bus. Logist. 40, 56–65. doi: 10.1111/jbl.12202
Rahman, S., Aquib, A. A., Jyoty, W. B., Rahman, M., and Dewan, T. (2021). A blockchain-driven framework designed for pharmaceutical community to secure and trace the trail of drug supply chain. (Doctoral dissertation). Dhaka: Brac University.
Rai, B. K. (2023). BBTCD: blockchain based traceability of counterfeited drugs. Health Serv. Outcomes Res. Methodol. 23, 337–353. doi: 10.1007/s10742-022-00292-w
Raj, R., Rai, N., and Agarwal, S. (2019). Anticounterfeiting in pharmaceutical supply chain by establishing proof of ownership. In: TENCON 2019–2019 IEEE region 10 conference (TENCON), pp. 1572–1577.
Rana, S. K., Kim, H. C., Pani, S. K., Rana, S. K., Joo, M. I., Rana, A. K., et al. (2021). Blockchain-based model to improve the performance of the next-generation digital supply chain. Sustain. For. 13:10008. doi: 10.3390/su131810008
Rana, S. K., and Rana, S. K. (2020). Blockchain based business model for digital assets management in trust less collaborative environment. J. Crit. Rev. 7, 738–750.
Rana, S. K., and Rana, S. K. (2021). “Intelligent amalgamation of Blockchain technology with industry 4.0 to improve security” in Internet of things. ed. S. K. Rana (Boca Raton, FL: CRC Press), 165–175.
Rana, S. K., Rana, A. K., and Dhawan, S. (2022). “A vital fusion of internet of medical things and Blockchain to transform data privacy and security” in Convergence of deep learning and artificial intelligence in internet of things. ed. S. K. Rana (Boca Raton, FL: CRC Press), 293–308.
Rana, S. K., Rana, S. K., Nisar, K., Ag Ibrahim, A. A., Rana, A. K., Goyal, N., et al. (2022). Blockchain technology and artificial intelligence based decentralized access control model to enable secure interoperability for healthcare. Sustain. For. 14:9471. doi: 10.3390/su14159471
Rana, S. K., Rana, S. K., Rana, A. K., and Islam, S. M. (2022). A Blockchain supported model for secure exchange of land ownership: an innovative approach. In: 2022 international conference on computing, communication, and intelligent systems (ICCCIS). IEEE, pp. 484–489.
Rana, S. K., Rana, S. K., Rana, A. K., Nisar, K., Soomro, T. R., and Nisar, S. (2022). A survey on Blockchain technology supported approaches for healthcare system, open issues and challenges. In: 2022 14th International Conference on Mathematics, Actuarial Science, Computer Science and Statistics (MACS). IEEE, pp. 1–7.
Reegu, F. A., Abas, H., Gulzar, Y., Xin, Q., Alwan, A. A., Jabbari, A., et al. (2023). Blockchain-based framework for interoperable electronic health records for an improved healthcare system. Sustain. For. 15:6337. doi: 10.3390/SU15086337
Reegu, F. A., Ayoub, S., Dar, A. A., Hussain, G., Gulzar, Y., and Fatima, U. (2024). Building trust: IoT security and Blockchain integration. Proceedings of the 18th INDIAcom; 2024 11th International Conference on Computing for Sustainable Global Development, INDIACom 2024, 1429–1434.
Sakshi, V. K., and Ahuja, S. (2021). Recognition and classification of mathematical expressions using machine learning and deep learning methods. In: 2021 9th International Conference on Reliability, Infocom Technologies and Optimization (Trends and Future Directions), ICRITO, 2021.
Shi, J., Yi, D., and Kuang, J. (2019). “Pharmaceutical supply chain management system with integration of iot and blockchain technology” in International conference on smart Blockchain. ed. J. Shi (Cham: Springer), 97–108.
Singla, D., and Rana, S. K. (2022). “A systematic review on Blockchain-based e-healthcare for collaborative environment” in International conference on emergent converging technologies and biomedical systems. ed. D. Singla (Singapore: Springer Nature Singapore), 361–376.
Singla, D., and Rana, S. (2023). “Blockchain-based platform for smart tracking and tracing the pharmaceutical drug supply chain” in Examining multimedia forensics and content integrity. ed. D. Singla (Pennsylvania, US: IGI Global), 144–172.
Soundarya, K., Pandey, P., and Dhanalakshmi, R. (2018). A counterfeit solution for pharma supply chain. EAI Endorsed Transactions on Cloud Systems, No. 3.
Tripathi, R., and Kumar, R. (2019). Supply chain traceability of fake medications using blockchain technology. The 11th international conference on communication systems and networks (COMSNETS) is scheduled for 2019, pp. 568–570.
Wani, S., Imthiyas, M., Almohamedh, H., Alhamed, K. M., Almotairi, S., and Gulzar, Y. (2021). Distributed denial of service (Ddos) mitigation using Blockchain–a comprehensive insight. Symmetry (Basel) 13:227. doi: 10.3390/sym13020227
WHO. (2020). Technical package for primary care treatment of cardiovascular disease. Available at: https://apps.who.int/iris/handle/10665/252661 may be accessible online as of December 19. Available at: apps.who.int/iris/handle.
Keywords: smart contracts, supply chain, polygon near field communication, collaborative environment, blockchain
Citation: Singla D, Kumar S, Gulzar Y, Mir MS, Gupta D, Jaziri W, Sassi N and Arora S (2024) Designing and implementing a resilient immutability mechanism for enhanced supply chain management in E-healthcare systems. Front. Sustain. Cities. 6:1403809. doi: 10.3389/frsc.2024.1403809
Edited by:
Nitin Goyal, Central University of Haryana, IndiaReviewed by:
Nuria Novas Castellano, University of Almeria, SpainV. B. Murali Krishna, National Institute of Technology, Andhra Pradesh, India
John Zaki, German International University, Egypt
Copyright © 2024 Singla, Kumar, Gulzar, Mir, Gupta, Jaziri, Sassi and Arora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonis Gulzar, eWd1bHphckBrZnUuZWR1LnNh; Deepak Singla, c2luZ2xhZGVlcGFrMjExQGdtYWlsLmNvbQ==
 Deepak Singla
Deepak Singla Sanjeev Kumar1
Sanjeev Kumar1 Yonis Gulzar
Yonis Gulzar Mohammad Shuaib Mir
Mohammad Shuaib Mir Deepali Gupta
Deepali Gupta