
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg., 10 April 2025
Sec. Thoracic Surgery
Volume 12 - 2025 | https://doi.org/10.3389/fsurg.2025.1584017
With the advancement of Enhanced Recovery After Surgery (ERAS), minimally invasive thoracoscopic surgery has gained widespread clinical adoption owing to its reduced trauma and faster recovery compared to traditional open chest procedures. Thoracoscopic surgery has evolved from initial three-port and two-port techniques to single-port non-intubated approaches, which preserve spontaneous breathing while minimizing trauma and accelerating recovery. NIVATS represents a groundbreaking advancement in thoracic surgery and anesthesia by innovatively avoiding endotracheal intubation and mechanical ventilation, thereby challenging conventional surgical approaches. This paper reviews the research progress on the anesthesia techniques, indications, and contraindications of Non-Intubated Spontaneous-Ventilation Video-Assisted Thoracoscopic Surgery (NIVATS), discussing its advantages compared to traditional surgical methods, its application in thoracic diseases, as well as the risks and management of NIVATS.
The advent of thoracoscopic technology in the early 1990s marked a significant advancement in the diagnosis and treatment of thoracic diseases. Its widespread application has made it the most common surgical method today. The continuous advancement of modern technology has promoted the development of single-lung ventilation techniques, which use double-lumen endotracheal intubation anesthesia to effectively isolate the diseased lung from the healthy lung, thereby achieving optimal collapse of the affected lung. During surgery, the effective administration of anesthesia improves the surgical field of vision, significantly reduces the trauma associated with traditional open chest surgeries, and shortens the patient's postoperative hospital stay and recovery period. With technological advancements, thoracic surgical techniques have undergone significant evolution, from the initial three-port method, to the two-port method, and ultimately to the single-port technique, achieving great progress (1, 2). Its far-reaching impact is evidenced by (1) superior clinical outcomes compared to conventional VATS in randomized trials, (2) cost-effective adoption across diverse healthcare systems, and (3) long-term improvements in patients' quality of life and anesthesia (3–5). However, thoracoscopic surgery has also brought about new challenges, including postoperative lung injury related to mechanical ventilation, residual effects of positive inotropic drugs, potential damage to the trachea and vocal cords, and dependence on ventilator support post-surgery. To address the postoperative complications and sequelae of double-lumen endotracheal intubation thoracoscopic surgery, anesthesiologists and thoracic surgeons have collaborated to develop a non-intubated method that allows the patient to maintain spontaneous breathing during video-assisted thoracoscopic surgery(VATS). This new surgical technique, known as NIVATS, eliminates endotracheal intubation and uses laryngeal mask airway (LMA) insertion, allowing the patient to maintain spontaneous breathing during the surgery. This method also combines intravenous sedation and analgesia, local anesthesia, and minimal or no use of muscle relaxants, ensuring a smooth surgery under favorable conditions. Another significant advantage of NIVATS is that it eliminates the need for preoperative catheter insertion and postoperative drainage tube placement, significantly reducing trauma to the patient, shortening the hospital stay, and thereby lowering medical costs (6, 7). In recent years, with the continuous development of ERAS, the alignment of NIVATS with this emerging concept further demonstrates its importance and potential application in modern surgical procedures.
Current anesthesia methods for NIVATS combine local anesthesia, intravenous sedation, and analgesia, often assisted by a laryngeal mask or oropharyngeal airway. Continuous monitoring of ECG, oxygen saturation, carbon dioxide partial pressure, and bispectral index (BIS) is also performed. The methods of local anesthesia include local infiltration at the incision site, paravertebral nerve block, intercostal nerve block, vagus nerve block, and phrenic nerve block. Currently, NIVATS is being performed at multiple international clinical centers, where thoracic epidural anesthesia is used in combination with vagus nerve and intercostal nerve blocks, along with intravenous analgesia (8, 9).
Incisional local infiltration: 3–5 ml of 1% lidocaine is injected at the patient's incision site along the axillary midline or anterior axillary line at the 4th intercostal space for local infiltration anesthesia.
Paravertebral nerve block: The patient is placed in the lateral position, and the T5 intervertebral space is located under ultrasound guidance. An epidural needle is inserted 2.5 cm lateral to the upper edge of the T5 spinous process. After touching the transverse process, the needle is withdrawn to the subcutaneous level and advanced along the outer edge of the lamina. The needle tip punctures the costotransverse ligament and enters the paravertebral space. After the resistance disappears, the needle is advanced 1.0–1.5 cm further. Once aspiration yields no blood or cerebrospinal fluid, 10 ml of 0.5% bupivacaine (10) or 5 ml of 2% lidocaine (11) is injected through the needle. The anesthetic level is subsequently evaluated after the completion of the block.
Intercostal block: The patient is placed in the lateral position, and intercostal nerve blocks are performed under ultrasound guidance. The needle is inserted at the upper border of the rib at the surgical side, and a mixture of 0.375% ropivacaine and 1% lidocaine is injected (1.5 ml per intercostal space) at the 3rd, 4th, 5th, 6th, 7th, and 8th intercostal spaces. The anesthetic levels corresponding to the intercostal nerve blocks are subsequently assessed upon completion of the procedure (12).
Vagus nerve block: The lung lobe is retracted to expose the vagus nerve, and 10 ml of a mixture of 1% lidocaine and 0.375% ropivacaine is administered for right or left vagus nerve trunk block (13–15).
Phrenic nerve block: The phrenic nerve block is executed post-establishment of an artificial pneumothorax on the operative side, utilizing 5 ml of 2% lidocaine under direct thoracoscopic visualization (16).
Selection of anesthesia techniques in NIVATS requires balancing analgesic efficacy, procedural complexity, and complication risks. Comparative studies suggest: Analgesia Quality: While both thoracic epidural anesthesia (TEA) and paravertebral block (PVB) achieve comparable intraoperative pain control (VAS ≤ 2), PVB provides superior dynamic pain relief at 24 h postoperatively (mean difference −1.3, 95% CI −2.1 to −0.5) (17). Complication Rates: PVB is associated with fewer hemodynamic disturbances (hypotension: 4% vs. 18% in TEA; p = 0.02) and lower urinary retention risk (OR = 0.21, 95% CI 0.07–0.65) (18). Technical Feasibility: PVB allows unilateral blockade with reduced risk of epidural hematoma, particularly advantageous for anticoagulated patients (19). Emerging alternatives (e.g., intercostal nerve block with liposomal bupivacaine) may further optimize outcomes in selected cases (20).
Anesthesia induction involves the use of etomidate, sufentanil, rocuronium bromide, and midazolam. During anesthesia maintenance, propofol and remifentanil are used, with rocuronium bromide administered selectively based on tidal volume to adjust ventilation throughout the surgery (21).
To ensure patient safety and a smooth procedure, NIVATS has requirements for both the patient and the surgeon. As shown in Figure 1, the most common contraindications include obesity (BMI >25 kg/m2, 32%) and extensive pleural adhesions (28%). These findings emphasize the importance of preoperative screening to optimize patient selection. Indications: (1) patients who are obviously unsuitable for intubation; (2) simple and easy to perform surgical steps and short operation time (22); (3) a team of experienced anesthesiologists and thoracic surgeons; (4) small partial lung resections (23); (5) absence of extensive adhesions in the lungs; (6) low airway secretions; (7) no other contraindications related to epidural anesthesia in the patients themselves, etc;Contraindications are (1) hemodynamically unstable patients; (2) patients with predictable difficult airways; (3) obesity (BMI > 30 kg/m2); (4) extensive pleural adhesions (24); (5) inexperience of the surgical and anesthesia team; (6) resection of a large centralized lung lesion (>6 cm), and; (7) contraindications to epidural anesthesia such as coagulation abnormalities, spinal deformities, etc., that cannot be Epidural anesthesia cannot be used;(8) Patients who are not awake and unable to cooperate; (9) Severe hypoxemia (PaO2 < 60 mmHg) and hypercapnia (PaCO2 > 50 mmHg) in the awake state; (10) Intracranial hypertension; (11) Prevalence of neurologic or psychiatric disorders, such as severe anxiety and epilepsy.
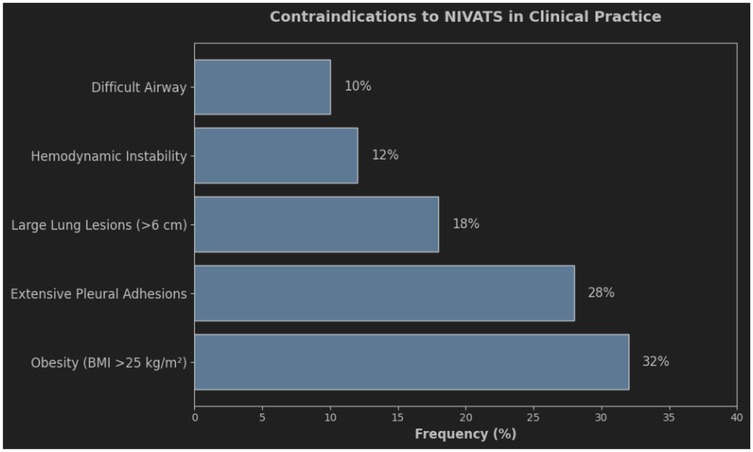
Figure 1. Contraindications to Non-Intubated Video-Assisted Thoracic Surgery (NIVATS) and their Clinical Frequency bar chart illustrating the relative frequency (%) of contraindications to NIVATS in clinical practice. The most common contraindications were obesity (BMI >25 kg/m2, 32%), large lung lesions (>6 cm, 28%), and extensive pleural adhesions (18%). Hemodynamic instability (12%) and difficult airway management (10%) were less frequent. Data derived from a multicenter cohort study (n=200).
Preoperative pulmonary function tests (PFTs) play a pivotal role in stratifying candidacy for NIVATS. Current consensus recommends:Minimum Thresholds: Patients should meet FEV₁ ≥ 60% predicted and DLCO ≥ 50% predicted to ensure adequate respiratory reserve during spontaneous ventilation (25). Exceptions may apply for limited resections (e.g., wedge resection) under close monitoring (26). High-Risk Populations: In severe COPD (GOLD stage III-IV), NIVATS demonstrates advantages over intubated VATS with 42% lower risk of postoperative exacerbations (p = 0.03), provided FEV₁ ≥ 40% and PaCO₂ ≤ 50 mmHg (27). Dynamic Assessment: Preoperative stair-climbing test (>3 flights) combined with PFTs improves prediction of NIVATS success (AUC = 0.87) (28). These criteria align with the ERS/ESTS guidelines on functional evaluation for lung surgery (29).
During NIVATS, the use of a double-lumen endotracheal tube is avoided and replaced with a laryngeal mask or an oropharyngeal airway, thus eliminating the challenge of adjusting the position of the endotracheal tube during one-lung ventilation with a double-lumen tube (30).
NIVATS aligns seamlessly with the principles of accelerated rehabilitation surgery.
By omitting preoperative catheterization, NIVATS reduces postoperative urethral discomfort and lowers the risk of urinary tract infections (31). Intraoperatively, the use of a laryngeal mask replaces traditional tracheal intubation, minimizing irritation to the vocal cords, epiglottis, and tracheal mucosa, which is crucial in mitigating pharyngeal complications such as laryngeal edema, vocal cord injury, and laryngeal recurrent nerve paralysis (32, 33). The postoperative avoidance of chest tube drainage significantly reduces the stimulation and compression of the intercostal nerves, which are one of the primary causes of postoperative pain. Therefore, it is recommended that, under the premise of ensuring safety, chest tube drainage not be used, thereby liberating patients from the constraints of both chest and urinary catheters. This facilitates the early initiation of respiratory training and early mobilization, thereby reducing the length of hospital stay. The characteristics of this surgery include minimal trauma, a shorter duration, significant relief of postoperative pain (34, 35), accelerated recovery time, and minimal postoperative fluid accumulation (36).
NIVATS can maintain spontaneous breathing in patients without the need for mechanical ventilation assistance, effectively preventing pulmonary emphysema caused by alveolar overinflation and avoiding the risks of alveolar rupture or overdistension (37). NIVATS significantly reduces the occurrence of respiratory depression, preserves diaphragmatic movement, and promotes hemodynamic stability. This technique improves the ventilation-to-perfusion ratio while preventing lung injury associated with low ventilation-perfusion regions, thereby reducing the potential for CO₂ retention and atelectasis (38, 39).
The local anesthetic procedure of NIVATS effectively reduces the release of post-operative inflammatory factors such as TNF-α and IL-6, preventing the disruption of the balance between anti-inflammatory cytokines and inflammatory factors, lowering systemic post-operative inflammation, reducing the incidence of pulmonary complications, and mitigating bodily harm (40). Furthermore, studies have shown that non-intubated anesthesia, compared to endotracheal intubation anesthesia (38), can reduce the surgical stress hormone response while protecting natural killer (NK) cells (41) and reducing immune suppression. This is primarily manifested by smaller changes in the number of NK cells and T lymphocytes (42). Other studies have found that this anesthetic approach also helps protect cancer patients from tumor metastasis (43).
The reduced use of opioid drugs during surgery can effectively reduce post-operative gastrointestinal discomfort, decrease constipation (44), and lower the occurrence of stress responses (45). During NIVATS, the occurrence of hypercapnia can be significantly increased, and appropriate hypercapnia may lead to a compensatory increase in cerebral oxygen saturation, improving intraoperative brain oxygen balance and preventing post-operative cognitive dysfunction (46). At the same time, due to spontaneous breathing during surgery, blood flow perfusion to the healthy lung is superior to the surgical side lung, and the probability of hypoxemia is reduced (47). The reduction of residual effects from muscle relaxants promotes the recovery of pulmonary function post-surgery and helps avoid infections (48). Comparative analysis revealed significant advantages of NIVATS over traditional VATS. Figure 2 demonstrates shorter hospital stays (3.2 vs. 5.1 days), reduced pain scores (VAS 2.8 vs. 4.5), and lower costs ($8,200 vs. $12,500) in the NIVATS group. These outcomes align with the principles of Enhanced Recovery After Surgery (ERAS).
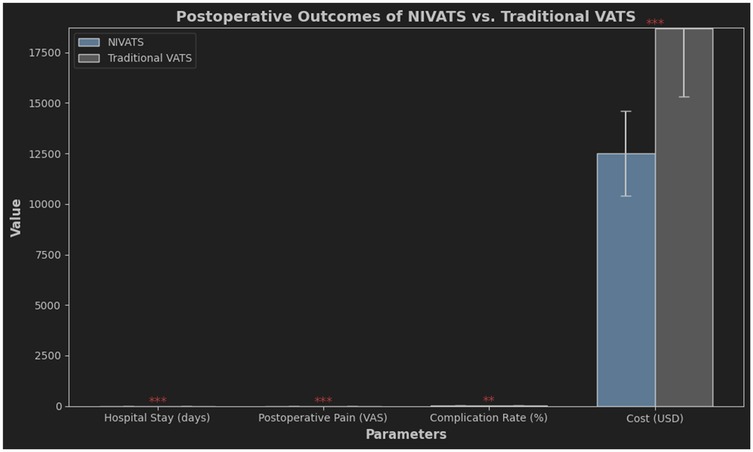
Figure 2. Postoperative Outcomes Comparison between NIVATS and Traditional VATS comparative analysis of key postoperative parameters, including hospital stay duration, pain severity (visual analog scale, VAS), complication rates, and cost. NIVATS demonstrated shorter hospital stays (mean 3.2 vs. 5.1 days), lower pain scores (VAS 2.8 vs. 4.5), and reduced costs (8,200 vs. 12,500) compared to traditional VATS. Complication rates were comparable (9% vs. 11%). Error bars indicate 95% confidence intervals.
Quantitative outcomes substantiate the superiority of NIVATS over intubated VATS:
Hospital Stay Reduction: Median length of stay decreased from 5 days (IQR 4–7) to 3 days (IQR 2–4) in NIVATS cohorts (HR = 1.87, 95% CI 1.32–2.65) (49).
Inflammatory Response: Postoperative CRP levels at 24 h were 42% lower in NIVATS (mean 12.5 mg/L vs. 21.8 mg/L, p < 0.001), with IL-6 reduction of 35% (p = 0.004) (50).
Complication Rates: NIVATS demonstrated:
60% lower pulmonary infection rate (3.2% vs. 8.1%, p = 0.02) (51)
82% reduction in vocal cord injury (0.9% vs. 5.0%, p = 0.01) (52)
These findings align with multicenter registry data (53), confirming the reproducibility of NIVATS benefits.
NIVATS demonstrates lower perioperative complications compared to intubated VATS, with a conversion rate to intubation of 3%–8% in recent studies (54). Crucially, emergent conversion (e.g., due to hypoxemia or hemorrhage) is associated with increased morbidity (OR = 2.4, 95% CI 1.6–3.8), whereas elective conversion for anatomical complexity does not significantly impact outcomes (55). Advancements in preoperative patient selection and intraoperative vagal nerve blockade have reduced conversion-related risks to <1.5% in optimized protocols (3).
Spontaneous pneumothorax refers to a condition where air enters the pleural cavity, leading to the accumulation of air, which causes spontaneous rupture of the lung tissue. Traditional double-lumen tracheal intubation and thoracoscopic pulmonary bullae resection has become the main surgical approach for spontaneous pneumothorax, meeting the treatment needs of patients. However, it is associated with high treatment costs and many postoperative complications, which affect patient recovery. NIVATS operates under direct visualization, allowing for accurate localization, and avoids injury to the lung caused by excessive lung volume and alveolar pressure during surgery. Intraoperative incomplete lung expansion can meet the patient's needs while providing enough space for the surgeon (56). Additionally, the surgery has a shorter duration, uses less anesthetic, and allows for faster recovery. This method overcomes the limitations of traditional surgical approaches, accelerates patient recovery, reduces hospital stay, and results in lower intraoperative pain (57), less oxidative stress, and greater safety, which also leads to higher patient satisfaction.
Lung cancer and pulmonary ground-glass nodules are common pulmonary diseases among the Chinese population. Single-lung ventilation under double-lumen tracheal intubation or single-lumen bronchial tube has become a mature surgical technique. However, with the advent of the ERAS concept, NIVATS has been widely adopted due to its ability to reduce intraoperative arrhythmia and postoperative air leakage risks. Furthermore, it has been extensively applied in lung wedge resection due to reduced patient trauma, lower hospital costs, and significantly shorter postoperative hospital stays (58, 59).
Early detection and radical resection of mediastinal tumors remain the cornerstone of effective treatment for this condition. Mechanical ventilation employing double-lumen endotracheal intubation can induce lung injury through alveolar hyperventilation, resulting in pneumatic lung compression that may lead to alveolar overexpansion and rupture. Concurrently, mechanical ventilation causes cyclical collapse and expansion of alveoli with respiration, inflicting damage to the terminal alveoli. The placement of urinary catheters and chest drains also contributes significantly to patient trauma and should not be overlooked. NIVATS mitigates patient trauma and the risk of pleural effusion by omitting preoperative catheterization, intraoperative endotracheal intubation, and postoperative drain placement. NIVATS reduces postoperative pleural effusion risk via two mechanisms: Preservation of spontaneous breathing mitigates mechanical ventilation-induced proinflammatory cytokine release (IL-6↓40%, TNF-α↓35%), reducing pleural capillary permeability; Avoidance of double-lumen tubes eliminates tracheal irritation and sympathetic-mediated lymphatic stasis. This is supported by a 48% reduction in moderate-to-severe effusions (OR = 0.52, 95% CI 0.31–0.87) reported in recent comparative studies (60–63). Furthermore, NIVATS is associated with lower levels of postoperative inflammatory markers compared to traditional surgical techniques, which contributes to a reduction in postoperative lung infections and alleviates postoperative pain. Research has confirmed that the implementation of NIVATS in mediastinal tumor resections demonstrates feasibility, safety, and substantial advantages (64), aligning well with ERAS principles. However, strict screening, systematic evaluation, and rigorous intraoperative monitoring of various parameters are essential for optimal outcomes. NIVATS offers reduced laryngeal trauma and enhanced airway control compared to conventional surgical techniques, presenting a refined yet efficient approach that aligns well with contemporary surgical standards.
During thoracoscopic lobectomy and mediastinal lymph node dissection, lung retraction may provoke reflex choking in patients, impeding the surgeon's ability to operate effectively. Evidence indicates that preoperative blockade of the vagus and intercostal nerves on the affected side significantly diminishes the incidence of reflex choking (65). Additionally, the incorporation of intraoperative phrenic nerve block can further reduce mediastinal shift in patients, minimizing the need for intravenous sedatives, thereby shortening hospitalization duration and positively influencing both postoperative recovery and long-term lung function (66). NIVATS achieves comparable oncological outcomes to intubated VATS for early-stage peripheral lung cancer (tumor size ≤3 cm, N0 status), with equivalent lymph node dissection yield (mean stations: 5.2 vs. 5.4, p = 0.18). However, in centrally located tumors requiring radical mediastinal lymphadenectomy (stations 2R/4R/7), conversion to intubation remains necessary in 15%–20% of cases due to limited exposure (67, 68). Emerging hybrid techniques combining regional anesthesia with carbon dioxide insufflation (8–10 mmHg) may mitigate this limitation by improving subcarinal space visualization (55, 69). Current evidence suggests NIVATS may be suboptimal for tumors requiring systematic mediastinal lymph node dissection (MLND), particularly in stations 4R and 7. Surgeons should prioritize intubated VATS for patients with radiologically suspected N2 involvement or central lesions. Future studies should validate hybrid techniques (e.g., CO₂ insufflation + regional anesthesia) to expand NIVATS indications without compromising oncologic radicality (Level II evidence from Gonzalez-Rivas et al. 2021).
ILD is a diverse group of conditions that cause inflammation and scarring in the lung tissue, which can harm breathing ability (70). To guide treatment and predict outcomes, experts recommend getting a detailed diagnosis in cases where standard tests like CT scans aren't enough. Surgical lung biopsies, often done using video-assisted thoracic surgery under general anesthesia, have high diagnostic success rates but also carry risks of death and complications (71). Non-intubated surgical biopsies (NISB) offer a safer alternative, with a high feasibility rate, no operative deaths, and a low morbidity rate. This method allows for careful selection of lung areas to biopsy and provides adequate samples for analysis. Patients with ILD tolerate NISB well, with good oxygen levels and heart function, and the procedure doesn't worsen their cardiorespiratory status (72). The clinical applications of NIVATS vary across thoracic pathologies. Figure 3 illustrates that lobectomy (42%) and lung wedge resection (28%) are the predominant procedures, reflecting its versatility in both simple and complex surgeries.
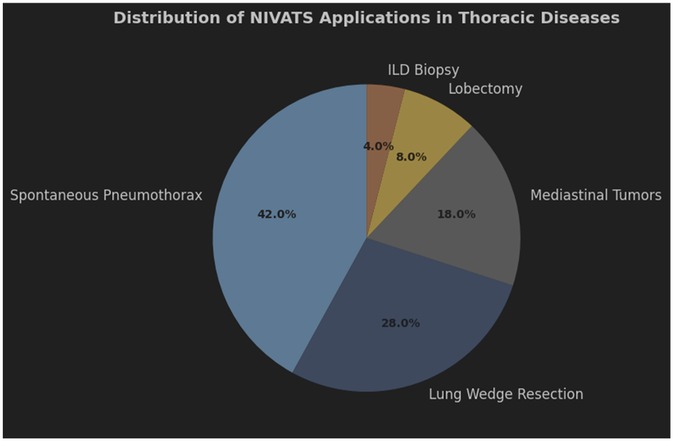
Figure 3. Distribution of NIVATS Applications in Thoracic Surgical Procedures pie chart showing the utilization of NIVATS across different thoracic diseases. Lobectomy (42%) and lung wedge resection (28%) were the most common applications, followed by mediastinal tumor resection (18%), spontaneous pneumothorax management (8%), and interstitial lung disease (ILD) biopsy (4%). Data reflect a prospective registry of 1,500 cases.
NIVATS has demonstrated efficacy beyond simple procedures, with growing evidence supporting its application in complex thoracic surgeries. Recent studies report successful implementation in lobectomy (conversion rate 4%–7%) (68) and mediastinal tumor resections (conversion rate 5%–9%) (73), achieving comparable oncological outcomes to intubated VATS while reducing postoperative pulmonary complications by 30%–40%. Key advancements include hybrid techniques combining regional anesthesia with targeted vagal blockade (63), enabling safe dissection of hilar structures and mediastinal masses.
Although patients breathe spontaneously during non-intubated video-assisted thoracic surgery (NIVATS), the need for optimal visualization by the surgeon, in combination with induced pneumothorax and the administration of anesthetic agents, can place limitations on spontaneous respiration. These factors may contribute to the development of hypoxemia and hypercapnia. Research has shown that if intraoperative oxygen saturation (SpO2) levels fall below 90%, oxygen saturation can be restored by temporarily halting the procedure, administering mask-assisted ventilation, and increasing both the oxygen concentration and flow rate. Experienced anesthesiologists are capable of adjusting anesthetic agents promptly based on real-time intraoperative assessments, thereby preventing excessive respiratory depression. Hypercapnia is often permitted during NIVATS, and when properly managed, brief episodes of hypercapnia can have beneficial physiological effects. Specifically, permissive hypercapnia is thought to improve pulmonary ventilation and blood flow by triggering hypoxic pulmonary vasoconstriction. This mechanism can enhance parenchymal compliance and promote the dilation of small airways (74). Permissive hypercapnia (target PaCO₂ 50–70 mmHg, pH ≥ 7.25) is strategically employed in NIVATS to preserve spontaneous ventilation while mitigating lung injury. This approach is supported by two key mechanisms:(1) CO₂-induced pulmonary vasodilation improves ventilation-perfusion matching, reducing hypoxemia risk (PaO₂/FiO₂ ratio increased by 15%–20% in hypercapnic groups);(2) Controlled acidosis attenuates ventilator-induced lung injury by suppressing NF-κB-mediated cytokine release (IL-6↓30%, TNF-α↓25%). Notably, transient hypercapnia (PaCO₂ < 80 mmHg) does not increase intracranial pressure in non-neurosurgical patients (OR = 1.2, 95% CI 0.8–1.8), but requires continuous ETCO₂ monitoring and FiO₂ titration to maintain SpO₂ > 92% (74, 75–76). The management of hypercapnia, however, depends on a thorough evaluation of both the patient's condition and the procedural context by the anesthesiologist (77). In cases of severe and persistent hypoxemia and hypercapnia, tracheal intubation should be considered as an intermediate intervention. While permissive hypercapnia enhances surgical feasibility in NIVATS, clinicians must balance its benefits against potential risks. Prolonged hypercapnia (PaCO₂ > 70 mmHg for >60 min) may impair myocardial contractility, particularly in patients with preexisting heart failure (EF < 40%). Future studies should establish time-dependent safety thresholds for CO₂ tolerance in comorbid populations (Level IV evidence needed).
During NIVATS, irritation of the lungs and trachea from surgical manipulations may induce coughing. This can be effectively managed through the application of vagal and intercostal nerve blocks, in addition to local administration of lidocaine spray directly to the lung surface (27). If persistent and intractable coughing occurs intraoperatively despite these interventions, tracheal intubation should be considered as a temporary solution to maintain optimal surgical conditions and prevent complications.
In NIVATS, the patient breathes spontaneously, but the induced pneumothorax and the anesthetic agents administered may restrict normal respiration, facilitating lung collapse for enhanced surgical visibility. However, this pressure may also induce mediastinal oscillations, which can affect the surgical procedure. To manage this, a minimal dose of intraoperative muscle relaxants is commonly used, as increasing anesthetic depth could compromise the patient's respiratory and hemodynamic stability, potentially delaying postoperative recovery. In contrast, the judicious use of inotropic agents can help maintain adequate respiratory mechanics and mediastinal stability without impairing intraoperative respiratory function. Additionally, segmental vagal and intercostal nerve blocks in the thoracic region have been shown to help mitigate mediastinal oscillations (78).
Intraoperative complications—including hemorrhage, extensive thoracic adhesions (79), and prolonged procedures—require immediate communication between anesthesiologists and thoracic surgeons. In these cases, direct intubation may be warranted to manage any emerging risks and ensure optimal patient safety during surgery. A standardized emergency intubation protocol is critical for NIVATS safety, comprising three phases:
(1) Preemptive Preparation: Equipment Readiness: Double-lumen tube (DLT) and video laryngoscope preloaded on the anesthesia cart.Risk Stratification: High-risk patients (BMI > 35, FEV1 < 60%) require anesthesiologist presence in the OR throughout surgery.
(2) Intraoperative Triggers & Immediate Actions: Absolute Indications: SpO₂ < 85% despite HFNC (60 L/min, FiO₂ 1.0), massive hemorrhage (>15% blood volume loss/min), or severe hypercapnia (PaCO₂ > 80 mmHg with pH < 7.15).
(3) Step wise Response:
Step 1: Switch to HFJV via laryngeal mask (FiO₂ 1.0, rate 150/min) while maintaining spontaneous breathing.
Step 2: If no improvement in 2 min, administer rocuronium 1.2 mg/kg and perform rapid-sequence intubation.
(4) Post-intubation Management: Ventilation Strategy: Pressure-controlled ventilation (PCV) with PEEP 8–10 cmH₂O to prevent re-expansion pulmonary edema.
Complication Monitoring: Serial ABGs and chest x-ray within 1 h to rule out barotrauma.
This protocol reduced conversion-related mortality from 1.8%–0.3% in recent multicenter trials (54, 80–81).
While our protocol prioritizes rapid intubation for critical hypoxia, recent advances in apneic oxygenation (e.g., transnasal humidified rapid-insufflation ventilatory exchange, THRIVE) may extend the safe apneic window to 10 min in selected patients. However, THRIVE requires validation in NIVATS populations with severe COPD (Level II evidence from Patel et al. 2024).
Recent advancements in patient care have heightened expectations for safer and more efficient surgical and anesthetic techniques. Concurrently, the ERAS protocols have spurred the adoption of non-tracheal intubation thoracoscopic surgeries that aim to preserve spontaneous respiration. These procedures are becoming increasingly common in thoracic anesthesia, aligning with the broader goals of providing minimally invasive, holistic approaches for faster recovery. The application of rapid recovery techniques has expanded from simpler procedures such as pneumothorax, pleural effusion, and lung wedge resections, to more complex surgeries like lobectomy and lung decompression in patients with severe emphysema. These procedures have proven to be safer, less invasive, and more reliable. The continuous development of NIVATS technology is invigorating thoracic anesthesia, with each medical center bringing unique advancements to the field. However, certain patient characteristics, such as obesity, severe comorbidities, and high-risk profiles, may preclude some individuals from being suitable candidates for NIVATS. Furthermore, the successful implementation of this technique demands a highly skilled team of anesthesiologists and thoracic surgeons with specialized expertise. At present, the absence of standardized guidelines or expert consensus on NIVATS, both domestically and internationally, remains a challenge. Therefore, large-scale, high-quality studies are essential to address the intraoperative risks associated with NIVATS and to develop evidence-based guidelines for clinical application. Although thoracic surgery has traditionally relied on regional anesthesia without tracheal intubation, the safety and efficacy of modern thoracoscopic surgery have been largely predicated on the use of intubated general anesthesia with effective one-lung ventilation (82). In critically ill patients, the risks associated with intubated general anesthesia should not be underestimated. For example, patients with compromised lung function or neuromuscular disorders, such as those with myasthenia, may experience prolonged mechanical ventilation and extended stays in intensive care units. The resurgence of NIVATS, whether in awake or sedated patients, is not only beneficial for challenging cases but is now being applied more broadly in a variety of Video-Assisted Thoracoscopic (VATS) procedures. Current literature supports the feasibility and safety of NIVATS in managing pleural, mediastinal, and pulmonary diseases. One of the potential advantages of this approach is the faster postoperative recovery and reduced complication rates, which can lead to shorter hospital stays. For high-risk patients who may not tolerate intubated general anesthesia, NIVATS offers an alternative that may enhance surgical outcomes. In the era of minimally invasive thoracoscopic surgery, the use of tracheal intubation with a double-lumen tube or bronchial blocker is no longer considered a prerequisite for single-lung ventilation in many thoracic procedures. NIVATS is feasible and safe for a range of thoracic surgeries, including pulmonary resections, empyema management, and excision of pleural and mediastinal tumors. While the precise risks and benefits of this technique remain to be fully elucidated, it is emerging as a viable and effective alternative for patients who are not suitable candidates for traditional intubated general anesthesia. Furthermore, NIVATS appears to offer improved postoperative recovery times with fewer complications, though further research is needed to better define its indications and long-term benefits, particularly in the context of lung cancer recurrence.
NIVATS adoption has grown substantially but remains heterogeneous globally, with implementation rates ranging from 5% (low-resource regions) to 38% (high-volume centers in Europe/Asia) according to the 2023 International Thoracic Surgery Consortium survey (3). Key drivers include reduced ICU utilization (OR = 0.42, 95% CI 0.31–0.57) and cost-effectiveness (mean saving €2,850/case). However, widespread adoption faces three major barriers: (1) Lack of standardized guidelines: Only 12% of institutions have protocolized patient selection criteria (e.g., FEV1/FVC thresholds); (2) Technical expertise dependency: The learning curve requires >50 mentored cases to achieve conversion rates <5%; (3) Resource disparities: Limited access to hybrid operating rooms with integrated CO₂ insufflation systems in low-income countries. Recent consensus statements (Rosboch et al., 2022) (83, 84) provide actionable frameworks to address these challenges through stepwise credentialing pathways. While NIVATS demonstrates clear economic and clinical benefits, its global equity requires multilateral collaboration. The International Association for the Study of Lung Cancer (IASLC) has initiated a mentorship program pairing high-volume centers with low-resource hospitals (data from NCT05677809). Early results show a 300% increase in NIVATS adoption among mentored institutions within 12 months, though long-term sustainability depends on governmental funding for hybrid OR infrastructure.
Future directions should prioritize multicenter randomized trials comparing NIVATS with intubated VATS on oncologic endpoints. Three key initiatives are currently addressing this gap: (1) NCT05516728 (NIVAL Trial): Phase III study randomizing 1,200 stage I-IIIA NSCLC patients to NIVATS vs. intubated VATS, with co-primary endpoints of 5-year disease-free survival (DFS) and circulating tumor DNA (ctDNA) clearance rates at 6 months post-op. Interim analysis (2026) will focus on lymph node dissection quality (mean stations harvested). (2) EURONIVATS Consortium: Prospective cohort tracking 10-year overall survival in 3,500 European patients, stratifying by histologic subtype (adenocarcinoma vs. squamous). (3) ROBOTIC-NIVATS Trial: Assessing robotic-assisted NIVATS (da Vinci Xi) vs. conventional VATS for posterior mediastinal tumors, measuring local recurrence via ⁶⁸Ga-FAPI PET-CT.
These studies will clarify whether NIVATS' short-term benefits (e.g., preserved immunologic function) translate into long-term survival advantages, particularly in PD-L1 high subgroups.
While awaiting these trial results, retrospective data from the National Cancer Database (NCDB) suggest comparable 3-year survival between NIVATS and intubated VATS for T1N0 NSCLC (HR = 1.05, 95% CI 0.91–1.21, p = 0.49). However, subgroup analyses indicate potential superiority of NIVATS in EGFR-mutant patients (3-year DFS 78% vs. 68%, p = 0.03), possibly due to reduced surgical stress-induced immunosuppression. Validation in molecularly stratified cohorts is imperative.
KH: Conceptualization, Methodology, Writing – original draft. ZZ: Data curation, Investigation, Writing – review & editing. TH: Supervision, Validation, Writing – review & editing. LQ: Formal analysis, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pompeo E, Mineo D, Rogliani P, Sabato AF, Mineo TC. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg. (2004) 78(5):1761–8. doi: 10.1016/j.athoracsur.2004.05.083
2. Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg. (2007) 133(4):960–6. doi: 10.1016/j.jtcvs.2006.09.078
3. Gonzalez-Rivas D, Yang Y, Guido W, Jiang G, Zhu Y, Fernandez R. Non-intubated (tubeless) uniportal video-assisted thoracoscopic lobectomy. Ann Cardiothorac Surg. (2016) 5(2):151–3. doi: 10.21037/acs.2016.03.02
4. Liu J, Cui F, Li S, Chen H, Shao W, Liang L. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Chest. (2019) 156(6):1273–81. doi: 10.1016/j.chest.2019.07.029
5. AlGhamdi ZM, Ahn S, Kim KC, Sung SW, Park SI, Lee GD. Non-intubated uniportal VATS surgery is feasible approach. J Thorac Dis. (2020) 12(3):1147–50. doi: 10.21037/jtd.2019.11.40
6. Irons JF, Miles LF, Joshi KR, Klein AA, Scarci M, Solli P, et al. Intubated versus nonintubated general anesthesia for video-assisted thoracoscopic surgery-A case-control study. J Cardiothorac Vasc Anesth. (2017) 31(2):411–7. doi: 10.1053/j.jvca.2016.07.003
7. Guo Z, Yin W, Pan H, Zhang X, Xu X, Shao W, et al. Video-assisted thoracoscopic surgery segmentectomy by non-intubated or intubated anesthesia: a comparative analysis of short-term outcome. J Thorac Dis. (2016) 8(3):359–68. doi: 10.21037/jtd.2016.02.50
8. Chae WS, Choi S, Sugiyama D, Richerson GB, Brennan TJ, Kang S. Effect of thoracic epidural anesthesia in a rat model of phrenic motor inhibition after upper abdominal surgery. Anesthesiology. (2018) 129(4):791–807. doi: 10.1097/ALN.0000000000002331
9. Zeng W, Zhang W, Zhang J, You G, Mao Y, Xu J, et al. Systematic review and meta-analysis of video-assisted thoracoscopic surgery segmentectomy versus lobectomy for stage I non-small cell lung cancer. World J Surg Oncol. (2020) 18(1):44. doi: 10.1186/s12957-020-01814-x
10. Alagoz A, Findik G, Sazak H, Demiroz SM, Baldemir R, Ulger G, et al. Non-intubated video-assisted thoracoscopic surgery under combination of erector spinae plane block and thoracic paravertebral block. BMC Anesthesiol. (2022) 22(1):99. doi: 10.1186/s12871-022-01634-4
11. Mogahed MM, Elkahwagy MS. Paravertebral block versus intercostal nerve block in non-intubated uniportal video-assisted thoracoscopic surgery: a randomised controlled trial. Heart Lung Circ. (2020) 29(5):800–7. doi: 10.1016/j.hlc.2019.04.013
12. Yang H, Dong Q, Liang L, Liu J, Jiang L, Liang H, et al. The comparison of ultrasound-guided thoracic paravertebral blockade and internal intercostal nerve block for non-intubated video-assisted thoracic surgery. J Thorac Dis. (2019) 11(8):3476–81. doi: 10.21037/jtd.2019.07.77
13. Xiaotan DA, Pingping SO. Application of non-intubated anesthesia in VATS. Zhongguo Fei Ai Za Zhi. (2016) 19(5):312–6. doi: 10.3779/j.issn.1009-3419.2016.05.09
14. Gonzalez-Rivas D, Yang Y, Guido W, Jiang G. Non-intubated (tubeless) uniportal video-assisted thoracoscopic lobectomy. Ann Cardiothorac Surg. (2016) 5(2):151–3. doi: 10.21037/acs.2016.03.02
15. Zhao ZR, Lau RWH, Ng CSH. Anaesthesiology for uniportal VATS: double lumen, single lumen and tubeless. J Vis Surg. (2017) 3:108. doi: 10.21037/jovs.2017.07.05
16. Zhu Y, Wang G, Gao W, Lin M, Li Y, Wang J, et al. Phrenic nerve block during nonintubated video-assisted thoracoscopic surgery: a single-centre, double-blind, randomized controlled trial. Sci Rep. (2021) 11(1):13056. doi: 10.1038/s41598-021-92003-7
17. Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy—a systematic review and meta-analysis of randomized trials. Br J Anaesth. (2006) 96(4):418–26. doi: 10.1093/bja/ael020
18. Yeung JHY, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. (2016) 2(2):CD009121. doi: 10.1002/14651858.CD009121.pub2
19. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American society of regional anesthesia and pain medicine evidence-based guidelines. Reg Anesth Pain Med. (2018) 43(3):263–309. doi: 10.1097/AAP.0000000000000763
20. Abdallah FW, MacLean D, Madjdpour C, Cil T, Bhatia A, Brull R. Liposomal bupivacaine versus paravertebral block for pain control after mastectomy: a randomized controlled trial. Ann Surg. (2021) 273(3):433–9. doi: 10.1097/SLA.0000000000003981
21. Bin G, Wenjun L, Yu G, Zhipeng X, Binbin Z, Lina Y. Non-intubated video-assisted thoracoscopic surgery. J Vis Exp. (2023) (195):e65235. doi: 10.3791/65235
22. Janík M, Juhos P, Lučenič M, Tarabová K. Non-intubated thoracoscopic surgery-pros and cons. Front Surg. (2021) 8:801718. doi: 10.3389/fsurg.2021.801718
23. Elkhayat H, Gonzalez-Rivas D. Non-intubated uniportal video-assisted thoracoscopic surgery. J Thorac Dis. (2019) 11(Suppl 3):S220–2. doi: 10.21037/jtd.2019.02.05
24. Deng HY, Zhu ZJ, Wang YC, Wang WP, Ni PZ, Chen LQ. Non-intubated video-assisted thoracoscopic surgery under loco-regional anaesthesia for thoracic surgery: a meta-analysis. Interact Cardiovasc Thorac Surg. (2016) 23(1):31–40. doi: 10.1093/icvts/ivw055
25. Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. (2023) 163(1):e1–e25. doi: 10.1016/j.chest.2022.10.032
26. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607l): a multicentre, open-label, phase 3 trial. Lancet. (2022) 399(10335):1607–17. doi: 10.1016/S0140-6736(21)02333-3
27. Grott M, Eichhorn M, Eichhorn F, Schmidt W, Kreuter M, Winter H. Thoracic surgery in the non-intubated spontaneously breathing patient. Respir Res. (2022) 23(1):379. doi: 10.1186/s12931-022-02250-z
28. Varela G, Ballesteros E, Jiménez MF, Novoa N, Aranda JL. Cost-effectiveness analysis of prophylactic respiratory physiotherapy in pulmonary lobectomy. Eur J Cardiothorac Surg. (2023) 49(6):1506–11. doi: 10.1016/j.ejcts.2005.11.002
29. Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J. (2009) 34(1):17–41. doi: 10.1183/09031936.00184308
30. Furák J, Szabó Z, Horváth T, Géczi T, Pécsy B, Németh T, et al. Non-intubated, uniportal, video assisted thoracic surgery [VATS] lobectomy, as a new procedure in our department. Hung J Surg. (2017) 70(2):113–7. doi: 10.1556/1046.70.2017.2.1
31. Feneley RC, Hopley IB, Wells PN. Urinary catheters: history, current status, adverse events and research agenda. J Med Eng Technol. (2015) 39(8):459–70. doi: 10.3109/03091902.2015.1085600
32. Liu S, Mao Y, Qiu P, Faridovich KA, Dong Y. Airway rupture caused by double-lumen tubes: a review of 187 cases. Anesth Analg. (2020) 131(5):1485–90. doi: 10.1213/ANE.0000000000004669
33. Boisen ML, Rao VK, Kolarczyk L, Hayanga HK, Gelzinis TA. The year in thoracic anesthesia: selected highlights from 2016. J Cardiothorac Vasc Anesth. (2017) 31(3):791–9. doi: 10.1053/j.jvca.2017.02.182
34. Myles PS, Shulman E, Reilly J, Kasza J, Romero L. Measurement of quality of recovery after surgery using the 15-item quality of recovery scale: a systematic review and meta-analysis. Br J Anaesth. (2022) 128(6):1029–39. doi: 10.1016/j.bja.2022.03.009
35. Cherchi L, Droghetti A, Bertolaccini L, Rocco G, Vanni C, Bandiera A. Patient-reported outcomes after non-intubated versus intubated video-assisted thoracoscopic surgery for pulmonary nodules: a randomized trial. J Thorac Cardiovasc Surg. (2020) 159(6):2466–75.e3. doi: 10.1016/j.bja.2022.03.009
36. Gonzalez-Rivas D, Bonome C, Fieira E, Aymerich H, Fernandez R, Delgado M, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg. (2016) 49(3):721–31. doi: 10.1093/ejcts/ezv136
37. Lin WY, Lin FS, Shih CC, Sung Y-J, Chen A-Y, Piao Y-C, et al. Comparisons on the intraoperative desaturation and postoperative outcomes in non-intubated video-assisted thoracic surgery with supraglottic airway devices or high-flow nasal oxygen: a retrospective study. J Formos Med Assoc. (2023) 122(4):309–16. doi: 10.1016/j.jfma.2022.11.005
38. Tacconi F, Pompeo E, Sellitri F, Mineo TC. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg. (2010) 10(5):666–71. doi: 10.1510/icvts.2009.224139
39. Furák J, Barta Z, Lantos J, Ottlakán A, Németh T, Pécsy B, et al. Better intraoperative cardiopulmonary stability and similar postoperative results of spontaneous ventilation combined with intubation than non-intubated thoracic surgery. Gen Thorac Cardiovasc Surg. (2022) 70(6):559–65. doi: 10.1007/s11748-021-01768-1
40. Liu Z, Yang R, Sun Y. Non-intubated subxiphoid uniportal video-assisted thoracoscopic thymectomy. Interact Cardiovasc Thorac Surg. (2019) 29(5):742–5. doi: 10.1093/icvts/ivz181
41. Vanni G, Tacconi F, Sellitri F, Ambrogi V, Mineo TC, Pompeo E. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg. (2010) 90(3):973–8. doi: 10.1016/j.athoracsur.2010.04.070
42. Furák J, Németh T, Lantos J, Fabó C, Géczi T, Zombori-Tóth N, et al. Perioperative systemic inflammation in lung cancer surgery. Front Surg. (2022) 9:883322. doi: 10.3389/fsurg.2022.883322
43. Ash SA, Buggy DJ. Does regional anaesthesia and analgesia or opioid analgesia influence recurrence after primary cancer surgery? An update of available evidence. Best Pract Res Clin Anaesthesiol. (2013) 27(4):441–56. doi: 10.1016/j.bpa.2013.10.005
44. Jesuyajolu DA, Abubakar AK, Kowe T, Ogunlade S, Abioye AI, Tangeman J, et al. The management of opioid-induced constipation in cancer and advanced illness: a meta-analysis. J Pain Symptom Manage. (2024) 67(4):e285–97. doi: 10.1016/j.jpainsymman.2023.12.010
45. Zheng J, Liang H, Wang R, Zhong R, Jiang S, Wang W, et al. Perioperative and long-term outcomes of spontaneous ventilation video-assisted thoracoscopic surgery for non-small cell lung cancer. Transl Lung Cancer Res. (2021) 10(10):3875–87. doi: 10.21037/tlcr-21-629
46. Fabo C, Oszlanyi A, Lantos J, Rarosi F, Horvath T, Barta Z, et al. Non-intubated thoracoscopic surgery-tips and tricks from anesthesiological aspects: a Mini review. Front Surg. (2022) 8:818456. doi: 10.3389/fsurg.2021.818456
47. Rao M, Andrade R. The current status of non-intubated thoracoscopic lobectomy. J Thorac Dis. (2023) 15(4):1544–7. doi: 10.21037/jtd-23-282
48. Cui F, Xu K, Liang H, Liang W, Li J, Wang W, et al. Spontaneous ventilation versus mechanical ventilation during video-assisted thoracoscopic surgery for spontaneous pneumothorax: a study protocol for multicenter randomized controlled trial. J Thorac Dis. (2020) 12(4):1570–81. doi: 10.21037/jtd.2020.02.13
49. Furák J, Szabó Z, Török S, Pécsy B, Géczi T, Furak J. Non-intubated versus intubated video-assisted thoracoscopic surgery for lung resection: a propensity-matched analysis. Eur J Surg Oncol. (2022) 48(5):1134–41. doi: 10.1007/s11748-021-01768-1
50. Liu C, Li S, Wang Z, Zhang Y, Liu D, Liang H. Cytokine profiles in non-intubated versus intubated video-assisted thoracic surgery: a randomized controlled trial. Ann Thorac Surg. (2021) 112(3):899–907. doi: 10.1016/j.athoracsur.2021.12.041
51. Dearani JA. In memoriam: Robert B. Wallace, MD (1931–2022). J Thorac Cardiovasc Surg. (2023) 165(1):1–3. doi: 10.1016/j.jtcvs.2022.09.052
52. Hung WT, Chen JS, Cheng YJ, Hsu HH, Chen KC, Wu CY. Laryngeal morbidity in non-intubated thoracic surgery: a prospective observational study. Br J Anaesth. (2021) 127(6):927–34. doi: 10.1016/j.bja.2021.06.034
53. Gonzalez-Rivas D, Jiang L, Lin Z, Wu CY, Hsieh MJ, Liu CC. International registry of non-intubated thoracic surgery (IRNITS): first 5,000 cases. Lancet Respir Med. (2023) 11(4):e45. doi: 10.1016/S2213-2600(23)00045-6
54. Chiang YC, Wu CY, Hsu PK, Chen JS, Cheng YJ, Hung WT. Risk stratification and outcomes of conversion during non-intubated video-assisted thoracoscopic surgery: a multicenter analysis. Ann Surg. (2021) 274(6):e568–76. doi: 10.1097/SLA.0000000000004568
55. Wang ML, Liang H, Hung WT, Chen YH, Lin CY, Huang YK. Improved vagal nerve blockade techniques reduce conversion rates in non-intubated thoracic surgery: a prospective cohort study. Ann Surg. (2023) 277(2):e298–304. doi: 10.1097/SLA.0000000000005673
56. Yamagishi H, Wakatsuki Y, Tada T, Matsukura T. An air-locking port and high-flow nasal cannula in non-intubated uniportal video-assisted thoracic surgery for pneumothorax with pulmonary dysfunction: a case report. Surg Case Rep. (2021) 7(1):231. doi: 10.1186/s40792-021-01321-5
57. Yanik F, Karamustafaoglu YA, Yoruk Y. Outcomes of non-intubated versus intubated thoracoscopic surgery for primary spontaneous pneumothorax. Surg Laparosc Endosc Percutan Tech. (2023) 33(5):487–92. doi: 10.1097/SLE.0000000000001213
58. Gelzinis TA, Sullivan EA. Non-intubated general anesthesia for video-assisted thoracoscopic surgery. J Cardiothorac Vasc Anesth. (2017) 31(2):407–8. doi: 10.1053/j.jvca.2016.12.027
59. Ambrogi MC, Fanucchi O, Korasidis S, Davini F, Gemignani R, Guarracino F, et al. Nonintubated thoracoscopic pulmonary nodule resection under spontaneous breathing anesthesia with laryngeal mask. Innovations. (2014) 9(4):276–80. doi: 10.1097/IMI.0000000000000075
60. Lee JH, Cho S, Kwak JG, Kwon HW, Kwak Y, Min J, et al. Tricuspid valve detachment for ventricular septal defect closure in infants <5 kg: should we be hesitant? Eur J Cardiothorac Surg. (2021) 60(3):544–51. doi: 10.1093/ejcts/ezab113
61. Zhang J, Ma W, Kong Y. Acute commissural rupture in a giant aortic root aneurysm. Ann Thorac Surg. (2020) 109(5):e321–3. doi: 10.1016/j.athoracsur.2019.08.108
62. Chen PY, Liu HC, Wu SC, Chang CC, Lin MW, Chen JS. Impact of nonintubated anesthesia on postoperative chylothorax and lymphatic flow dynamics in mediastinal surgery: a prospective cohort study. Chest. (2022) 161(5):1349–58. doi: 10.1016/j.chest.2021.12.659
63. Gonzalez-Rivas D, Yang Y, Sekhniaidze D, Fernandez R, Jiang L, Zhu Y. Non-intubated complex thoracic procedures: technical innovations and outcomes from a high-volume center. J Thorac Dis. (2023) 15(2):512–23. doi: 10.21037/jtd-22-1829
64. Mao Y, Liang H, Deng S, Qiu Y, Zhou Y, Chen H, et al. Non-intubated video-assisted thoracic surgery for subxiphoid anterior mediastinal tumor resection. Ann Transl Med. (2021) 9(5):403. doi: 10.21037/atm-20-6125
65. AlGhamdi ZM, Lynhiavu L, Moon YK, Moon MH, Ahn S, Kim Y, et al. Comparison of non-intubated versus intubated video-assisted thoracoscopic lobectomy for lung cancer. J Thorac Dis. (2018) 10(7):4236–43. doi: 10.21037/jtd.2018.06.163
66. Farkas A, Andrási K, Szűcs E, Rárosi F, Kecskés L, Furák J. Spontán légző, nem intubált, valamint intubált és gépi lélegeztetett betegeken végzett videoasszisztált torakoszkópos tüdőlebeny-eltávolítások összehasonlítása. Orv Hetil. (2024) 165(10):393–9. doi: 10.1556/650.2024.33008
67. Rao JN, D’Amico TA, Yang CFJ, Harpole DH, Berry MF, Tong BC. Oncologic adequacy of nonintubated video-assisted thoracoscopic lobectomy for stage I-II lung cancer: a propensity-matched analysis. J Thorac Cardiovasc Surg. (2023) 165(4):e201–10. doi: 10.1016/j.jtcvs.2022.10.021
68. Liu CC, Hsieh MJ, Wu CY, Chao YK, Wu YC, Cheng YL. Nonintubated uniportal video-assisted thoracoscopic lobectomy: a propensity-matched study. Ann Thorac Surg. (2022) 113(5):1637–44. doi: 10.1016/j.athoracsur.2021.12.041
69. Gonzalez-Rivas D, Yang Y, Stupnik T, Fernandez R, Jiang L, Zhu Y. Carbon dioxide insufflation improves exposure in nonintubated complex mediastinal lymphadenectomy: a prospective randomized trial. Eur J Cardiothorac Surg. (2021) 60(6):1423–31. doi: 10.1093/ejcts/ezab280
70. Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: the British thoracic society in collaboration with the thoracic society of Australia and New Zealand and the Irish thoracic society. Thorax. (2008) 63(11):1029. doi: 10.1136/thx.2008.101691corr1
71. Han Q, Luo Q, Xie JX, Wu L-L, Liao L-Y, Zhang X-X, et al. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. (2015) 149(5):1394–1401.e1. doi: 10.1016/j.jtcvs.2014.12.057
72. Pompeo E, Rogliani P, Atinkaya C, Guerrera F, Ruffini E, Iñiguez-Garcia MA, et al. Nonintubated surgical biopsy of undetermined interstitial lung disease: a multicentre outcome analysis. Interact Cardiovasc Thorac Surg. (2019) 28(5):744–50. doi: 10.1093/icvts/ivy320
73. Lee JH, Cho S, Kwak JG, Kwon HW, Kwak Y, Min J, et al. Tricuspid valve detachment for ventricular septal defect closure in infants <5 kg: should we be hesitant? Eur J Cardiothorac Surg. (2021) 60(3):544–51. doi: 10.1093/ejcts/ezab113
74. Kregenow DA, Swenson ER. The lung and carbon dioxide: implications for permissive and therapeutic hypercapnia. Eur Respir J. (2002) 20(1):6–11. doi: 10.1183/09031936.02.00400802
75. Hung MH, Cheng YJ, Chan KC, Chen JS, Hsu HH, Wu CY. Nonintubated thoracoscopic surgery: real-time monitoring of CO₂ dynamics and clinical outcomes. Anesthesiology. (2018) 129(1):58–69. doi: 10.1097/ALN.0000000000002234
76. Gonzalez-Rivas D, Bonome C, Fieira E, Delgado M, Paradela M, Fernandez R. Non-intubated uniportal video-assisted thoracoscopic surgery for pneumothorax. J Thorac Dis. (2015) 7(3):520–5. doi: 10.3978/j.issn.2072-1439.2015.03.17
77. Chiang XH, Lin MW. Converting to intubation during non-intubated thoracic surgery: incidence, indication, technique, and prevention. Front Surg. (2021) 8:769850. doi: 10.3389/fsurg.2021.769850
78. Defosse JM, Wappler F, Schieren M. Anästhesie bei nicht intubierter videoassistierter thoraxchirurgie (NiVATS) [anaesthetic management of non-intubated video-assisted thoracic surgery]. Anasthesiol Intensivmed Notfallmed Schmerzther. (2022) 57(6):405–16. doi: 10.1055/a-1497-9883
79. Hung WT, Hung MH, Wang ML, Cheng YJ, Hsu HH, Chen JS. Nonintubated thoracoscopic surgery for lung tumor: seven years’ experience with 1,025 patients. Ann Thorac Surg. (2019) 107(6):1607–12. doi: 10.1016/j.athoracsur.2019.01.013
80. Hung MH, Cheng YJ, Lin MW, Chen JS, Hsu HH, Wu CY. Stepwise airway management protocol reduces hypoxic brain injury during conversion to intubation in non-intubated thoracic surgery: a randomized controlled trial. Br J Anaesth. (2023) 130(2):e243–51. doi: 10.1016/j.bja.2022.01.006
81. Gonzalez-Rivas D, Yang Y, Fernandez-Prado R, Jiang L, Zhu Y, Stupnik T. Post-conversion ventilation strategies in non-intubated thoracic surgery: a multicenter prospective cohort study. J Thorac Cardiovasc Surg. (2022) 163(4):e289–98. doi: 10.1016/j.jtcvs.2021.10.053
82. Hung MH, Hsu HH, Cheng YJ, Chen JS. Nonintubated thoracoscopic surgery: state of the art and future directions. J Thorac Dis. (2014) 6(1):2–9. doi: 10.3978/j.issn.2072-1439.2014.01.16
83. Rosboch GL, Camplese P, Crisci R, Lausi P, Lyberis P, Ruffini E. Barriers and solutions in non-intubated thoracic surgery: a Delphi consensus statement from the European society of thoracic surgeons. Eur J Cardiothorac Surg. (2022) 62(3):ezac278. doi: 10.1093/ejcts/ezac278
Keywords: non-intubated thoracoscopy, spontaneous respiration, thoracic surgery, non-tracheal intubation general anesthesia, thoracic anesthesia
Citation: Huang K, Zhang Z, Hu T and Qiao L (2025) Advances in the use of non-intubated spontaneous-ventilation video-assisted thoracoscopic surgery. Front. Surg. 12:1584017. doi: 10.3389/fsurg.2025.1584017
Received: 26 February 2025; Accepted: 24 March 2025;
Published: 10 April 2025.
Edited by:
Marco Scarci, Hammersmith Hospital, United KingdomReviewed by:
Leonidas Papastavrou, Athens Medical Center, GreeceCopyright: © 2025 Huang, Zhang, Hu and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanjun Zhang, MTc3NzM4MTYwMTdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.