- 1National Demonstration Center for Experimental General Medicine Education, Xianning Medical College, Hubei University of Science and Technology, Xianning, China
- 2Department of Bone and Joint Surgery, (Guangxi Diabetic Foot Salvage Engineering Research Center), The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 3Department of Ultrasound Medicine, The Second Hospital Affiliated to Hubei University of Science and Technology, Xianning, China
Objective: This study aims to compare the effects of tibial cortex transverse transport (TTT) and platelet-rich plasma (PRP) on the healing of severe diabetic foot ulcers, evaluate the clinical efficacy of TTT, and explore its potential impact on lower limb circulation.
Methods: A retrospective analysis was conducted on two patient groups treated at our hospital between July 2019 and June 2022. One group underwent TTT, while the other received PRP therapy. Both groups had Wagner level 3 or higher ulcers. An 18-month follow-up was performed for both groups, during which we documented wound healing progress and healing times to assess clinical efficacy. To investigate lower limb blood flow recovery, lower limb arterial ultrasound was used to measure blood flow velocities in the affected popliteal and dorsalis pedis arteries. Additionally, ELISA was employed to measure the stromal cell-derived factor-1 (SDF-1) levels of angiogenic factors in peripheral blood.
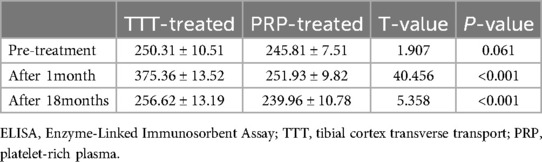
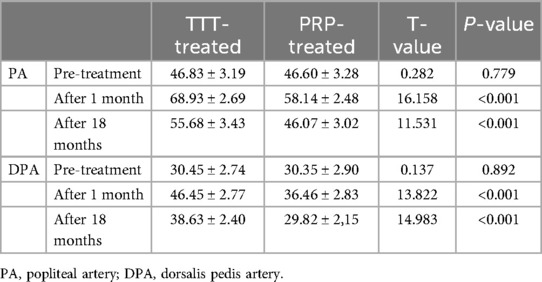
Results: A total of 60 diabetic foot ulcers (DFUs) patients were enrolled in our study, with 30 patients in each group: TTT-treated and PRP-treated. During the 18-month follow-up, the wound healing rate in the TTT-treated group was significantly higher than in the PRP-treated group [96.67% (29/30) vs. 80% (24/30), p < 0.05]. Furthermore, the healing time in the TTT-treated group was shorter (3.02 ± 0.84 vs. 6.04 ± 0.85 months, p < 0.001). The amputation rate [3.33% (1/30) vs. 20% (6/30), p < 0.05] and recurrence rate [6.67% (2/30) vs. 26.67% (8/30), p < 0.05] in the TTT-treated group were lower than those in the PRP-treated group. After 1 month and 18 months of treatment, the flow velocities in the popliteal artery (68.93 ± 2.69 vs. 58.14 ± 2.48 cm/s, p < 0.001; 55.68 ± 3.43 vs. 46.07 ± 3.02 cm/s, p < 0.001) and dorsalis pedis artery (46.45 ± 2.77 vs. 36.46 ± 2.83 cm/s, p < 0.001; 38.63 ± 2.40 vs. 29.82 ± 2.15 cm/s, p < 0.001) in the TTT-treated group were significantly higher than in the PRP-treated group. Additionally, the TTT-treated group showed higher levels of SDF-1 expression (375.36 ± 13.52 vs. 251.93 ± 9.82 pg/ml, p < 0.001; 256.62 ± 13.19 vs. 239.96 ± 10.78 pg/ml, p < 0.001).
Conclusion: Our results suggest that TTT treatment is more clinically effective than PRP for treating severe DFUs. This increased efficacy may be attributed to enhanced lower limb blood flow, which is potentially driven by elevated SDF-1 levels.
Introduction
Currently, the global population of individuals with diabetes is estimated at around 425 million, with approximately one-third expected to develop diabetic foot ulcers (DFUs) (1). Shockingly, one in five DFU patients faces the risk of amputation, a condition that significantly intensifies the burden on both families and society (2).
The factors contributing to the occurrence and progression of diabetic foot ulcers (DFUs) include not only physical factors, such as long-term hyperglycemia, neuropathy, and peripheral vascular disease, but also social and cultural factors (3–5). Clinical practice employs a variety of approaches to address diabetic foot complications, including conservative drug replacement, debridement, vascular reconstruction, tendon transposition, and skin coverage (6–10). Despite these interventions, outcomes often remain unsatisfactory, particularly in severe cases of diabetic foot, where secondary infections frequently arise, exacerbating the condition and leading to amputation in a significant number of patients (10). As a result, limb preservation remains a primary objective for both patients and healthcare practitioners.
As medical technology advances, an increasing number of treatment methods are being utilized to manage diabetic foot ulcers (DFUs). One such method, tibial cortex transverse transport (TTT), has proven to be an effective treatment for severe DFUs. A clinical study involving 136 patients with severe DFUs demonstrated that after two years of follow-up, the wound healing rate reached 96%, with a recurrence rate of only 2.9% (11). TTT, a surgical approach derived from the Ilizarov technique, is based on the core principle of continuously and gradually stretching bone tissue to stimulate both local and systemic regenerative potential, thereby promoting foot wound healing. Previous studies have suggested that TTT can enhance wound healing in DFUs by improving angiogenesis and reducing local inflammation (12–14). Compared to traditional surgical approaches, this innovative technique not only promotes wound healing and limb preservation in patients with persistent diabetic foot ulcers, but also exhibits minimal postoperative complications (11, 15).
In recent years, studies have shown that platelet-rich plasma (PRP) can promote collagen synthesis, the formation of new blood vessels, and the generation of fibrous and granulation tissue by releasing biologically active substances. This, in turn, stimulates wound tissue epithelialization and accelerates tissue regeneration and repair (16). The clinical application of PRP has been expanding, now encompassing bone and soft tissue repair, chronic refractory wounds (CRWs), facial rejuvenation, and other therapeutic areas (17, 18). Numerous clinical studies, both domestically and internationally, have confirmed the positive role of PRP in the treatment of chronic wounds (19, 20).
However, there are no clinical reports comparing the efficacy of TTT and PRP in treating diabetic foot ulcers (DFUs). In this study, we retrospectively compared the wound healing outcomes of 60 DFU patients who received either TTT or PRP treatment. Additionally, we assessed the expression of stromal cell-derived factor-1 (SDF-1) factors in their peripheral blood to evaluate the clinical efficacy of these two treatment strategies.
Methods
Study design
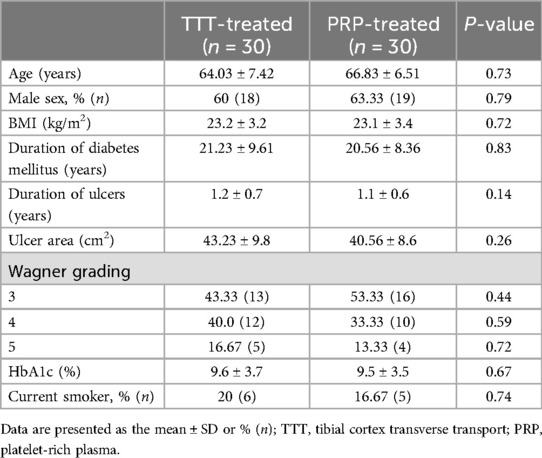
The study retrospectively analyzed 60 patients with ulcerations affecting the tendon, joint capsule, or bone, who were treated at our institution between July 2019 and June 2022. One group received tibial cortex transverse transport (TTT), while the other PRP treatment. The study was approved by the hospital's Ethics Committee, and informed consent was obtained from the patients and their families (Table 1).
Setting
Inclusion criteria were as follows: patients aged >18 years, diagnosed with type II diabetes according to the American Diabetes Association criteria (21), and with foot ulcers classified as severe (grade 3 or higher) diabetic foot ulcers based on the Wagner grading system (22).
Exclusion criteria included patients with lower extremity arterial ultrasound or computed tomography angiography (CTA) results suggesting popliteal artery outflow tract stenosis >85% or occlusion; patients with psychiatric disorders unable to cooperate with surgery; patients with recent cardiovascular or cerebrovascular events, or those at high risk thereof, who were unable to tolerate surgery. We maintained detailed records of the foot ulcer's location, duration, and complications. Preoperatively, we collected wound secretions for culture to identify pathogenic bacteria and assess their antibiotic susceptibility, thereby enabling the selection of appropriate antibiotics. If the foot ulcer was open, we performed a bone probe test and a flatfoot x-ray to determine the presence of diabetic foot osteomyelitis (23). We defined peripheral arterial hypoperfusion as the absence of a palpable dorsalis pedis and posterior tibial arteries, and/or an ankle-brachial index (ABI) < 0.9 (24). Preoperatively, we assessed lower extremity vascular status using CTA. If CTA indicated severe arterial stenosis (>50% diameter reduction) or arterial occlusion due to atherosclerosis, further evaluation by a vascular surgeon and potential revascularization were required (25).
Participants
The PRP-treated group received a PRP topical wound dressing following debridement, while the TTT-treated group underwent tibial cortex transverse transport (Figure 1). Both groups received comprehensive medical treatment to control diabetes mellitus, with prophylactic antibiotics administered intraoperatively and continued for 1 day postoperatively. Patients in the TTT-treated group underwent minimally invasive osteotomy in the operating room to create a bone fragment, and a specialized external fixator was placed under fluoroscopy.
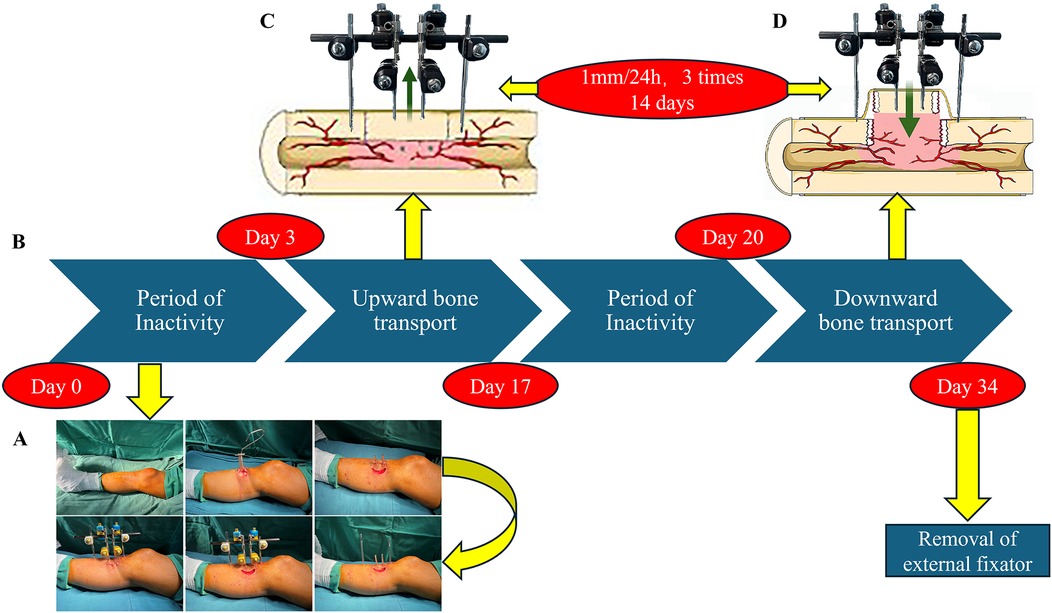
Figure 1. The TTT procedure and postoperative management. (A) TTT Surgical Steps: Initially, a fragment is excised from the medial side of the proximal tibia. This is followed by the placement of fixation pins at both ends and the center of the fragment, and the attachment of an external fixator. (B–D) Postoperative Process: After three days of immobilization, the patient undergoes 14 days of upward transport (C) and downward transport (D), interspersed with three days of rest. Finally, the external fixator is removed completely.
On postoperative day 3, bone transfer was initiated using the accordion technique, performed three times daily at regular intervals, resulting in a total outward transfer of 1 mm over 2 weeks (26) Lower limb x-rays were used to confirm whether the bone fragment had reached its highest point. Following a three-day consolidation period, the 14-day reverse transport phase began. Ultimately, the bone fragment returned to its original position, which was verified by x-ray. To prevent postoperative pin tract infections, we routinely applied 75% alcohol to the pin sites. After completing the transport process, the external fixator was removed.
Patients in the PRP-treated group had 30–60 ml of peripheral venous blood collected prior to surgical debridement for PRP extraction. The blood was placed into an anticoagulation tube and centrifuged at a radius of 15 cm at 3,600 rpm for 5 min. This process removed most of the lower layer of erythrocytes and leukocytes, while retaining the plasma and platelet layer. The plasma and platelet layer were then centrifuged again for 5 min, yielding 5–10 ml of PRP. Once the necrotic tissue in the foot was debrided, the prepared autologous PRP was directly applied to the wound, and a sterile dressing was then placed over it. Subsequently, sterile dressings were changed every 7 days and covered with PRP, while monitoring the wound healing process.
Follow up
All patients were followed up at the outpatient clinic at 4 and 12 weeks after surgery. Follow-up continued with monthly telephone calls for 18 months. The follow-up included an assessment of wound healing, recording healing time, recurrence of foot ulcers, amputation status, and any complications. In the TTT-treated group, complications included local fractures, pin tract infections, and issues arising from prolonged bed rest, such as pressure sores, pneumonia, and lower limb venous embolism. In the PRP-treated group, complications were primarily related to prolonged bed rest. To evaluate blood flow in the lower limbs, we performed lower limb arterial ultrasound to detect blood flow velocity in the affected side's popliteal and dorsalis pedis arteries. We collected peripheral venous blood preoperatively, one month postoperatively, and at the last follow-up, and assessed serum SDF-1 levels using Enzyme-Linked ImmunoSorbent Assay (ELISA). Recurrent ulcers were defined as the reoccurrence of ulcers at the same or neighboring site of a previously healed foot wound.
Enzyme-Linked ImmunoSorbent Assay
Venous blood was collected in a serum separator tube without anticoagulants and left at room temperature for 2 h. The blood was then centrifuged at 1,000 × g for 20 min to collect the serum. Serum samples and diluted standards were added to a pre-coated ELISA plate (Abcam, ab100637), followed by incubation, washing, addition of enzyme-conjugated secondary antibody, another washing step, and color development. Absorbance at each well was measured using a microplate reader, and the SDF-1 concentration was calculated from the standard curve.
Statistical analyses
All data were analyzed statistically using SPSS 26.0 software (Chicago, IL, USA). Continuous variables are presented as mean ± SD (for normal distribution) or median (P25, P75) (for non-normal distribution), while categorical variables are expressed as numbers and percentages. To compare the differences between two groups, the Student's t-test (for normal distribution), Mann–Whitney U test (for non-parametric variables), and chi-square test or Fisher's exact test (for categorical data) were used. Unless otherwise stated, all p-values were two-tailed, and a p-value of less than 0.05 was considered statistically significant.
Result
Observation of clinical efficacy
A total of 60 DFU patients were included in the study, with 30 patients in the TTT-treated group and 30 in the PRP-treated group. The baseline data of these patients are presented in Table 1. After 18 months of follow-up, the healing rate in the TTT-treated group was significantly higher than in the PRP-treated group [96.67% (29/30) vs. 80% (24/30), p < 0.05].
As illustrated in Figures 2, 3, DFU patients frequently present with deep tissue infections, which underscores the importance of thorough debridement in promoting wound healing. After undergoing TTT surgery, the patient's foot wound progressed through several stages: first, soft tissue necrosis, followed by the expansion of fresh granulation tissue, and eventually reepithelialization, all culminating in complete wound healing.
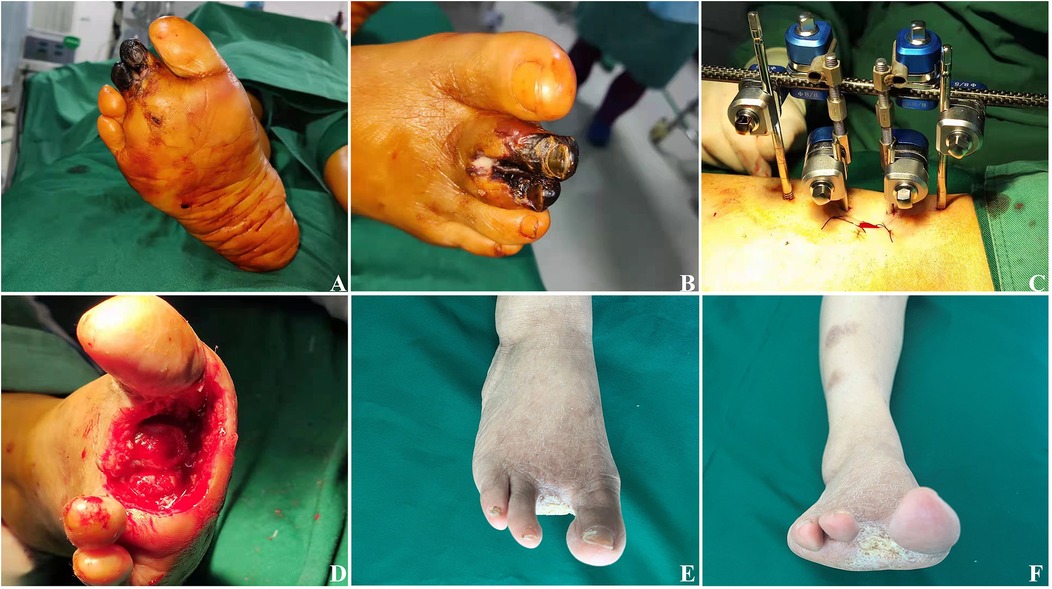
Figure 2. Effects of TTT surgery in a 68-year-old woman with severe and resistant plantar diabetic foot ulcer. (A,B) Preoperative images showing the ulcers, with evident necrosis of the second and third toes of the right foot. (C) An external fixator is placed on the tibia. (D) Intraoperative removal of the necrotic second and third toes, exposing the deep wound down to the cartilage surface. (E) After four weeks, the wound is nearly fully healed. (F) After six weeks, the original wound is completely healed.
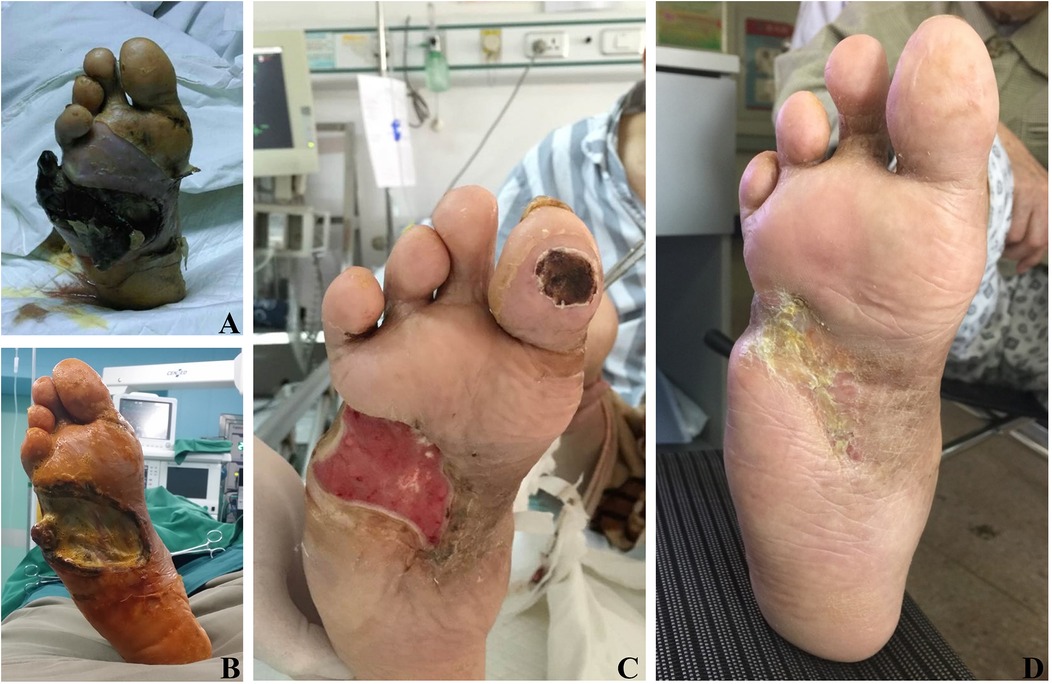
Figure 3. Effects of TTT surgery in a 75-year-old man with severe and resistant plantar diabetic foot ulcer. (A) Preoperative images showing gangrene of the fifth toe of the right foot and extensive tissue necrosis on the right plantar surface. (B) Ten days post-surgery, secondary tissue necrosis developed in the foot. (C) Four weeks post-surgery, healthy plantar granulation tissue is evident. (D) Eight weeks post-surgery, complete healing of the ulcers, demonstrating the effectiveness of TTT in promoting ulcer healing. Although secondary necrosis persists in the foot ulcer 10 days post-surgery, the wound continues to heal following reoperation and necrotic tissue removal.
Moreover, the average healing time in the TTT-treated group was shorter than in the PRP-treated group (3.02 ± 0.84 months vs. 6.04 ± 0.85 months, p < 0.001) (Figure 4). The TTT-treated group had only one patient who required amputation [3.33% (1/30)], while the PRP-treated group had six patients who underwent amputation [20% (6/30), p < 0.05]. Regarding foot ulcer recurrence, 2 patients in the TTT-treated group and 8 patients in the PRP-treated group experienced recurrence [6.67% (2/30) vs. 26.67% (8/30), p < 0.05]. Therefore, the TTT-treated group had lower amputation and ulcer recurrence rates compared to the PRP-treated group. Throughout the 18 months of follow-up, no treatment-related complications were observed in either group.
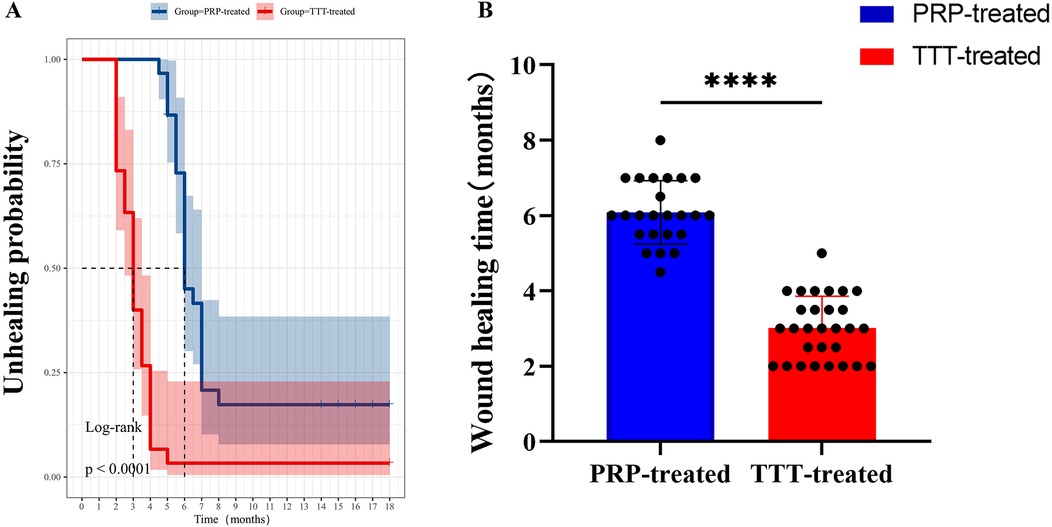
Figure 4. Comparison of wound healing times between the TTT-treated group and the PRP-treated group. (A) Kaplan–Meier curve analysis of the probability of healing in patients. (B) Comparison of foot healing times between the two groups of DFU patients. ****P < 0.0001.
Serum SDF-1
As shown in Figure 5A, the levels of SDF-1 were not significantly different between the two groups before treatment (P > 0.05). After treatment, the serum levels of SDF-1 in both groups increased significantly (P < 0.05), with the TTT-treated group showing significantly higher levels than the PRP-treated group (375.36 ± 13.52 vs. 251.93 ± 9.82 pg/ml, p < 0.001). At the last follow-up, the SDF-1 level in the TTT-treated group remained higher than in the PRP-treated group (256.62 ± 13.19 vs. 239.95 ± 10.78 pg/ml, p < 0.001) (Table 2).
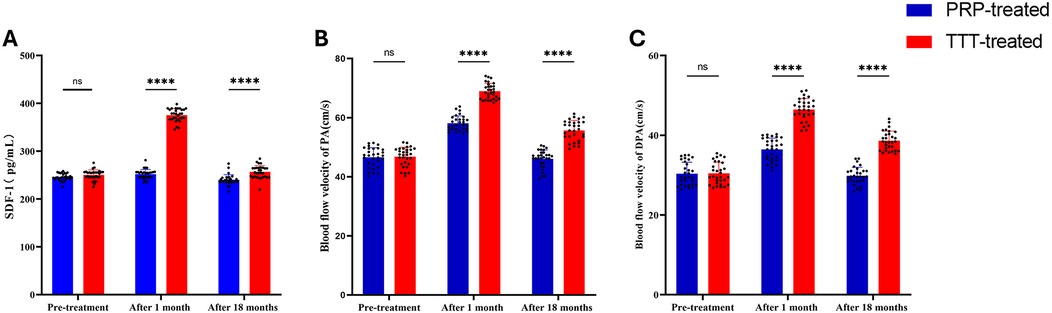
Figure 5. Comparison of preoperative and postoperative serum SDF-1 levels and lower limb arterial blood flow velocities between the TTT-treated and PRP-treated groups. (A) Serum SDF-1 concentrations. (B and C) Blood flow velocity in the popliteal artery (B) and dorsalis pedis artery (C) PA, popliteal artery; DPA, dorsalis pedis artery. NS, no significant difference. ****P < 0.0001.
Lower limb blood flow velocity
When we measured the blood flow velocity in the popliteal and dorsalis pedis arteries of both groups using a lower limb blood flow ultrasound system, no significant difference was found between the TTT-treated and PRP-treated groups before treatment (p > 0.05). After one month of treatment, the lower limb arterial blood flow velocity increased significantly in both groups, with the TTT-treated group showing faster velocities than the PRP-treated group (popliteal artery: 68.93 ± 2.69 vs. 58.14 ± 2.48 cm/s, p < 0.001; dorsalis pedis artery: 46.45 ± 2.77 vs. 36.46 ± 2.83 cm/s, p < 0.001). After 18 months of treatment, the lower limb blood flow velocity in both groups stabilized, but the TTT-treated group continued to exhibit faster blood flow than the PRP-treated group (popliteal artery: 55.68 ± 3.43 vs. 46.06 ± 3.02 cm/s, p < 0.05; dorsalis pedis artery: 38.63 ± 2.40 vs. 29.82 ± 2.15 cm/s, p < 0.05) (Figures 5B,C; Table 3).
Discussion
Recent studies indicate that TTT is an effective treatment for DFUs, promoting ulcer healing and reducing amputation rates, which aligns with our findings (11, 27, 28). Although TTT demonstrates significant effectiveness in treating DFUs, its wider adoption has been hindered by unclear mechanisms underlying its action.
In our study, one patient in the TTT-treated group experienced a foot wound that failed to heal properly, ultimately necessitating amputation. During our follow-up, however, the residual limb healed successfully. Upon further investigation, we found that the patient had inadequate blood sugar control post-surgery and began weight-bearing on the lower limb prematurely, which led to the wound's failure to heal. Therefore, proper postoperative care and patient compliance are critical factors in ensuring the successful healing of foot ulcers.
A study indicated that TTT applies the tension-stress law, stimulating tissue regeneration (29). In animal experiments, researchers discovered that during the healing process in the fracture distraction area, the regeneration of the microvascular network occurred prior to bone tissue healing, a finding confirmed by angiography (30). Although TTT involves the manipulation of tibial fragments, this process also promotes local microvascular regeneration, which generates numerous angiogenesis-promoting substances that may positively affect the distant ulcer tissue.
Stromal cell-derived factor-1 (SDF-1), also known as CXCL12, is the exclusive ligand for the hematopoietic receptor CXCR4 and acts as a chemotactic cytokine (31). SDF-1 plays a critical role in promoting cell migration, differentiation, and vascular formation and repair (32, 33). Research indicates that activation of the SDF-1/CXCR4 axis can enhance stem cell proliferation, differentiation, and survival, while also mediating stem cell homing behavior (34). In injured tissues, the upregulation of SDF-1 recruits stem cells or progenitor cells to the damaged area, facilitating tissue repair (35). As an angiogenesis-associated factor, the increase in SDF-1 is strongly correlated with angiogenesis. In our study, we observed a significant increase in SDF-1 levels in the peripheral blood of DFU patients following TTT, along with a noticeable rise in lower limb blood flow velocity, suggesting that TTT may promote wound healing by enhancing angiogenesis in the lower limbs. A clinical study found that after TTT treatment, SDF-1 levels in the peripheral blood of DFU patients continued to rise, positively influencing angiogenesis in foot ulcers, which aligns with our findings (36).
To verify the clinical efficacy of TTT, we used PRP as a reference, as PRP is also an effective treatment for DFUs. PRP is a platelet-rich plasma concentrate obtained by centrifuging autologous whole blood, first proposed by hematologists in the 1970s as a treatment for patients with thrombocytopenia (37). PRP contains various growth factors, including platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), and insulin-like growth factor 1 (IGF-1), which are released upon activation of PRP and help promote tissue repair and regeneration (38). With further research, the applications of PRP have expanded to areas such as facial trauma, skin diseases, and facial rejuvenation (39). Due to its strong regenerative properties, PRP has been applied in the treatment of DFUs, yielding good results and significantly improving the healing rate of DFUs (40, 41). Our study results demonstrate that while PRP is effective in treating DFUs, both in terms of healing rate and healing time, TTT outperforms PRP. This is because TTT induces small artificial injuries that activate the body's regenerative potential, stimulate the generation of microvessels in the lower limbs, and promote ulcer healing.
Limitations
This study has several limitations. First, this study was retrospective and involved a relatively small sample size, which may limit the generalizability of the results. Second, uncontrolled or unidentified confounding factors, such as the patient's age, gender, duration of illness, and presence of other comorbidities, may influence the effectiveness of the treatment. Third, the use of TTT and PRP varies across different medical institutions, potentially affecting the evaluation of treatment outcomes. Variations in standardization could account for differences in study results. Additionally, this study was not registered in the clinical trial registry for Randomized Controlled Trials (RCTs).
Conclusion
In conclusion, we found that TTT promotes the healing of DFUs, preserves limbs, and reduces the recurrence of severe diabetic foot ulcers. Additionally, the increase in peripheral blood SDF-1 levels and enhanced lower limb blood flow suggest that the mechanism of TTT may be linked to angiogenesis. These findings indicate that TTT is an effective treatment for severe DFUs. Although no significant surgical complications were observed, further large-scale clinical trials are needed to confirm the efficacy and safety of this procedure.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Hubei University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by Hubei University of Science and Technology. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
P-XZ: Data curation, Writing – original draft. H-JS: Data curation, Funding acquisition, Writing – original draft. S-JY: Formal Analysis, Validation, Writing – review & editing. XC: Investigation, Software, Writing – original draft. Z-ML: Conceptualization, Project administration, Writing – review & editing. S-NL: Funding acquisition, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TTT, tibial cortex transverse transport; PRP, platelet-rich plasma; DFUs, diabetic foot ulcers; SDF-1, stromal cell-derived factor-1; PA, popliteal artery; DPA, dorsalis pedis artery; ELISA, enzyme-linked immunosorbent assay.
References
1. Standl E, Khunti K, Hansen T, Schnell O. The global epidemics of diabetes in the 21st century: current situation and perspectives. Eur J Prev Cardiol. (2019) 26:7–14. doi: 10.1177/2047487319881021
2. Armstrong D, Boulton A, Bus S. Diabetic foot ulcers and their recurrence. N Engl J Med. (2017) 376:2367–75. doi: 10.1056/NEJMra1615439
3. Jeffcoate W, Boyko E, Game F, Cowled P, Senneville E, Fitridge R. Causes, prevention, and management of diabetes-related foot ulcers. Lancet Diabetes Endocrinol. (2024) 12:472–82. doi: 10.1016/S2213-8587(24)00110-4
4. Costa D, Ielapi N, Caprino F, Giannotta N, Sisinni A, Abramo A, et al. Social aspects of diabetic foot: a scoping review. Soc Sci. (2022) 11:149. doi: 10.3390/socsci11040149
5. Costa D, Gallelli G, Scalise E, Ielapi N, Bracale UM, Serra R. Socio-cultural aspects of diabetic foot: an ethnographic study and an integrated model proposal. Societies. (2024) 14:240. doi: 10.3390/soc14110240
6. Crews R, Candela J. Decreasing an offloading device’s size and offsetting its imposed limb-length discrepancy lead to improved comfort and gait. Diabetes Care. (2018) 41:1400–5. doi: 10.2337/dc17-2584
7. Bus S, van Deursen R, Armstrong D, Lewis J, Caravaggi C, Cavanagh P, et al. Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes-Metab Res Rev. (2016) 32:99–118. doi: 10.1002/dmrr.2702
8. Tamir E, Vigler M, Avisar E, Finestone A. Percutaneous tenotomy for the treatment of diabetic toe ulcers. Foot Ankle Int. (2014) 35:38–43. doi: 10.1177/1071100713509604
9. Greenman R, Panasyuk S, Wang X, Lyons T, Dinh T, Longoria L, et al. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet. (2005) 366:1711–7. doi: 10.1016/S0140-6736(05)67696-9
10. Suh H, Oh T, Hong J. Innovations in diabetic foot reconstruction using supermicrosurgery. Diabetes-Metab Res Rev. (2016) 32:275–80. doi: 10.1002/dmrr.2755
11. Chen Y, Kuang X, Zhou J, Zhen P, Zeng Z, Lin Z, et al. Proximal tibial cortex transverse distraction facilitating healing and limb salvage in severe and recalcitrant diabetic foot ulcers. Clin Orthop Relat Res. (2020) 478:836–51. doi: 10.1097/CORR.0000000000001075
12. Yang S, Pan K, Hua Q, Su H, Hou J, Liu K, et al. Correlation analysis of patients with diabetic foot ulcers treated with tibial cortex transverse transport surgery and platelet-to-lymphocyte ratio and monocyte-to-neutrophil ratio. Adv Clin Exp Med. (2024) 34. doi: 10.17219/acem/187765
13. Liu J, Huang X, Su H, Yu J, Nie X, Liu K, et al. Tibial cortex transverse transport facilitates severe diabetic foot wound healing via HIF-1α-induced angiogenesis. J Inflamm Res. (2024) 17:2681–96. doi: 10.2147/JIR.S456590
14. Yang Y, Li Y, Pan Q, Bai S, Wang H, Pan X, et al. Tibial cortex transverse transport accelerates wound healing via enhanced angiogenesis and immunomodulation. Bone Joint Res. (2022) 11:189–99. doi: 10.1302/2046-3758.114.BJR-2021-0364.R1
15. Yuan T, Zhang C, Zeng B. Treatment of chronic femoral osteomyelitis with platelet-rich plasma (PRP): a case report. Transfus Apher Sci. (2008) 38:167–73. doi: 10.1016/j.transci.2008.01.006
16. Verma R, Kumar S, Garg P, Verma Y. Platelet-rich plasma: a comparative and economical therapy for wound healing and tissue regeneration. Cell Tissue Bank. (2023) 24:285–306. doi: 10.1007/s10561-022-10039-z
17. Zhang Y, Xing F, Luo R, Duan X. Platelet-rich plasma for bone fracture treatment: a systematic review of current evidence in preclinical and clinical studies. Front Med (Lausanne). (2021) 8. doi: 10.3389/fmed.2021.676033
18. Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. (2018) 11:624–34. doi: 10.1007/s12178-018-9527-7
19. Huang H, Sun X, Zhao Y. Platelet-rich plasma for the treatment of burn wounds: a meta-analysis of randomized controlled trials. Transfus Apher Sci. (2021) 60. doi: 10.1016/j.transci.2020.102964
20. Peng Y, Wang J, Liu X, Zhou Y, Jia S, Xu J, et al. Efficacy of platelet-rich plasma in the treatment of diabetic foot ulcers: a systematic review and meta-analysis. Ann Vasc Surg. (2024) 98:365–73. doi: 10.1016/j.avsg.2023.05.045
21. American Diabetes Association. Executive summary: standards of medical care in diabetes-2009 (vol 32, suppl 1, pg S6, 2009). Diabetes Care. (2009) 32:754. doi: 10.2337/dc09-er04a
22. Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. (1981) 2:64–122. doi: 10.1177/107110078100200202
23. Lozano R, Fernández M, Hernández D, Montesinos J, Jiménez S, Jurado M. Validating the probe-to-bone test and other tests for diagnosing chronic osteomyelitis in the diabetic foot. Diabetes Care. (2010) 33:2140–5. doi: 10.2337/dc09-2309
24. Potier L, Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial Index in patients with diabetes. Eur J Vasc Endovasc Surg. (2011) 41:110–6. doi: 10.1016/j.ejvs.2010.09.020
25. Gerhard-Herman M, Gardin JM, Jaff M, Mohler E, Roman M, Naqvi TZ, et al. Guidelines for noninvasive vascular laboratory testing: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr. (2006) 19(8):955–72. doi: 10.1016/j.echo.2006.04.019
26. Fu R, Feng Y, Liu Y, Gao X, Bertrand D, Du T, et al. Effect of the accordion technique on bone regeneration during distraction osteogenesis: a computational study. Comput Methods Programs Biomed. (2022) 227. doi: 10.1016/j.cmpb.2022.107232
27. Jianda X, Maosheng B, Chenjian P, Xiaojing Y, Changhui W, Junhao L, et al. An novel and alternative treatment method for large heel ulceration in diabetic patients: proximal tibial cortex transverse distraction. Int Wound J. (2023) 20:732–9. doi: 10.1111/iwj.13916
28. Ding Y, Yu D, Huang H, Peng X, Yang S, Lin Z, et al. Combining tibial cortex transverse transport (TTT) and endovascular therapy (EVT) for limb salvage in chronic limb-threatening ischemia. Orthop Surg. (2024) 16:2132–9. doi: 10.1111/os.14222
29. Ferguson J, Sutherland M, Pandit H, McNally M. The rate of symptomatic venous thromboembolism in patients undergoing elective ilizarov surgery and the cost of chemical prophylaxis. Bone Joint J. (2014) 96B:426–30. doi: 10.1302/0301-620X.96B3.32939
30. Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. (1989):263–85.2912628
31. Li J, Chen H, Zhang D, Xie J, Zhou X. The role of stromal cell-derived factor 1 on cartilage development and disease. Osteoarthritis Cartilage. (2021) 29:313–22. doi: 10.1016/j.joca.2020.10.010
32. Gilbert W, Bragg R, Elmansi AM, McGee-Lawrence ME, Isales CM, Hamrick MW, et al. Stromal cell-derived factor-1 (CXCL12) and its role in bone and muscle biology. Cytokine. (2019) 123:154783. doi: 10.1016/j.cyto.2019.154783
33. Li J, Li Y, Huang D, Yao M. Role of stromal cell-derived factor-1 in endothelial progenitor cell-mediated vascular repair and regeneration. Tissue Eng Regen Med. (2021) 18:747–58. doi: 10.1007/s13770-021-00366-9
34. Ling L, Hou J, Liu D, Tang D, Zhang Y, Zeng Q, et al. Important role of the SDF-1/CXCR4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (hAD-MSCs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (POI). Stem Cell Res Ther. (2022) 13. doi: 10.1186/s13287-022-02759-6
35. Ceradini D, Kulkarni A, Callaghan M, Tepper O, Bastidas N, Kleinman M, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. (2004) 10:858–64. doi: 10.1038/nm1075
36. Ou S, Wu X, Yang Y, Xia C, Zhang W, Qu Y, et al. Tibial cortex transverse transport potentiates diabetic wound healing via activation of SDF-1/CXCR4 signaling. Peerj. (2023) 11. doi: 10.7717/peerj.15894
37. Sharun K, Pawde A, Amarpal . Classification and coding systems for platelet-rich plasma (PRP): a peek into the history. Expert Opin Biol Ther. (2021) 21:121–3. doi: 10.1080/14712598.2021.1846715
38. Patel H, Pundkar A, Shrivastava S, Chandanwale R, Jaiswal AM. A comprehensive review on platelet-rich plasma activation: a key player in accelerating skin wound healing. Cureus. (2023) 15(11):e48943. doi: 10.7759/cureus.48943
39. Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. (2018) 4:18–24. doi: 10.1159/000477353
40. Rui S, Dai L, Zhang X, He M, Xu F, Wu W, et al. Exosomal miRNA-26b-5p from PRP suppresses NETs by targeting MMP-8 to promote diabetic wound healing. J Controlled Release. (2024) 372:221–33. doi: 10.1016/j.jconrel.2024.06.050
Keywords: tibial cortex transverse transport, platelet-rich plasma, diabetic foot ulcers, stromal cell derived factor-1, wound healing
Citation: Zhen P-X, Su H-J, Yang S-J, Chen X, Lin Z-M and Liu S-N (2025) Comparison of clinical efficacy between tibial cortex transverse transport and platelet-rich plasma treatment for severe diabetic foot ulcers. Front. Surg. 12:1507982. doi: 10.3389/fsurg.2025.1507982
Received: 8 October 2024; Accepted: 24 February 2025;
Published: 17 March 2025.
Edited by:
Keng Lin Wong, National University of Singapore, SingaporeReviewed by:
Davide Costa, Magna Græcia University, ItalyDavid O. Osei-Hwedieh, Massachusetts General Hospital and Harvard Medical School, United States
Copyright: © 2025 Zhen, Su, Yang, Chen, Lin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sai-Nan Liu, NDk4MTA4OTYxQHFxLmNvbQ==
 Pu-Xiang Zhen
Pu-Xiang Zhen Hong-Jie Su2
Hong-Jie Su2