- 1Thoracic Oncology Department, Baotou Cancer Hospital, Baotou, Inner Mongolia, China
- 2Oncology and Palliative Care Department, Baotou Cancer Hospital, Baotou, Inner Mongolia, China
- 3Thoracic Oncology Surgery Department, Baotou Cancer Hospital, Baotou, Inner Mongolia, China
Background: A meta-analysis study was done to figure out how to predict the prognosis of people with resectable non-small-cell lung cancer (NSCLC) who had a significant pathological response following neoadjuvant immunotherapy.
Methods: Up until August 2024, a comprehensive literature study was completed, and 2,386 connected studies were revised. The 35 selected studies included 3,118 resectable non-small-cell lung tumor participants at the beginning of the study. Using dichotomous techniques and a fixed or random model, the odds ratio (OR) and 95% confidence intervals (CIs) were used to assess the prediction using significant pathological response following neoadjuvant immunotherapy in resectable NSCLC.
Results: Individuals with resectable NSCLC had significantly higher major pathological response when comparing neoadjuvant chemo-immunotherapy to neoadjuvant chemotherapy (OR, 5.07; 95% CI, 4.09–6.27, p < 0.001), objective response rate to non-objective response rate (OR, 7.02; 95% CI, 4.28–11.50, p < 0.001), and programmed death-ligand 1 ≥1% to programmed death-ligand ≤1% (OR, 2.49; 95% CI, 1.44–4.30, p = 0.001). However, no significant difference was found in major pathological response between stage III and stage I-II (OR, 1.43; 95% CI, 0.88–2.33, p = 0.15), and squamous cell cancer and non-squamous cell cancer (OR, 1.35; 95% CI, 0.95–1.92, p = 0.09) in individuals with resectable NSCLCs.
Conclusion: Individuals with resectable NSCLCs had significantly higher major pathological response when comparing neoadjuvant chemo-immunotherapy to neoadjuvant chemotherapy, objective response rate to non-objective response rate, and programmed death-ligand 1≥1% to programmed death-ligand 1 ≤1%, however, no significant difference was found between stage III and stage I-II, and squamous cell cancer and non-squamous cell cancer. To validate this discovery, more research is required since most of the selected studies had a low sample size, and caution must be implemented when interacting with its values.
Introduction
One of the most prevalent and fatal malignancies worldwide is lung cancer (1). The cornerstone of care for non-small cell lung cancer (NSCLC) that is both locally progressed and in its early stages is still surgical resection. Nevertheless, despite curative resection, 30%–55% of patients with NSCLC have recurrence and ultimately pass away from their illness, even in the early stages of the disease (2, 3). According to a meta-analysis of NSCLC, adding chemotherapy to neoadjuvant care could result in a 5% increase in survival after five years (4). Immune checkpoint inhibitors that target the programmed cell death protein 1/programmed death-ligand 1 (PDL 1) axis are currently the mainstay of treatment for metastatic NSCLC, either used alone or in conjunction with chemotherapy. Numerous phase 2 neoadjuvant immunotherapy trials have demonstrated positive results, indicating that immune checkpoint inhibitors, either in combination with chemotherapy or on their own, can significantly minimize the growth of cancers that have spread locally or enhance their pathological regression (5). In the neoadjuvant setting, the major pathological response (MPR), which is defined as 10% or less viable tumor, ranges from 19% to 45% with a single drug and varies from 33% to 83% when paired with chemotherapy (6). Adjuvant nivolumab with chemotherapy demonstrated statistically significant improvements in pathological complete response rate, MPR rate, and event-free survival when compared to chemotherapy alone (6). The transition of possibly successful treatment to clinical practice may be delayed by the requirement for an extended follow-up time, even though the gold standard of outcome measurement for phase 3 trials which is overall survival. It has also been suggested to use a MPR as a potential surrogate endpoint to quickly assess the clinical effectiveness of neoadjuvant chemotherapy. From 2008 to 2012, 151 patients with NSCLC were managed with neoadjuvant chemotherapy and then experienced total surgical resection (7). Multivariable analysis of the data showed that MPR was related to long-term overall survival. According to Hellman et al. (8), MPR was highly correlated with increased survival, accurately depicted the effect of treatment, and adequately measured the extent of treatment benefit on survival. As of yet, the immunotherapy age has not shown the evidence-based validity of MPRs.
Objectives
This time, we used a meta-analysis to assess the reliability of MPRs as a proxy for survival following neoadjuvant immunotherapy.
Methods
Eligibility criteria
To provide an overview, the studies that showed the prognosis prediction using significant pathological response following neoadjuvant immunotherapy in resectable NSCLCs were picked (9).
Information sources
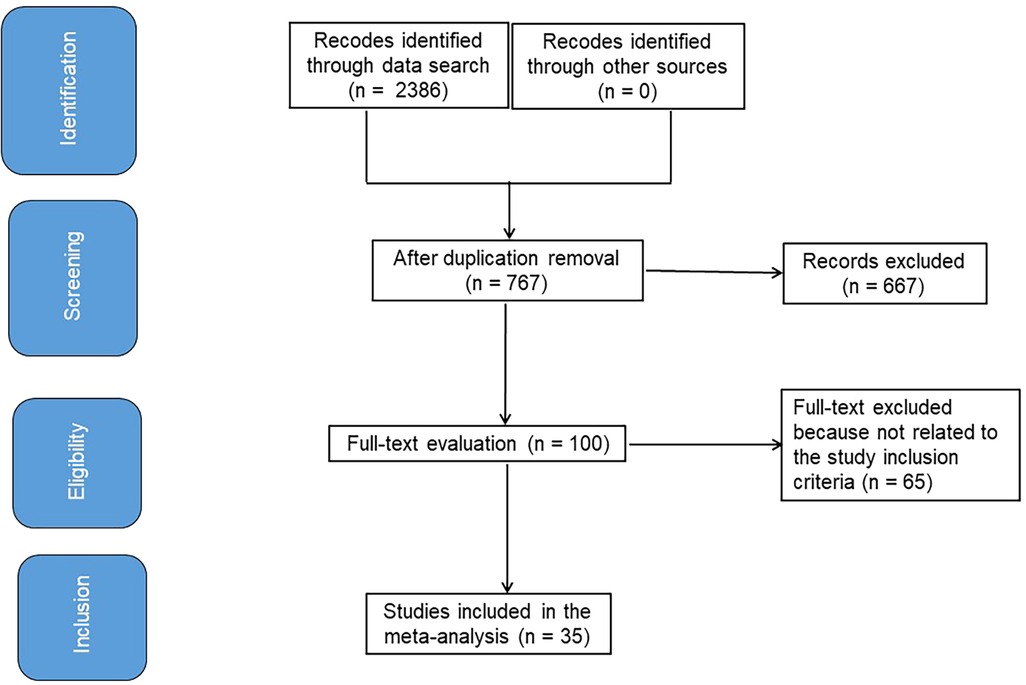
Figure 1 symbolizes the entirety of the study. When the following inclusion criteria were satisfied, the literature was incorporated into the study (10, 11):
1. The study was a randomized controlled trial (RCT), observational, prospective, or retrospective study.
2. The people who were chosen for investigation have resectable NSCLCs.
3. MPR was integrated into the intervention.
4. The study made a distinction of the prognosis prediction using significant pathological response following neoadjuvant immunotherapy in resectable NSCLCs.
Studies that did not check the possessions of the prognosis prediction using significant pathological response following neoadjuvant immunotherapy in resectable NSCLCs, studies on MPR in individuals without neoadjuvant immunotherapy, and studies with no comparison significance were also omitted (12, 13).
Search strategy
A search protocol process was identified using the PICOS view, and we defined it as follows: the “population” consisted of people with resectable NSCLCs, P; MPR was the “intervention” or “exposure,” and the “comparison” involved correlation between MPR and different patients’ variables; the “outcome” was the effect on MPR; and the “research design” was without boundaries (14).
We have thoroughly searched the databases of Google Scholar, Embase, the Cochrane Library, PubMed, and OVID through August 2024 using a set of keywords and additional terms as shown in Table 1 (15, 16). To prevent the inclusion of a study that was unable to establish a link between the effects of MPR in resectable NSCLCs and its prognosis prediction, the replications of the papers were eliminated, assembled into an EndNote file, and their titles and abstracts were once again assessed (17, 18).
Selection process
The meta-analysis method was then used to organize and assess the method that followed the epidemiological proclamation (19, 20).
Data collection process
Some of the criteria utilized to gather data were the name of the first author, research data, research year, nation or region, population type, categories, quantitative and qualitative estimation methods, data sources, outcome estimation, medical and therapy physiognomies, and statistical analysis (21).
Data items
When a study yielded differing values, we independently gathered the data founded on a valuation of prognosis prediction using significant pathological response following neoadjuvant immunotherapy in resectable NSCLCs.
Research risk of bias assessment
Two authors looked into the opportunity for bias in the studies and the standard of approaches utilized in papers elected for supplementary analysis. The two authors (Fang Nie, and Ying Wang) conducted unbiased reviews of techniques used for each test.
Effect measures
Sensitivity analysis was limited to studies that assessed and documented the prognosis prediction using significant pathological response following neoadjuvant immunotherapy in resectable NSCLCs. A subclass analysis was used to compare the correlation between MPRs and different patients’ variables in resectable NSCLCs individuals’ sensitivity.
Synthesis methods
Using a dichotomous approach and a random or fixed-effect model, the odds ratio (OR) and a 95% confidence interval (CI) were determined. A range of 0%–100% was used to determine the I2 index. At 0%, 25%, 50%, and 75% of the data, respectively, there was no, low, moderate, and significant heterogeneity visible (22). To ensure that the exact model was used, additional structures that show a high degree of similarity with the related inquiry were also examined. The fixed-effect rose was an option if I2 was less than 50%; otherwise, the random effect was used (22). A subclass analysis was performed by splitting the original estimation into the previously specified consequence groups. A p-value of less than 0.05 was utilized in the analysis to define the statistical significance of differences across subcategories.
Reporting bias assessment
Both quantitative and qualitative methods were employed to measure the bias in the investigations: the Egger regression test and funnel plots, which display the logarithm of the ORs against their standard errors. The presence of investigation bias was determined by p ≥ 0.05 (23).
Certainty assessment
We looked at each p-value with two-tailed testing. Graphs and statistical analyses were created using Reviewer Manager Version 5.3 (The Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark).
Results
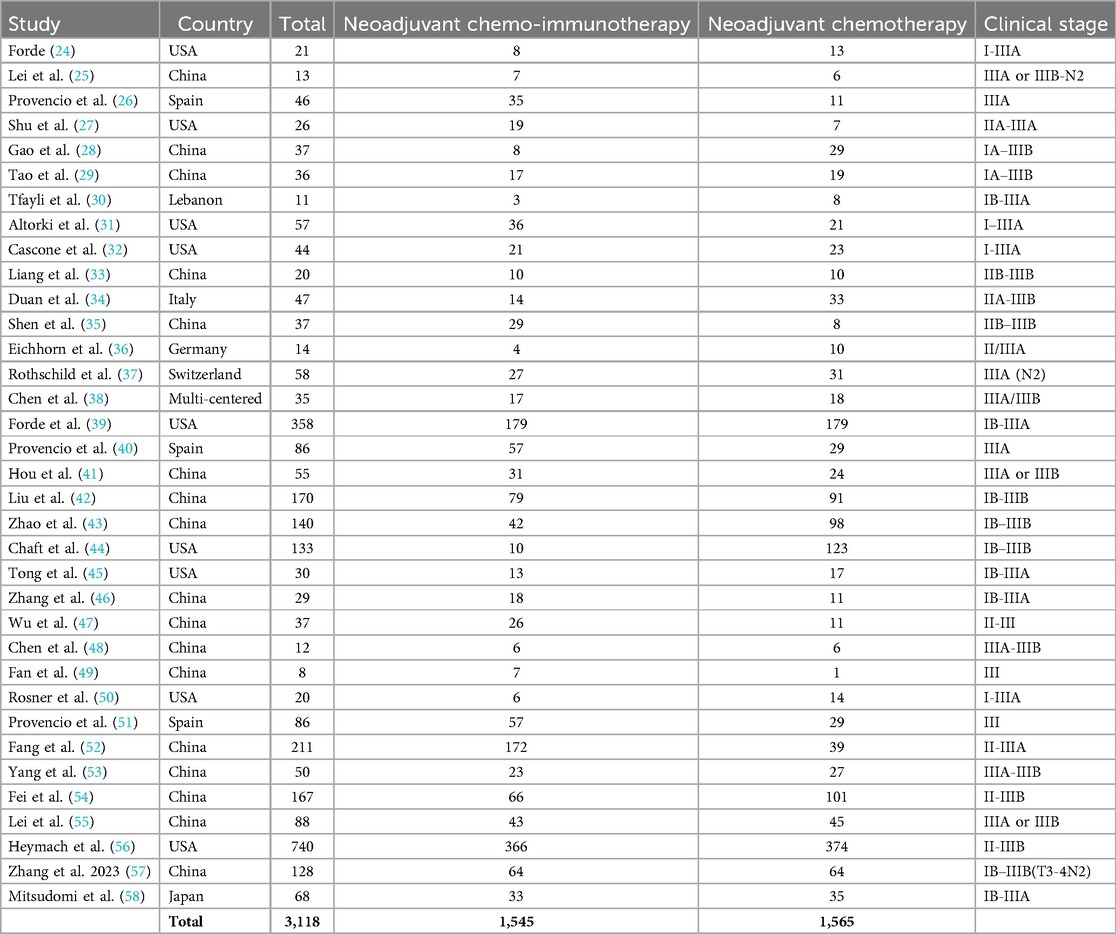
Out of 2,386 connected studies, 35 papers that were published between 2018 and 2024 and satisfied the inclusion criteria were selected for the study (24–58). Table 2 provides access to the findings of these inquiries. At the beginning of the studies that were used, there were 3,118 resectable NSCLC participants. There were between 8 and 740 subjects as a sample size.
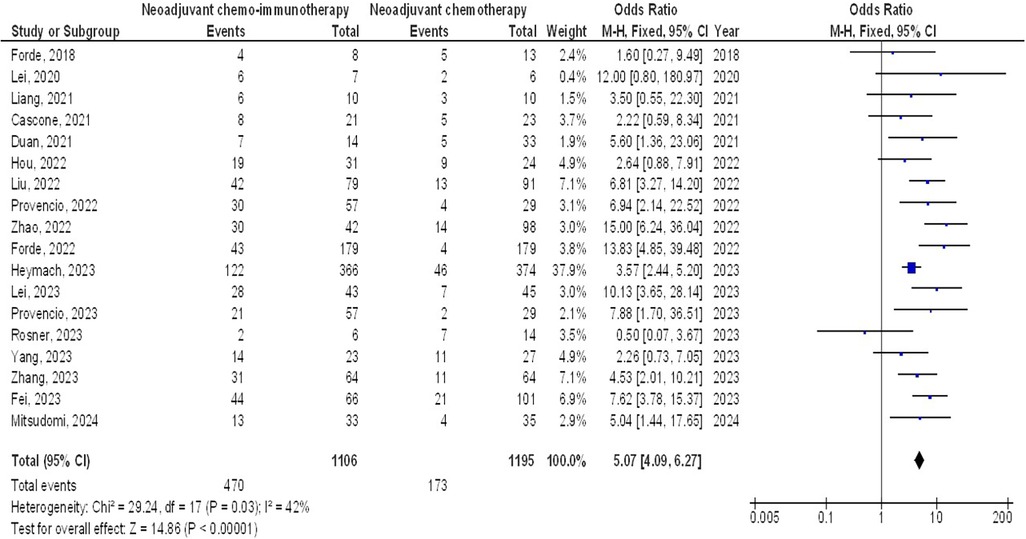
As illustrated in Figures 2–4, Individuals with resectable NSCLCs had significantly higher MPR when comparing neoadjuvant chemo-immunotherapy to neoadjuvant chemotherapy (OR, 5.07; 95% CI, 4.09–6.27, p < 0.001) with low heterogeneity (I2 = 42%), objective response rate to non-objective response rate (OR, 7.02; 95% CI, 4.28–11.50, p < 0.001) with no heterogeneity (I2 = 19%), and PDL 1 ≥ 1% to PDL 1 ≤ 1% (OR, 2.49; 95% CI, 1.44–4.30, p = 0.001) with no heterogeneity (I2 = 0%).
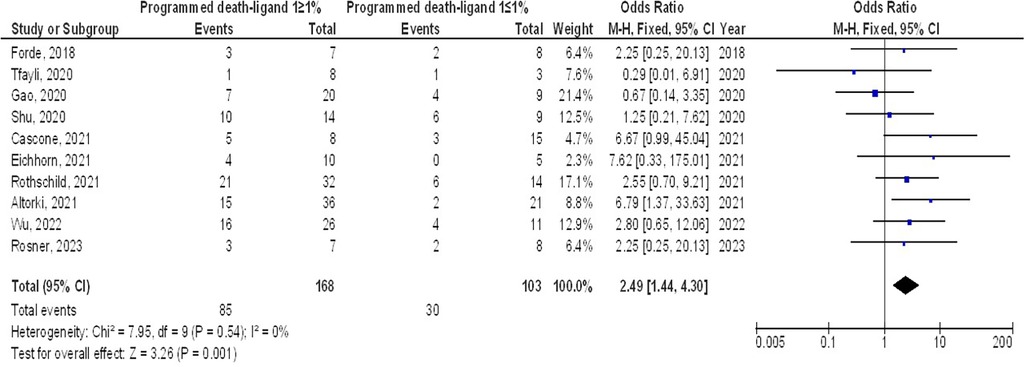
Figure 2. The neoadjuvant chemo-immunotherapy compared to neoadjuvant chemotherapy's forest plot influence on MPR in resectable NSCLC.

Figure 3. The objective response rate compared to non-objective response rate's forest plot influence on MPR in resectable NSCLC.
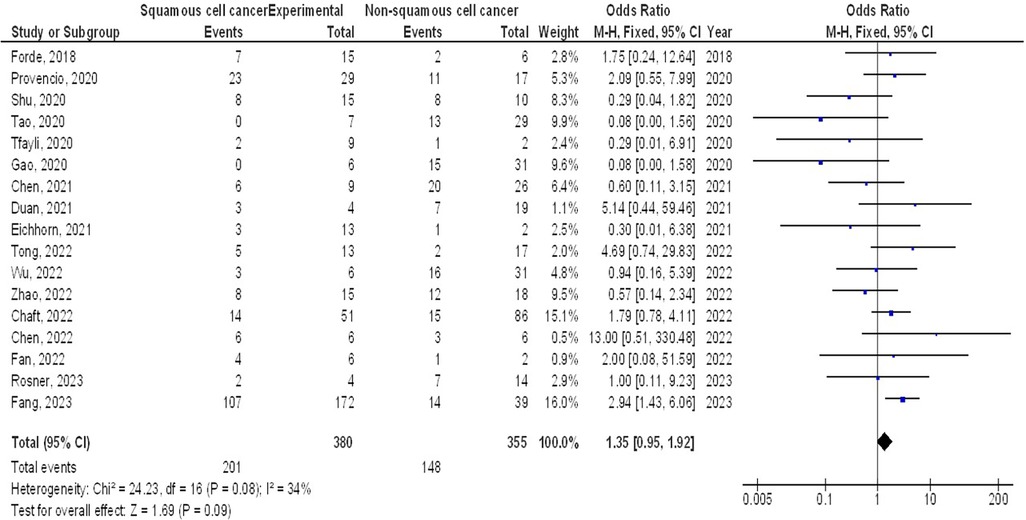
However, no significant difference was found in MPR between stage III and stage I-II (OR, 1.43; 95% CI, 0.88–2.33, p = 0.15) with no heterogeneity (I2 = 0%), and squamous cell cancer and non-squamous cell cancer (OR, 1.35; 95% CI, 0.95–1.92, p = 0.09) with low heterogeneity (I2 = 34%) in resectable NSCLCs, as shown in Figures 5, 6.
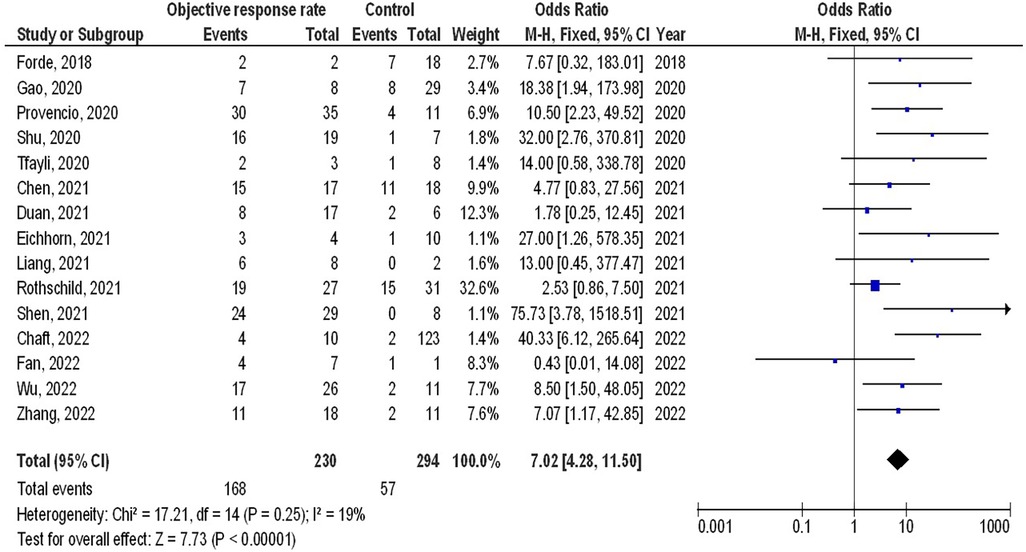
Figure 6. The squamous cell cancer compared to non-squamous cell cancer's forest plot influence on MPR in resectable NSCLC.
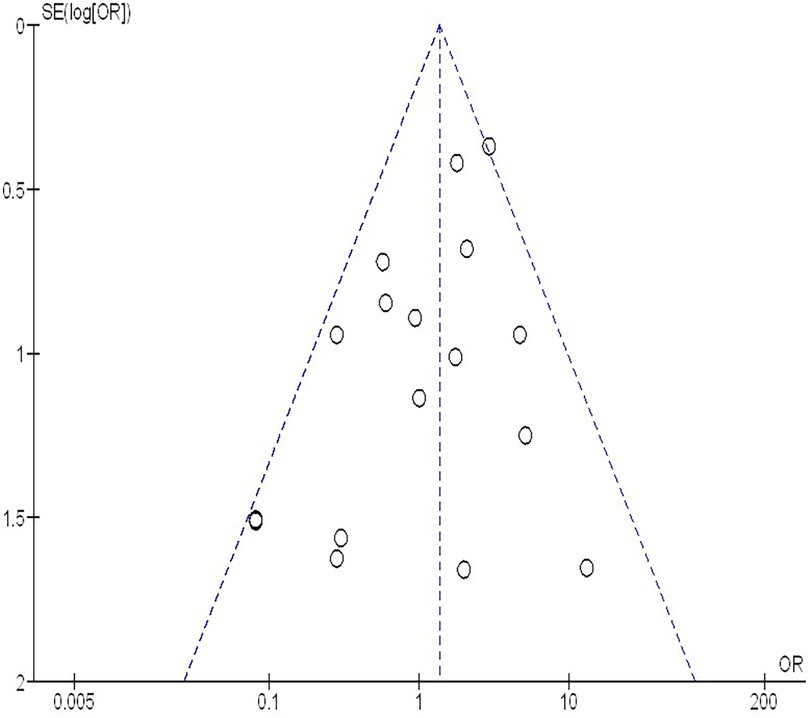
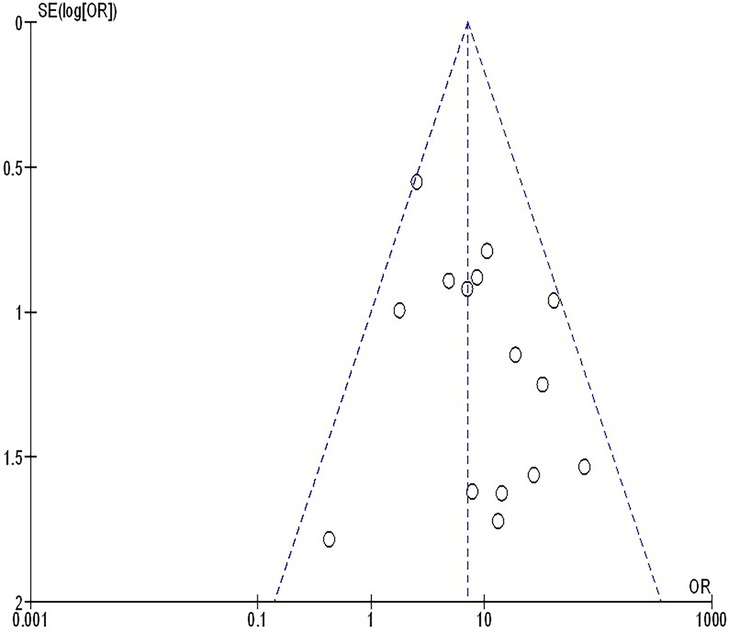
The insufficiency of data, e.g., age, ethnicity, and gender, on comparative results precluded the application of stratified models to investigate the impacts of particular components. Using the quantitative Egger regression test and the visual interpretation of the funnel plot, no evidence of research bias was detected (p = 0.89) as shown in Figures 7–11. However, it was shown that there was no bias in the selective reporting and that the majority of concerned RCTs had poor technical quality.
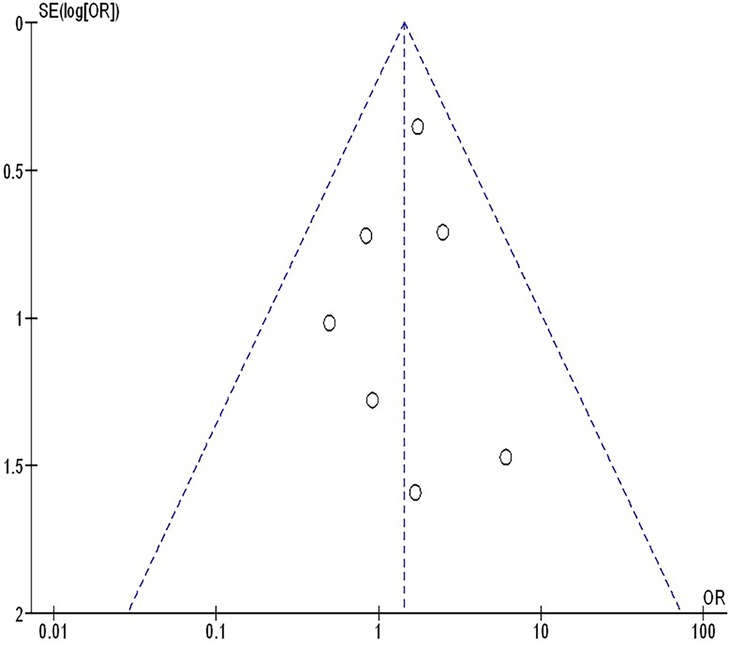
Figure 7. The neoadjuvant chemo-immunotherapy compared to neoadjuvant chemotherapy's funnel plot influence on MPR in resectable NSCLC.
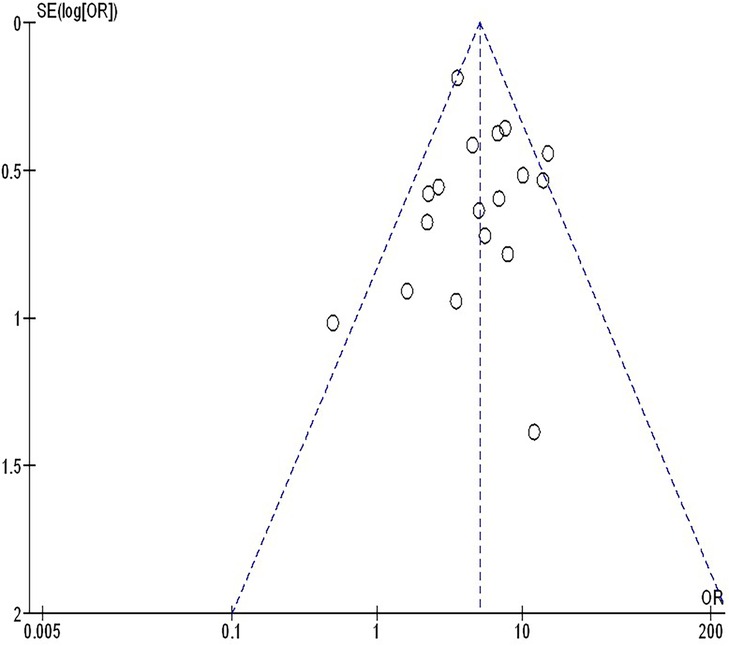
Figure 8. The objective response rate compared to non-objective response rate's funnel plot influence on MPR in resectable NSCLC.
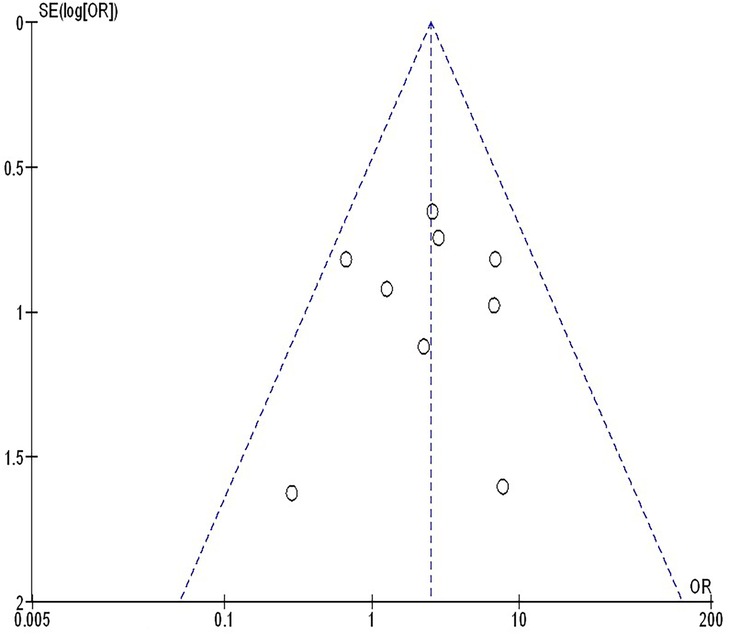
Figure 11. The squamous cell cancer compared to non-squamous cell cancer's funnel plot influence on MPR in resectable NSCLC.
Discussions
3,118 resectable NSCLC participants were at the starting point of the studies that were utilized for the meta-analysis (24–58). Individuals with resectable NSCLCs had a significantly higher MPR when comparing neoadjuvant chemo-immunotherapy to neoadjuvant chemotherapy, the objective response rate to non-objective response rate, and PDL 1 ≥ 1% to PDL 1 ≤ 1%. However, no significant difference was found in MPRs between stage III and stage I-II, and squamous cell cancer and non-squamous cell cancer in resectable NSCLCs. To validate this discovery, more studies are required since most of the selected studies had a low sample size (27 out of 35 studies were <100 subjects), and thoughtfulness must be exercised when interrelating with its values. That would have an impact on how significant the evaluated assessments were (59–69).
Patients with resectable NSCLC may fare better with neoadjuvant treatment. Finding the ideal neoadjuvant strategy to attain a high response rate and manageable toxicity is still a challenge. The use of immune checkpoint inhibitors in early-stage NSCLC has gained attention as immunotherapy has lately emerged as a possible therapeutic approach for the disease. Due to the cancer's total antigen load before surgical resection, the administration of early immune checkpoint inhibitor therapy may elicit a profound pathological response (39, 70). 15% was the MPR rate after neoadjuvant chemotherapy, according to Brandt et al. (71) Neoadjuvant nivolumab produced a significant pathological response in 45% of individuals, according to the CheckMate 159 study (24). The efficacy of nivolumab in conjunction with carboplatin/paclitaxel as neoadjuvant therapy for patients with stage IIIa resectable NSCLC was assessed in the phase II RESECTABLE NON-SMALL-CELL LUNG CANCER research. Patients with locally advanced NSCLC may now have neoadjuvant chemo-immunotherapy as a novel option, according to a high MPR rate of 82.9% (26). In the phase III CheckMate-816 trial, it was found that nivolumab plus chemotherapy improved the MPR as a neoadjuvant treatment for resectable NSCLC by 36.9 vs. 8.9%, respectively, when compared to neoadjuvant platinum-based chemotherapy alone (39). The high rates of substantial pathological response may be explained by the synergistic action of immune checkpoint inhibitors and chemotherapy, with cytotoxic chemotherapy boosting the recognition of these drugs as immunotherapies (72, 73). Lack of surrogate endpoints of clinical success typically prevented innovative perioperative treatment options for resectable NSCLC from being widely accepted. A thorough assessment of the ongoing neoadjuvant therapy trials including patients with NSCLC is necessary, as pathological response has demonstrated a patient-level connection with survival in a variety of malignancies (74, 75). A combined analysis of two neoadjuvant chemotherapy studies revealed that pathological complete response was a favorable prognostic factor of overall survival (76). Five-year overall survival was 80.0% in the pathological complete response group compared to 55.8% in the non-pathological complete response group. Its use as a surrogate endpoint was severely limited, possibly due to the low pathological complete response rate following neoadjuvant therapies and the lack of appropriate data available for study. MPR appears to be more common than pathological complete response. MPR has been recognized as an additional predictor of survival in patients with NSCLC who underwent neoadjuvant chemotherapy, despite the lack of mediastinal downstaging assessment. Following neoadjuvant chemotherapy, Waser et al. observed that the main pathological response rate was 30% and that the histopathologic response was a strong predictor of overall survival. The MPR was suggested by the College of American Pathologists as one of the research endpoints for clinical trials including neoadjuvant immunotherapy for lung cancer (8). Nevertheless, there is still much to learn about the association between substantial pathological response and overall survival in patients with resectable NSCLC undergoing neoadjuvant immunotherapy. The MPR appeared to be a different measure of overall survival for individuals who underwent neoadjuvant chemotherapy for NSCLC. Neoadjuvant chemotherapy produced a good radiological response rate in the multicenter randomized trial MRC LU22/NVALT 2/EORTC 08012. On the other hand, no proof of any advantage in terms of overall survival was found. It was common to see differences between the pathological and radiographic assessments. When compared to traditional chemotherapeutic drugs, the tumor response patterns of immune agents may vary (77). With neoadjuvant chemo-immunotherapy, the incidence of radiographic partial response and complete response varied from 38 to 72% (26, 35, 37). Pseudo progression was initially reported in melanoma patients receiving ipilimumab treatment. It was defined as the radiologic advancement of the tumor burden followed by an objective response (78). According to certain research, immune checkpoint inhibitor-treated cancer types may have pseudoprogression. Conventional cytotoxic treatment does not usually produce this unusual effect. The traditional response evaluation criteria in solid tumors is still a valid and useful way to evaluate immunotherapy response in the clinic, even if additional radiologic criteria tailored specifically to immunotherapy have been developed (79). There was currently no agreement despite recent trials evaluating putative prognostic biomarkers for significant pathological response. Lung adenocarcinoma and lung squamous carcinoma are the two predominant subtypes that account for about 80% of instances of NSCLC (80). In comparison to adenocarcinoma, squamous cell cancer patients have comparatively greater MPR rates, according to several studies (26, 57). Based on several sizable prospective trials and the Lung Adjuvant Cisplatin Evaluation meta-analysis, patients with early stages of the illness (stages IB to II) are typically advised to undergo upfront resection and adjuvant chemotherapy (81). Which stages of NSCLC respond best to neoadjuvant immune checkpoint inhibitor therapy is yet unknown. It is crucial to evaluate pathological responses according to stages since this could enable better trial designs in the future for particular disease stages (73). According to the results of the CheckMate-816 trial (NCT02998528), patients with stage IIIA disease had a larger event-free survival benefit than patients with stages IB to II disease, and patients with tumors expressing PDL 1 at 1% or higher had a larger benefit than patients with PDL 1 expression at less than 1%. With the inclusion of nivolumab in CheckMate-816, the primary benefit in terms of pathological response for patients in stage IIIA was more striking than the benefit for patients in stages IB to II (39). The predictive value of the PDL 1 status may differ in patients with non-metastatic earlier-stage lung cancer with less tumor burden, regardless of the results in metastatic stage IV patients. The Checkmate-816 trial (39) and the NEoverall survivalTAR (32) both demonstrated that increased PDL 1 expression was also connected to more pathologic reactions. Nevertheless, no correlation was discovered between PDL 1 expression and pathogenic response by Shu et al. (27) or the CLMC3 experiment (24).
Limitations
Given that a few of the researchers chosen for the meta-analysis were not included, a variety bias might have occurred. Nevertheless, the excluded studies did not encounter the necessary standards to be incorporated into the meta-analysis. Moreover, we did not have enough information to determine whether factors such as race and age had an impact on results. Pathological response following neoadjuvant immunotherapy in resectable NSCLCs was the aim of the study. Bias may have increased as a result of the incorporation of incomplete or erroneous data from earlier studies. The individuals’ age, gender, and race were likely sources of bias in addition to their nutritional status. Unintentionally skewed values might arise from incomplete data and unpublished research.
Conclusions
Individuals with resectable NSCLCs had a significantly higher MPR when comparing neoadjuvant chemo-immunotherapy to neoadjuvant chemotherapy, the objective response rate to non-objective response rate, and PDL 1 ≥ 1% to PDL 1 ≤ 1%. However, no significant difference was found in MPRs between stage III and stage I-II, and squamous cell cancer and non-squamous cell cancer in resectable NSCLCs. To validate this discovery, more studies are required since most of the selected studies had a low sample size (27 out of 35 studies were <100 subjects), and thoughtfulness must be exercised when interrelating with its values. That would have an impact on how significant the evaluated assessments were.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
FN: Writing – original draft, Writing – review & editing. YW: Writing – original draft, Writing – review & editing. WS: Writing – original draft, Writing – review & editing. LZ: Writing – original draft, Writing – review & editing. JH: Writing – original draft, Writing – review & editing. RT: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Carnio S, Novello S, Papotti M, Loiacono M, Scagliotti GV. Prognostic and predictive biomarkers in early stage non-small cell lung cancer: tumor based approaches including gene signatures. Transl Lung Cancer Res. (2013) 2(5):372–81. doi: 10.3978/j.issn.2218-6751.2013.10.05
3. The Lancet. Lung cancer treatment: 20 years of progress. Lancet. (2024) 403(10445):2663. doi: 10.1016/S0140-6736(24)01299-6
4. Gilligan D, Nicolson M, Smith I, Groen H, Dalesio O, Goldstraw P, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet. (2007) 369(9577):1929–37. doi: 10.1016/S0140-6736(07)60714-4
5. Sorin M, Prosty C, Ghaleb L, Nie K, Katergi K, Shahzad MH, et al. Neoadjuvant chemoimmunotherapy for NSCLC: a systematic review and meta-analysis. JAMA Oncol. (2024) 10(5):621–33. doi: 10.1001/jamaoncol.2024.0057
6. Liang W, Cai K, Chen C, Chen H, Chen Q, Fu J, et al. Expert consensus on neoadjuvant immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res. (2020) 9(6):2696–715. doi: 10.21037/tlcr-2020-63
7. Weissferdt A, Pataer A, Vaporciyan AA, Correa AM, Sepesi B, Moran CA, et al. Agreement on Major pathological response in NSCLC patients receiving neoadjuvant chemotherapy. Clin Lung Cancer. (2020) 21(4):341–8. doi: 10.1016/j.cllc.2019.11.003
8. Waser NA, Quintana M, Schweikert B, Chaft JE, Berry L, Adam A, et al. Pathological response in resectable non–small cell lung cancer: a systematic literature review and meta-analysis. JNCI Cancer Spectrum. (2024) 8(3):pkae021. doi: 10.1093/jncics/pkae021
9. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
10. Amin MA. A meta-analysis of the eosinophil counts in the small intestine and colon of children without obvious gastrointestinal disease. Int J Clin Med Res. (2023) 1(1):1–8. doi: 10.61466/ijcmr1010001
11. Emad M, Osama H, Rabea H, Saeed H. Dual compared with triple antithrombotics treatment effect on ischemia and bleeding in atrial fibrillation following percutaneous coronary intervention: a meta-analysis. Int J Clin Med Res. (2023) 1(2):77–87. doi: 10.61466/ijcmr1020010
12. Giong Z, Lie N. Phosphate-specific diet effect on serum phosphate levels in adults undergoing hemodialysis: a meta-analysis. Int J Clin Med Res. (2024) 2(4):135–42. doi: 10.61466/ijcmr2040005
13. Gu R, Xu G. A meta-analysis looking at the effects of continuous management for complications related to intraoperative pressure wound ulcers in women with breast cancer. Int J Clin Med Res. (2024) 2(4):100–6. doi: 10.61466/ijcmr2040001
14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006
15. Guo Y. Effect of resident participation in ophthalmic surgery on wound dehiscence: a meta-analysis. Int J Clin Med Res. (2024) 2(2):50–6. doi: 10.61466/ijcmr2020002
16. Jiany L, Xiu W. Effect of Chinese herbal medicine as an adjunctive technique to standard treatment for personal with diabetic foot ulcers: a meta-analysis. Int J Clin Med Res. (2024) 2(4):118–26. doi: 10.61466/ijcmr2040003
17. Koang Y. A meta-analysis on the use of photobiomodulation to regulate gingival wound healing in addition to periodontal therapies. Int J Clin Med Res. (2024) 2(4):107–15. doi: 10.61466/ijcmr2040002
18. Osama H, Saeed H, Nicola M, Emad M. Neuraxial anesthesia compared to general anesthesia in subjects with hip fracture surgery: a meta-analysis. Int J Clin Med Res. (2023) 1(2):66–76. doi: 10.61466/ijcmr1020009
19. Shaaban MEA, Mohamed AIM. Determining the efficacy of N-acetyl cysteine in treatment of pneumonia in COVID-19 hospitalized patients: a meta-analysis. Int J Clin Med Res. (2023) 1(2):36–42. doi: 10.61466/ijcmr1020006
20. Singh RK. A meta-analysis of the impact on gastrectomy versus endoscopic submucosal dissection for early stomach cancer. Int J Clin Med Res. (2023) 1(3):88–99. doi: 10.61466/ijcmr1030011
21. Gupta S, Rout G, Patel AH, Mahanta M, Kalra N, Sahu P, et al. Efficacy of generic oral directly acting agents in patients with hepatitis C virus infection. J Viral Hepat. (2018) 25(7):771–8. doi: 10.1111/jvh.12870
22. Sheikhbahaei S, Trahan TJ, Xiao J, Taghipour M, Mena E, Connolly RM, et al. FDG-PET/CT and MRI for evaluation of pathologic response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis of diagnostic accuracy studies. Oncologist. (2016) 21(8):931–9. doi: 10.1634/theoncologist.2015-0353
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
24. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. (2018) 378(21):1976–86. doi: 10.1056/NEJMoa1716078
25. Lei J, Yan X, Zhao J, Tian F, Lu Q, Jiang T. 62MO A randomised, controlled, multicenter phase II trial of camrelizumab combined with albumin-bound paclitaxel and cisplatin as neoadjuvant treatment in locally advanced NSCLC. Ann Oncol. (2020) 31:S1441–2. doi: 10.1016/j.annonc.2020.10.550
26. Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. (2020) 21(11):1413–22. doi: 10.1016/S1470-2045(20)30453-8
27. Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. (2020) 21(6):786–95. doi: 10.1016/S1470-2045(20)30140-6
28. Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, et al. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J Thorac Oncol. (2020) 15(5):816–26. doi: 10.1016/j.jtho.2020.01.017
29. Tao X, Li N, Wu N, He J, Ying J, Gao S, et al. The efficiency of 18F-FDG PET-CT for predicting the major pathologic response to the neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Eur J Nucl Med Mol Imaging. (2020) 47(5):1209–19. doi: 10.1007/s00259-020-04711-3
30. Tfayli A, Al Assaad M, Fakhri G, Akel R, Atwi H, Ghanem H, et al. Neoadjuvant chemotherapy and Avelumab in early stage resectable nonsmall cell lung cancer. Cancer Med. (2020) 9(22):8406–11. doi: 10.1002/cam4.3456
31. Altorki NK, McGraw TE, Borczuk AC, Saxena A, Port JL, Stiles BM, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. (2021) 22(6):824–35. doi: 10.1016/S1470-2045(21)00149-2
32. Cascone T, William WN Jr, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. (2021) 27(3):504–14. doi: 10.1038/s41591-020-01224-2
33. Liang H, Yang C, Gonzalez-Rivas D, Zhong Y, He P, Deng H, et al. Sleeve lobectomy after neoadjuvant chemoimmunotherapy/chemotherapy for local advanced non-small cell lung cancer. Transl Lung Cancer Res. (2021) 10(1):143–55. doi: 10.21037/tlcr-20-778
34. Duan H, Wang T, Luo Z, Tong L, Dong X, Zhang Y, et al. Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable non-small cell lung cancer: an open-label, multicenter, single-arm study. Transl Lung Cancer Res. (2021) 10(2):1020–8. doi: 10.21037/tlcr-21-130
35. Shen D, Wang J, Wu J, Chen S, Li J, Liu J, et al. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB-IIIB resectable lung squamous cell carcinoma. J Thorac Dis. (2021) 13(3):1760–8. doi: 10.21037/jtd-21-103
36. Eichhorn F, Klotz LV, Kriegsmann M, Bischoff H, Schneider MA, Muley T, et al. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: first clinical experience. Lung Cancer. (2021) 153:150–7. doi: 10.1016/j.lungcan.2021.01.018
37. Rothschild SI, Zippelius A, Eboulet EI, Prince SS, Betticher D, Bettini A, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non–small-cell lung cancer—a multicenter single-arm phase II trial. J Clin Oncol. (2021) 39(26):2872–80. doi: 10.1200/JCO.21.00276
38. Chen Y, Yan B, Xu F, Hui Z, Zhao G, Liu J, et al. Neoadjuvant chemoimmunotherapy in resectable stage IIIA/IIIB non-small cell lung cancer. Transl Lung Cancer Res. (2021) 10(5):2193–204. doi: 10.21037/tlcr-21-329
39. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170
40. Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio J, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non–small-cell lung cancer (NADIM phase II trial). J Clin Oncol. (2022) 40(25):2924–33. doi: 10.1200/JCO.21.02660
41. Hou X, Shi X, Luo J. Efficacy and safety of camrelizumab (a PD-1 inhibitor) combined with chemotherapy as a neoadjuvant regimen in patients with locally advanced non-small cell lung cancer. Oncol Lett. (2022) 24(1):1–8. doi: 10.3892/ol.2022.13336
42. Liu Z, Gao Z, Zhang M, Wang X, Gong J, Jiang S, et al. Real-World effectiveness and prognostic factors analysis of stages I–III non-small cell lung cancer following neoadjuvant chemo-immunotherapy or neoadjuvant chemotherapy. Ann Thorac Cardiovasc Surg. (2022) 28(2):111–20. doi: 10.5761/atcs.oa.21-00143
43. Zhao D, Xu L, Wu J, She Y, Su H, Hou L, et al. Comparison of perioperative outcomes among non-small cell lung cancer patients with neoadjuvant immune checkpoint inhibitor plus chemotherapy, EGFR-TKI, and chemotherapy alone: a real-world evidence study. Transl Lung Cancer Res. (2022) 11(7):1468–78. doi: 10.21037/tlcr-22-476
44. Chaft JE, Oezkan F, Kris MG, Bunn PA, Wistuba II, Kwiatkowski DJ, et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nat Med. (2022) 28(10):2155–61. doi: 10.1038/s41591-022-01962-5
45. Tong BC, Gu L, Wang X, Wigle DA, Phillips JD, Harpole DH, et al. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non–small cell lung cancer. J Thorac Cardiovasc Surg. (2022) 163(2):427–36. doi: 10.1016/j.jtcvs.2021.02.099
46. Zhang P, Dai J, Sun F, Xia H, He W, Duan L, et al. Neoadjuvant sintilimab and chemotherapy for resectable stage IIIA non-small cell lung cancer. Ann Thorac Surg. (2022) 114(3):949–58. doi: 10.1016/j.athoracsur.2022.01.039
47. Wu YL, Chen K, Xing W, Chen Q, Liu L, Zhang Q, et al. 84P SHR-1316 vs placebo in combination with chemotherapy as perioperative treatment in patients with resectable stage II-III NSCLC: a randomized, double-blind, multicenter, phase Ib/III trial. Ann Oncol. (2022) 33:S72. doi: 10.1016/j.annonc.2022.02.094
48. Chen T, Ning J, Campisi A, Dell’Amore A, Ciarrocchi AP, Li Z, et al. Neoadjuvant PD-1 inhibitors and chemotherapy for locally advanced NSCLC: a retrospective study. Ann Thorac Surg. (2022) 113(3):993–9. doi: 10.1016/j.athoracsur.2021.03.041
49. Fan BS, Wang XT, Di SY, Zhao JH, Chen SY, Zhou SH, et al. Short-term outcomes of neoadjuvant sintilimab with chemotherapy in stage III non-small cell lung cancer: a case series. Transl Cancer Res. (2022) 11(6):1697–704. doi: 10.21037/tcr-22-1194
50. Rosner S, Reuss JE, Zahurak M, Zhang J, Zeng Z, Taube J, et al. Five-year clinical outcomes after neoadjuvant nivolumab in resectable non–small cell lung cancer. Clin Cancer Res. (2023) 29(4):705–10. doi: 10.1158/1078-0432.CCR-22-2994
51. Provencio M, Nadal E, González-Larriba JL, Martínez-Martí A, Bernabé R, Bosch-Barrera J, et al. Perioperative nivolumab and chemotherapy in stage III non–small-cell lung cancer. N Engl J Med. (2023) 389(6):504–13. doi: 10.1056/NEJMoa2215530
52. Fang M, Hang Q, Jiang H, Cai L, Hu J, Ying H, et al. Efficacy and safety evaluation of neoadjuvant immunotherapy plus chemotherapy for resectable non–small cell lung cancer in real world. Front Oncol. (2023) 12:1055610. doi: 10.3389/fonc.2022.1055610
53. Yang Y, Liu Z. Neoadjuvant immune checkpoint inhibitor treatment + chemotherapy (vs. Chemotherapy alone) for locally advanced non-small cell lung cancer: a retrospective cohort study. Oncol Lett. (2023) 26(1):292. doi: 10.3892/ol.2023.13878
54. Fei K, Guo G, Wang J, Wang Z, Wang Y, Hao X, et al. Effectiveness of neoadjuvant immunochemotherapy compared to neoadjuvant chemotherapy in non-small cell lung cancer patients: real-world data of a retrospective, dual-center study. Front Oncol. (2023) 13:1145303. doi: 10.3389/fonc.2023.1145303
55. Lei J, Zhao J, Gong L, Ni Y, Zhou Y, Tian F, et al. Neoadjuvant camrelizumab plus platinum-based chemotherapy vs chemotherapy alone for Chinese patients with resectable stage IIIA or IIIB (T3N2) non–small cell lung cancer: the TD-FOREKNOW randomized clinical trial. JAMA Oncol. (2023) 9(10):1348–55. doi: 10.1001/jamaoncol.2023.2751
56. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Perioperative durvalumab for resectable non–small-cell lung cancer. N Engl J Med. (2023) 389(18):1672–84. doi: 10.1056/NEJMoa2304875
57. Zhang B, Xiao H, Pu X, Zhou C, Yang D, Li X, et al. A real-world comparison between neoadjuvant chemoimmunotherapy and chemotherapy alone for resectable non-small cell lung cancer. Cancer Med. (2023) 12(1):274–86. doi: 10.1002/cam4.4889
58. Mitsudomi T, Ito H, Okada M, Sugawara S, Shio Y, Tomii K, et al. Neoadjuvant nivolumab plus chemotherapy in resectable non-small-cell lung cancer in Japanese patients from CheckMate 816. Cancer Sci. (2024) 115(2):540–54. doi: 10.1111/cas.16030
59. Sayed AM, Khalaf AM, Abdelrahim MEA, Elgendy MO. Repurposing of some anti-infective drugs for COVID-19 treatment: a surveillance study supported by an in silico investigation. Int J Clin Pract. (2020) 75(4):e13877. doi: 10.1111/ijcp.13877
60. Saeed H, Salem HF, Rabea H, Abdelrahim ME. Effect of human error, inhalation flow, and inhalation volume on dose delivery from Ellipta® dry-powder inhaler. J Pharm Innov. (2019) 14(3):239–44. doi: 10.1007/s12247-018-9352-y
61. Nicola M, Elberry A, Sayed O, Hussein R, Saeed H, Abdelrahim M. The impact of adding a training device to familiar counselling on inhalation technique and pulmonary function of asthmatics. Adv Ther. (2018) 35(7):1049–58. doi: 10.1007/s12325-018-0737-6
62. Elgendy MO, Abdelrahim ME, Eldin RS. Potential benefit of repeated MDI inhalation technique counselling for patients with asthma. Eur J Hosp Pharm. (2015) 22(6):318–22. doi: 10.1136/ejhpharm-2015-000648
63. Saeed H, Mohsen M, Eldin AS, Elberry AA, Hussein RR, Rabea H, et al. Effects of fill volume and humidification on aerosol delivery during single-limb noninvasive ventilation. Respir Care. (2018) 63(11):1370–8. doi: 10.4187/respcare.06022
64. Hassan A, Rabea H, Hussein RR, Eldin RS, Abdelrahman MM, Said AS, et al. In vitro characterization of the aerosolized dose during non-invasive automatic continuous positive airway pressure ventilation. Pulm Ther. (2016) 2:115–26. doi: 10.1007/s41030-015-0010-y
65. Elgendy MO, Hassan AH, Saeed H, Abdelrahim ME, Eldin RS. Asthmatic children and MDI verbal inhalation technique counseling. Pulm Pharmacol Ther. (2020) 61:101900. doi: 10.1016/j.pupt.2020.101900
66. Harb HS, Elberry AA, Rabea H, Fathy M, Abdelrahim ME. Performance of large spacer versus nebulizer T-piece in single-limb noninvasive ventilation. Respir Care. (2018) 63(11):1360–9. doi: 10.4187/respcare.05976
67. Madney YM, Fathy M, Elberry AA, Rabea H, Abdelrahim ME. Nebulizers and spacers for aerosol delivery through adult nasal cannula at low oxygen flow rate: an in vitro study. J Drug Deliv Sci Technol. (2017) 39:260–5. doi: 10.1016/j.jddst.2017.04.014
68. Vecellio L, Abdelrahim ME, Montharu J, Galle J, Diot P, Dubus J-C. Disposable versus reusable jet nebulizers for cystic fibrosis treatment with tobramycin. J Cyst Fibros. (2011) 10(2):86–92. doi: 10.1016/j.jcf.2010.10.004
69. Zawbaa HM, Osama H, El-Gendy A, Saeed H, Harb HS, Madney YM, et al. Effect of mutation and vaccination on spread, severity, and mortality of COVID-19 disease. J Med Virol. (2022) 94(1):197–204. doi: 10.1002/jmv.27293
70. Stefani D, Plönes T, Viehof J, Darwiche K, Stuschke M, Schuler M, et al. Lung cancer surgery after neoadjuvant immunotherapy. Cancers (Basel). (2021) 13(16):4033. doi: 10.3390/cancers13164033
71. Brandt WS, Yan W, Zhou J, Tan KS, Montecalvo J, Park BJ, et al. Outcomes after neoadjuvant or adjuvant chemotherapy for cT2-4N0-1 non-small cell lung cancer: a propensity-matched analysis. J Thorac Cardiovasc Surg. (2019) 157(2):743–753.e3. doi: 10.1016/j.jtcvs.2018.09.098
72. Bailly C, Thuru X, Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer. (2020) 2(1):zcaa002. doi: 10.1093/narcan/zcaa002
73. Ulas EB, Dickhoff C, Schneiders FL, Senan S, Bahce I. Neoadjuvant immune checkpoint inhibitors in resectable non-small-cell lung cancer: a systematic review. ESMO Open. (2021) 6(5):100244. doi: 10.1016/j.esmoop.2021.100244
74. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. (2014) 384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8
75. Menzies AM, Amaria RN, Rozeman EA, Huang AC, Tetzlaff MT, van de Wiel BA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. (2021) 27(2):301–9. doi: 10.1038/s41591-020-01188-3
76. Mouillet G, Monnet E, Milleron B, Puyraveau M, Quoix E, David P, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol. (2012) 7(5):841–9. doi: 10.1097/JTO.0b013e31824c7d92
77. William WN Jr, Pataer A, Kalhor N, Correa AM, Rice DC, Wistuba II, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. (2013) 8(2):222–8. doi: 10.1097/JTO.0b013e3182774108
78. Di Giacomo AM, Danielli R, Guidoboni M, Calabrò L, Carlucci D, Miracco C, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. (2009) 58(8):1297–306. doi: 10.1007/s00262-008-0642-y
79. Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al. Novel patterns of response under immunotherapy. Ann Oncol. (2019) 30(3):385–96. doi: 10.1093/annonc/mdz003
80. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. (2021) 398(10299):535–54. doi: 10.1016/S0140-6736(21)00312-3
Keywords: resectable non-small-cell lung tumors, prognosis prediction, pathological response, programmed death-ligand 1, neoadjuvant immunotherapy, stage
Citation: Nie F, Wang Y, Shi W, Zhu L, Hao J and Tao R (2024) Prognosis prediction using significant pathological response following neoadjuvant immunotherapy in resectable non-small-cell lung tumors: a meta-analysis. Front. Surg. 11:1500593. doi: 10.3389/fsurg.2024.1500593
Received: 23 September 2024; Accepted: 4 November 2024;
Published: 22 November 2024.
Edited by:
Giulio Maurizi, Sapienza University of Rome, ItalyReviewed by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesFilipe Azenha, University of Lucerne, Switzerland
Copyright: © 2024 Nie, Wang, Shi, Zhu, Hao and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rancen Tao, dGFvcmFuY2VuQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Fang Nie1,†
Fang Nie1,† Rancen Tao
Rancen Tao