- Department of Medical Oncology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Traditional Chinese Medicine), Hangzhou, Zhejiang, China
Background: Tunneled peripherally inserted central catheters (PICC) have potential to reduce complications compared to non-tunneled PICC in previous studies. Which is better is debatable. Thus, the aim to compare the effect of tunneled and non-tunneled PICC for cancer patients undergoing chemotherapy.
Methods: Embase, PubMed, Cochrane Library database, and CNKI were searched from inception to March 15, 2024. Odds ratios (ORs) with 95% confidence intervals (95% CIs) was calculated to assess the complications of tunneled and non-tunneled PICC for cancer patients undergoing chemotherapy using random- or fixed-effects models.
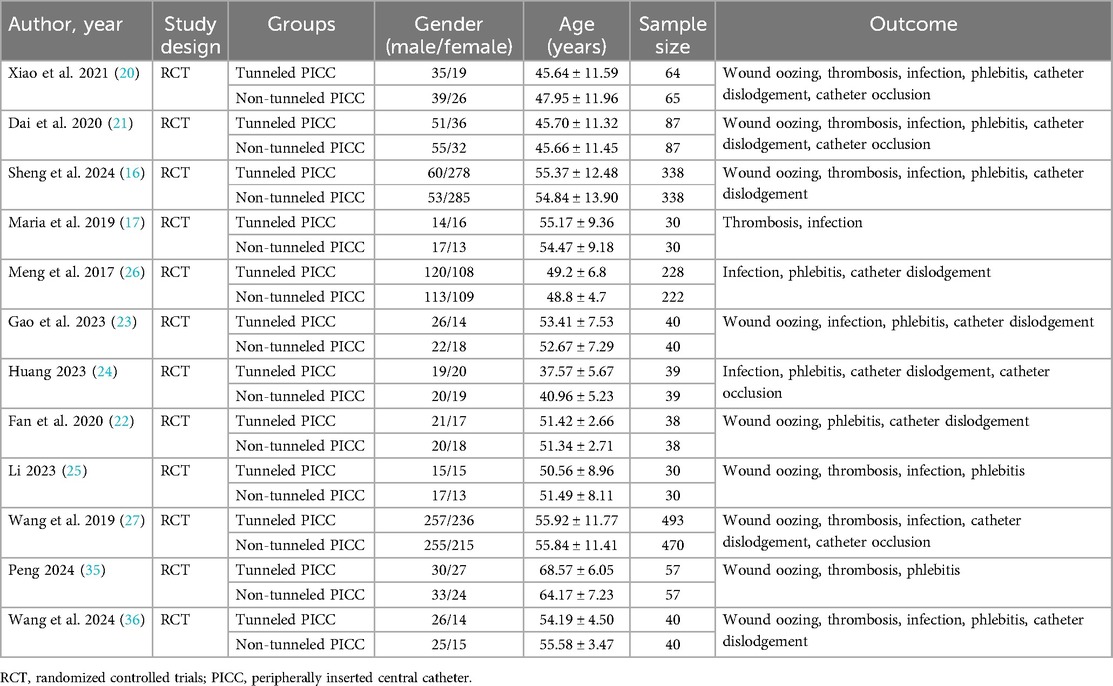
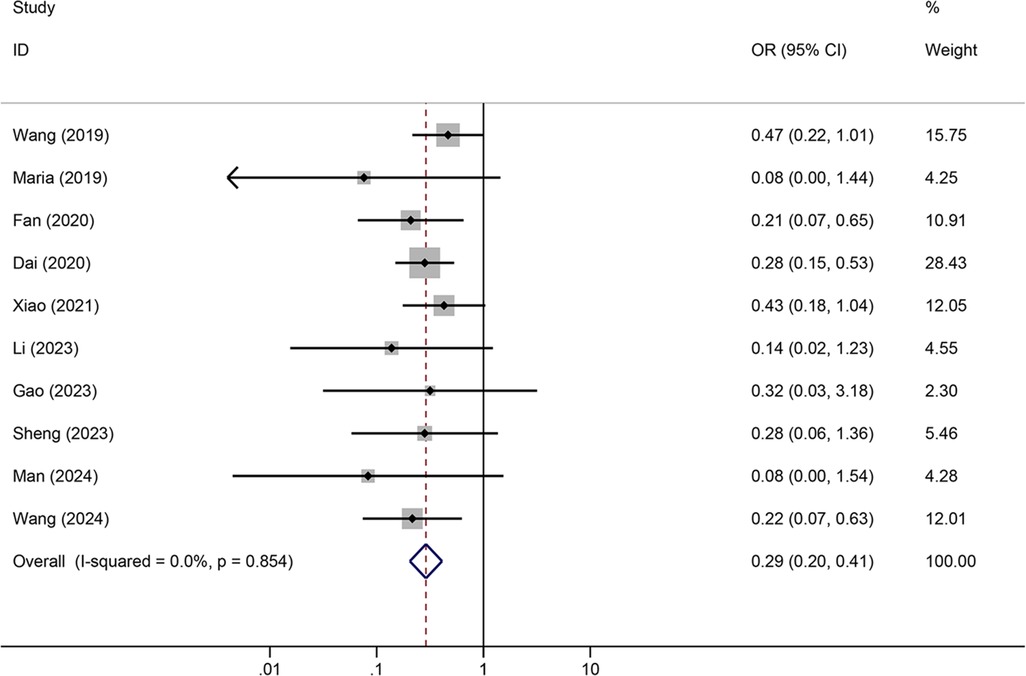
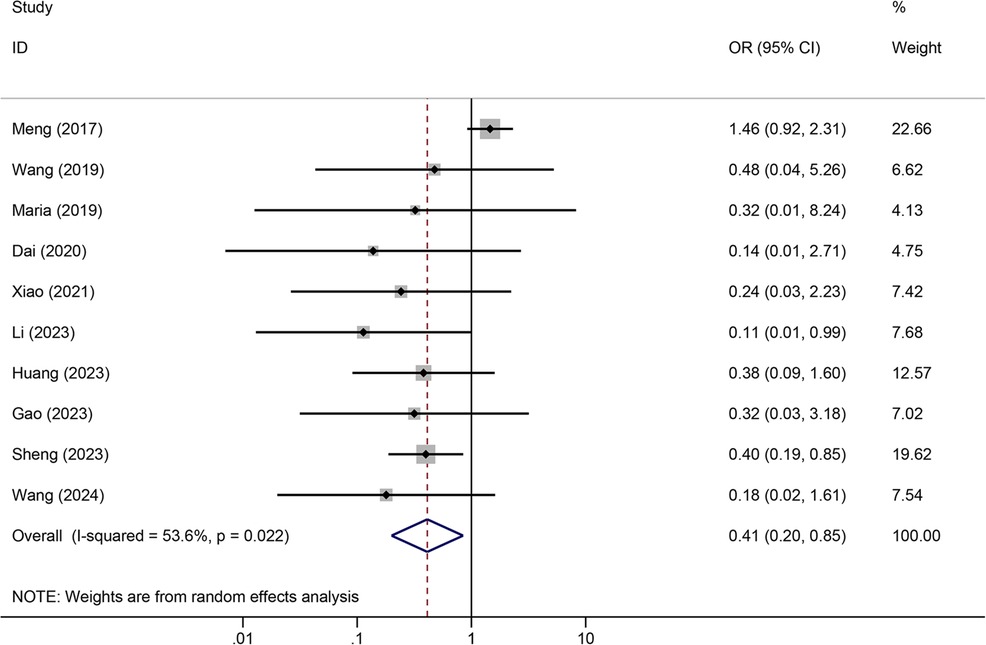
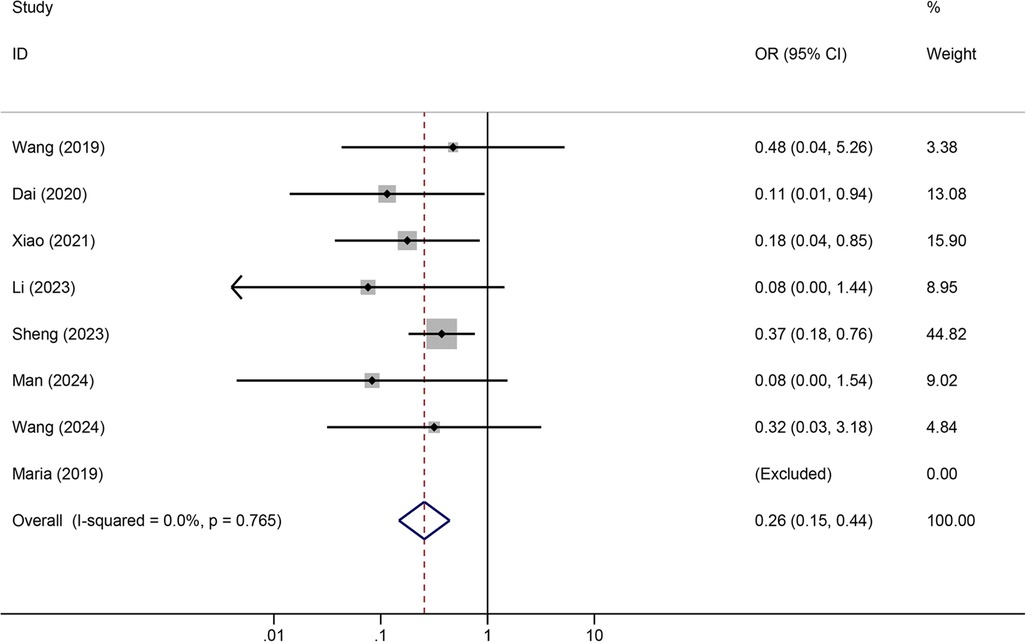
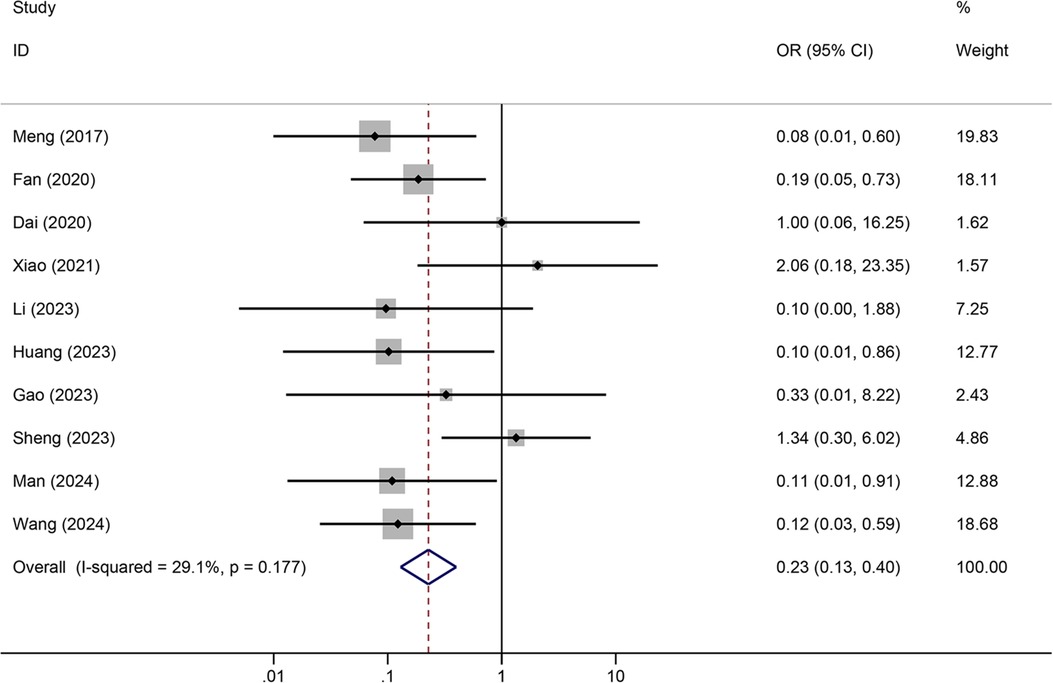
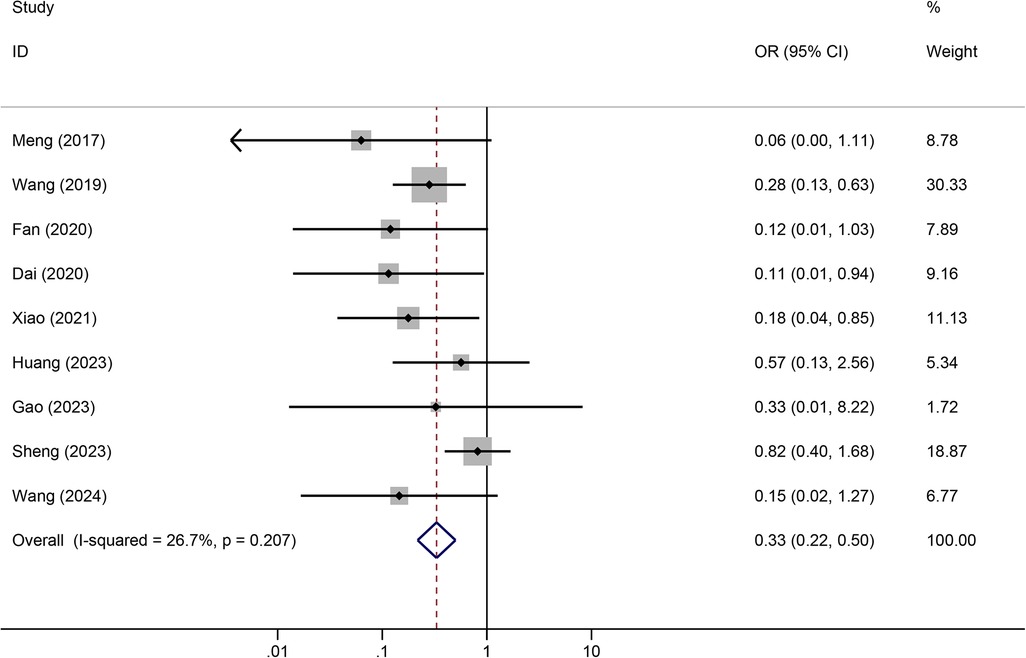
Results: A total of 12 articles were retrieved. Meta-analysis showed that tunneled PICC significantly decreased the risk of wound oozing (OR: 0.29, 95% CI: 0.20–0.41), infection risk (OR: 0.41, 95% CI: 0.20–0.85), thrombosis risk (OR: 0.26, 95% CI: 0.15–0.44), phlebitis risk (OR: 0.23, 95% CI: 0.13–0.40), and catheter dislodgement risk (OR: 0.33, 95% CI: 0.22–0.50) compared to non-tunneled PICC.
Conclusions: The subcutaneous tunneling technology has advantages over normal technique in decreasing PICC-related complications for cancer patients undergoing chemotherapy.
Systematic Review Registration: PROSPERO (CRD42024522862).
Introduction
Cancer has become the second leading cause of death worldwide, posing a serious threat to people's lives and health, and its incidence is increasing annually (1). Chemotherapy is the standard treatment for cancer patients and can prolong their survival (2). However, long-term chemotherapy causes significant damage to the body of cancer patients (3). Prolonged intravenous infusion can cause vascular injury and increase the risk of catheter-related bloodstream infections (4, 5). Currently, traditional peripherally inserted central catheter (PICC) techniques are widely used in the chemotherapeutic treatment of cancer patients (6, 7). Traditional PICC methods have advantages, but the issue of catheter-related infections cannot be ignored because they can lead to infective endocarditis, septic embolism, and even death (8, 9). Another infection complication that causes 30% of traditional PICC treatment failures is catheter-related thrombosis (CRT), which is associated with hypercoagulability and endothelial vascular injury (10). Furthermore, if there are poor vascular conditions in the mid-upper arm or scarred skin in the puncture area, traditional non-tunneled PICC may result in changes in the puncture area, ultimately leading to puncture near the axilla (11). However, puncture in this area increases the probabilities of catheter displacement, dislodgement, and bloodstream infections.
In recent years, subcutaneous tunneling techniques have attracted a considerable amount of attention. In tunneled PICC, the upper 1/3 of the arm is the puncture site, and the external part of the catheter is passed through a subcutaneous tunnel to the middle 1/3 of the arm, thus achieving the optimal exit position for the catheter (12, 13). This is because the middle arm provides the greatest stability, thereby lowering the risks of infection, venous thrombosis, catheter displacement, and other complications associated with conventional PICC placement (14). However, only a few studies have compared the outcomes of tunneled and non-tunneled PICC during adjuvant chemotherapy (15–17). Currently, there is no clear or consistent evidence suggesting which treatment is safer or preferable. Therefore, the aim of this meta-analysis was to compare the outcomes of tunneled and non-tunneled PICC placement during cancer chemotherapy treatment and provide useful information for physicians to better counsel cancer patients.
Materials and methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements (18). Ethical approval is not required due to all the data analysis based on the published data. This meta-analysis was registered in the International prospective register of systematic reviews (PROSPERO registration number: CRD42024522862).
Literature search
The literature search was performed using the Embase, PubMed, Cochrane Library database, and CNKI from inception to March 15, 2024. We used Boolean logic with keywords or MeSH terms included PICC, peripherally inserted central catheter, tunneled, cancer, and chemotherapy. References of the included studies were checked for additional potentials studies.
Study selection
The inclusion criteria were as follows: (1) population: chemotherapy-treated cancer patients; (2) intervention: tunneled PICC; (3) comparison: non-tunneled PICC; (4) outcome: wound oozing, thrombosis, infection, phlebitis, catheter dislodgement, and catheter occlusion; (5) study design: randomized controlled trial (RCT). The exclusion criteria were as follows: (1) incompletely reported data; (2) duplicate previous literature; (3) conference abstracts, comments, or reviews.
Data extraction
Two reviewers independently extracted information from included studies using a standardized electronic form. Any disagreements were resolved through discussion with a third reviewer. The following information was extracted: first author, study design, groups, gender, age, sample size, and outcome.
Quality assessment
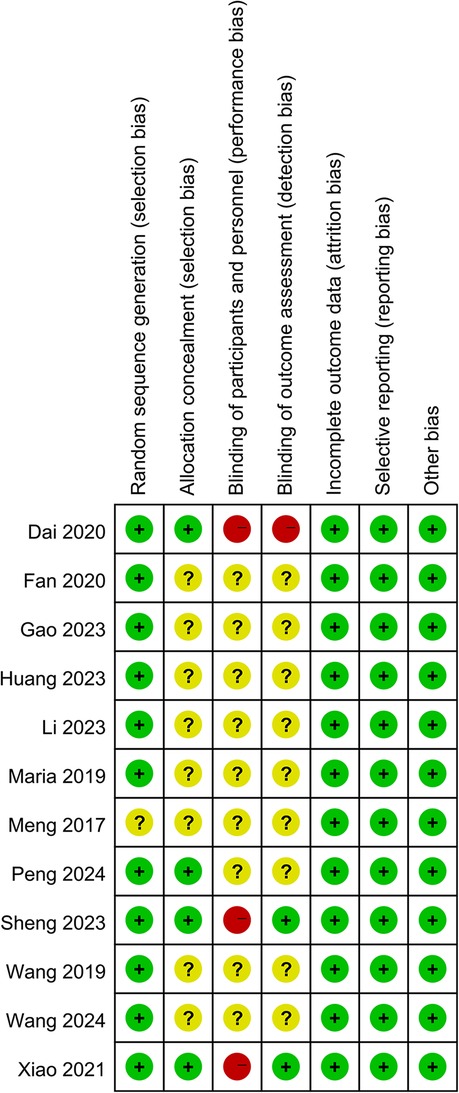
Two reviewers independently assessed the risk of bias of the included RCTs using the Cochrane Collaboration risk of bias tool (19). It contains six perspectives including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, selective reporting, and other bias risk. Each perspective was judged as “low”, “high”, or “unclear” risk.
Statistical analysis
Statistical analyses were undertaken using Stata software version 12.0 (Cochrane Collaboration, Oxford, UK). The random- or fixed effect model was used to calculate the odds ratio (OR) with a 95% confidence interval (95% CI). The I2 and chi-square tests were used to assess the heterogeneity of the studies. The I2 < 25%, 25% ≤ I2 < 50%, 50% ≤ I2 < 75%, and I2 ≥ 75% indicated no heterogeneity, low heterogeneity, moderate heterogeneity, and high heterogeneity, respectively. If heterogeneity is observed, the random-effects model is used, otherwise, a fixed effect model was selected for analysis. The publication biases were judged by Egger test and Begg test. Sensitivity tests were also conducted to examine the robustness of the disparities.
Results
Study selection
The literature search yielded 342 articles. After removing 22 duplicated studies, 320 articles that potentially investigated tunneled and non-tunneled PICC in chemotherapy-treated cancer patients were screened. After screening the title and abstract, 305 articles were removed, and 15 studies were eligible for full-text review. Of these, 3 studies were excluded. Finally, 12 RCTs (16, 17, 20–27) with 2,940 participants (tunneled PICC 1,484 vs. non-tunneled PICC 1,456) were included in this meta-analysis (Figure 1).
Study characteristics
The characteristics of the included studies were presented in Table 1. These studies were published between the years of 2017 and 2024. Eleven studies were conducted in the China and 1 in the Greece. The sample sizes of the trials ranged from 30 to 493. The mean age of the participants ranged from 37.57 to 68.57 in the tunneled PICC group and 40.96 to 64.17 in the non-tunneled PICC group. The 1,377 participants in the tunneled PICC group and 1,359 in the non-tunneled PICC group. The outcomes index contains wound oozing, thrombosis, infection, phlebitis, catheter dislodgement, and catheter occlusion.
Results of quality assessment
The Cochrane risk of bias assessment tool was used to evaluate risk in the included studies. Eight studies didn't describe allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. One study didn't report random sequence generation. Two studies had blinded outcome assessments and none blinded participants or personnel (Figure 2).
Meta-analysis results
Tunneled PICC significantly decreased the risk of wound oozing (OR: 0.29, 95% CI: 0.20–0.41, p < 0.001) with low heterogeneity (I2 = 0%), infection risk (OR: 0.41, 95% CI: 0.20–0.85, p = 0.032) with moderate heterogeneity (I2 = 54.6%), thrombosis risk (OR: 0.26, 95% CI: 0.15–0.44, p < 0.001) with low heterogeneity (I2 = 0%), phlebitis risk (OR: 0.23, 95% CI: 0.13–0.40, p < 0.001) with moderate heterogeneity (I2 = 29.1%), and catheter dislodgement risk (OR: 0.33, 95% CI: 0.22–0.50, p < 0.001) with moderate heterogeneity (I2 = 26.7%) compared to non-tunneled PICC (Figures 3–7). However, no significant difference was observed in catheter occlusion risk (OR: 0.82, 95% CI: 0.49–1.37, p = 0.450) with moderate heterogeneity (I2 = 47.6%).
Sensitivity analyses
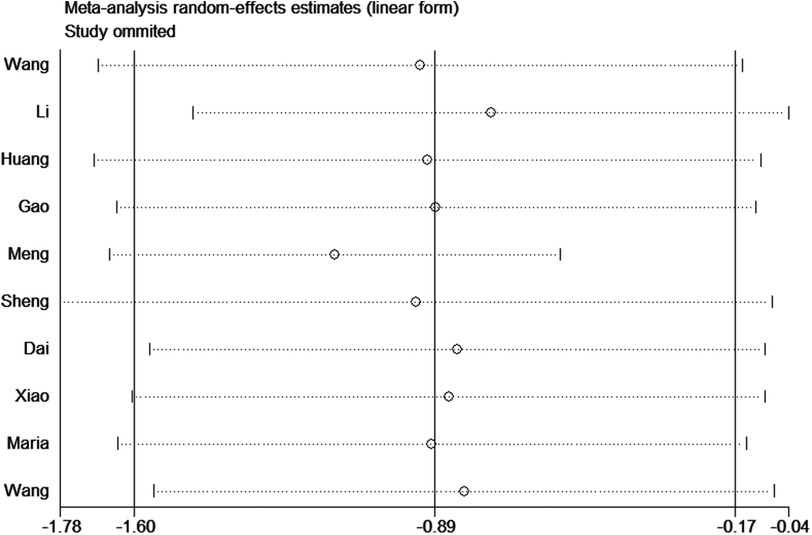
To assess the robustness of the results, we performed a sensitivity analysis by removing Meng et al. 2017. The heterogeneity significantly decreased (I2 = 0%) and the result was reliable (Figure 8).
Publication bias
To assess the presence of publication bias in this meta-analysis regarding infection risk, visual funnel plots and Egger's regression test were employed. The funnel plots exhibited a balanced and symmetrical shape, suggesting no substantial publication bias. The statistical analysis further confirmed this finding, with the calculated P-value of the Egger test being 0.325, endorsing the absence of significant publication bias in the study.
Discussion
Despite being an important decision in clinical practice, very little is known about the benefits and relative risks of various venous access methods for cancer patients receiving chemotherapy. This meta-analysis included 2,940 cancer patients receiving chemotherapy. Among these patients, 1,484 underwent tunneled PICC placement and 1,456 underwent non-tunneled PICC placement. Compared to non-tunneled PICC placement, tunneled PICC placement significantly reduced the incidences of wound leakage, infection risk, thrombosis, venous inflammation, and catheter dislocation.
In this study, the rates of wound leakage, infection, and catheter dislodgement were significantly lower in patients treated with the subcutaneous tunnel technique than in patients treated with the conventional method for placing PICCs, which is consistent with the findings of previous research (20). However, we found that the prevalence of catheter occlusion was not significantly different between tunneled PICC and non-tunneled PICC (OR: 0.82, 95% CI: 0.49–1.37, p = 0.450) in all subjects. Interestingly, although the study by Meng et al. (26) found that 7.4% of patients with tunneled PICC developed catheter occlusion compared with 6.7% of patients in the non-tunneled PICC group, other studies comparing tunneled PICC with non-tunneled PICC reported a decreased risk of PICC-associated catheter occlusion (24, 27). Part of the difference observed in rates of catheter occlusion among studies may be insufficient sample sizes and different tunnel lengths. Catheter dislodgement can be caused by coughing, vomiting, improper arm positioning, or skin issues. The presence of a subcutaneous tunnel increases the strength of the fixation of the catheter to the surrounding skin, reducing the mobility of the catheter and therefore reducing the dislodgement rate (28). Additionally, the tunnel that forms between the venous puncture site and the catheter exit site acts as a buffer and provides compression hemostasis, reducing the rate of bleeding. Furthermore, the subcutaneous tunnel increases the difficulty for microorganisms to travel retrograde along the catheter, reducing the incidence of infection (16). Moreover, the lower rate of catheter malpositioning reduces the introduction of pathogens due to repeated inward displacement of the catheter, and the lower rate of bleeding reduces the colonization of bacteria on the catheter, further controlling the occurrence of infection (29).
The ratio of catheter-to-vein diameter is an important factor affecting the occurrence of PICC-related venous thrombosis. The ratio of catheter to vein should be between 33% and 45% to reduce the incidence of thrombosis (30). The subcutaneous tunnel allows higher positioning of the puncture point, where the vein diameter is larger, and preserves an exit site in one-third of the arm for proper fixation (31). A larger vessel diameter not only helps reduce the incidence of thrombosis but also allows the use of multilumen catheters that would otherwise exceed the optimal ratio of catheter to vein for suitable vessels. A larger lumen diameter at the tunneled PICC puncture site reduces the incidence of thrombosis caused by repeated mechanical friction against the vessel wall and reduced blood flow (32). Additionally, vein wall damage is also a major cause of venous inflammation, and a lower catheter-to-vein diameter ratio reduces mechanical friction between the catheter and vessel wall, thereby reducing the occurrence of venous inflammation (12, 33).
Tunnel length is associated with the risk of PICC-related complications, and longer tunnel lengths increase the stability of tunnel passages (34). Earlier studies have found that PICC with a tunnel length of 5 cm significantly reduced the incidence of complications (20). However, PICC with a tunnel length of 6 cm did not show a greater advantage against in reducing bleeding event (15). In our included literatures, the length of the tunnel was between 3 and 5 cm. Recent study has compared different tunnel length for a tunneled PICC to reduce the risk of PICC-related complications and found that a longer tunnel length was associated with longer catheter residence times and fewer PICC-related complications, and a tunnel length longer than 4 cm was recommended for tunneled PICC (15).
Several limitations of this study should be noted. First, due to the limited number of studies, we were unable to determine whether the results were influenced by age or race. Second, there were insufficient data to compare the impact of different tunnel lengths on outcome measures; the nutritional status of the respondents as well as age, sex, and sex characteristics are potential sources of bias. Furthermore, our study did not evaluate the cost-effectiveness of these two regimens. Cost-effectiveness should also be considered when selecting tunnel PICC placement for cancer patients.
Conclusions
In conclusion, for cancer patients undergoing chemotherapy, subcutaneous tunneling technology are a safer treatment option than non-tunneled PICC. However, due to limitations in the number and quality of the included research, the conclusions of this study need to be confirmed by using larger sample sizes, multicenter and high-quality clinical trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JH: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XM: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Yang L, Yang J, Kleppe A, Danielsen HE, Kerr DJ. Personalizing adjuvant therapy for patients with colorectal cancer. Nat Rev Clin Oncol. (2024) 21(1):67–79. doi: 10.1038/s41571-023-00834-2
3. Carvalho C, Glynne-Jones R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol. (2017) 18(6):e354–e63. doi: 10.1016/S1470-2045(17)30346-7
4. Luvira V, Satitkarnmanee E, Pugkhem A, Kietpeerakool C, Lumbiganon P, Pattanittum P. Postoperative adjuvant chemotherapy for resectable cholangiocarcinoma. Cochrane Database Syst Rev. (2021) 9(9):CD012814. doi: 10.1002/14651858.CD012814.pub2
5. Giustozzi M, Franco L, Agnelli G, Verso M. Unmet clinical needs in the prevention and treatment of cancer-associated venous thromboembolism. Trends Cardiovasc Med. (2023) 33(6):336–43. doi: 10.1016/j.tcm.2022.02.003
6. Chopra V, Flanders SA, Saint S, Woller SC, O'Grady NP, Safdar N, et al. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. (2015) 163(6 Suppl):S1–40. doi: 10.7326/M15-0744
7. Chen J, Zhao H, Xia Z, Zhang Y, Lv X, Zhou X, et al. Knowledge, attitude, and practice toward the daily management of PICC in critically ill cancer patients discharged from intensive care units. Cancer Manag Res. (2018) 10:1565–74. doi: 10.2147/CMAR.S166008
8. Liu X, Tao S, Ji H, Chen S, Gu Y, Jin X. Risk factors for peripherally inserted central catheter (PICC)-associated infections in patients receiving chemotherapy and the preventive effect of a self-efficacy intervention program: a randomized controlled trial. Ann Palliat Med. (2021) 10(9):9398–405. doi: 10.21037/apm-21-1848
9. Liu R, Xu H, Pu L, Xie X, Chen H, Wu Z, et al. Clinical characteristics of peripherally inserted central catheter-related complications in cancer patients undergoing chemotherapy: a prospective and observational study. BMC Cancer. (2023) 23(1):894. doi: 10.1186/s12885-023-11413-0
10. Kang J, Chen W, Sun W, Ge R, Li H, Ma E, et al. Peripherally inserted central catheter-related complications in cancer patients: a prospective study of over 50,000 catheter days. J Vasc Access. (2017) 18(2):153–7. doi: 10.5301/jva.5000670
11. Zapletal O, Sirotek L, Coufal O. Venous access in cancer patients. Rozhl Chir. (2019) 98(11):427–33. doi: 10.33699/PIS.2019.98.11.427-433
12. Giustivi D, Gidaro A, Baroni M, Paglia S. Tunneling technique of PICCs and midline catheters. J Vasc Access. (2022) 23(4):610–4. doi: 10.1177/11297298211002579
13. Brescia F, Pittiruti M, Spencer TR, Dawson RB. The SIP protocol update: eight strategies, incorporating rapid peripheral vein assessment (RaPeVA), to minimize complications associated with peripherally inserted central catheter insertion. J Vasc Access. (2022) 11297298221099838. doi: 10.1177/11297298221099838
14. Dawson RB. PICC Zone insertion MethodTM (ZIMTM): a systematic approach to determine the ideal insertion site for PICCs in the upper arm. J Association for Vascular Access. (2011) 16(3):156–65. doi: 10.2309/java.16-3-5
15. Li J, Hu Z, Lin X, Huang W, Huang C, Luo J, et al. A randomized controlled trial to compare peripherally inserted central catheter tunnel lengths in adult patients with cancer. Clin J Oncol Nurs. (2023) 27(3):295–304. doi: 10.1188/23.CJON.295-304
16. Sheng Y, Yang LH, Wu Y, Gao W, Dongye SY. Implementation of tunneled peripherally inserted central catheters placement in cancer patients: a randomized multicenter study. Clin Nurs Res. (2024) 33(1):19–26. doi: 10.1177/10547738231194099
17. Maria K, Theodoros K, Maria B, Panagiotis K, Emmanouil S, Evangelos KA. Implementation of tunneled versus not tunneled peripherally inserted central catheters. J Vasc Nurs. (2019) 37(2):132–4. doi: 10.1016/j.jvn.2018.11.007
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. (2021) 18(3):e1003583. doi: 10.1371/journal.pmed.1003583
19. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
20. Xiao MF, Xiao CQ, Li J, Dai C, Fan YY, Cao HJ, et al. Subcutaneous tunneling technique to improve outcomes for patients undergoing chemotherapy with peripherally inserted central catheters: a randomized controlled trial. J Int Med Res. (2021) 49(4):3000605211004517. doi: 10.1177/03000605211004517
21. Dai C, Li J, Li QM, Guo X, Fan YY, Qin HY. Effect of tunneled and nontunneled peripherally inserted central catheter placement: a randomized controlled trial. J Vasc Access. (2020) 21(4):511–9. doi: 10.1177/1129729819888120
22. Fan B, Huang FM, Meng X. A comparative study of tunnel PICC catheterization and conventional PICC catheterization. China Modern Doctor. (2020) 58(27):166–9.
23. Gao WY, Yu WB, Yang GD, Li XN. Application of tunnel PICC catheterization in tumor patients. Chinese Evidence-Based Nursing. (2023) 9(4):713–5. doi: 10.12102/j.issn.2095-8668.2023.04.029
24. Huang XX. Comparative application of tunnel PICC catheterization and conventional PICC catheterization in tumor chemotherapy. The Medical Forum. (2023) 27(18):46–8. doi: 10.19435/j.1672-1721.2023.18.014
25. Li LX. Clinical application of tunnel PICC implantation. The Medical Forum. (2023) 27(27):4–6. doi: 10.19435/j.1672-1721.2023.27.002
26. Meng QB, Wang FL, Wang GX, Zhang WC, Li M, Ma YN. Clinical application of located subcutaneous jugular vein inserted central catheter in cancer patients. Chin J Nurs. (2017) 52(12):1464–8. doi: 10.3761/j.issn.0254-1769.2017.12.011
27. Wang AJ, Zheng X, Xu XF, Luan XR. Application of one-insertion subcutaneous tunneling technique for power PICC placement via ultrasound. Chin J Nurs. (2019) 54(11):1707–11. doi: 10.3761/j.issn.0254-1769.2019.11.022
28. Ostroff MD, Moureau NL. Report of modification for peripherally inserted central catheter placement: subcutaneous needle tunnel for high upper arm placement. J Infus Nurs. (2017) 40(4):232–7. doi: 10.1097/NAN.0000000000000228
29. Sriskandarajah P, Webb K, Chisholm D, Raobaikady R, Davis K, Pepper N, et al. Retrospective cohort analysis comparing the incidence of deep vein thromboses between peripherally-inserted and long-term skin tunneled venous catheters in hemato-oncology patients. Thromb J. (2015) 13:21. doi: 10.1186/s12959-015-0052-2
30. Sharp R, Cummings M, Fielder A, Mikocka-Walus A, Grech C, Esterman A. The catheter to vein ratio and rates of symptomatic venous thromboembolism in patients with a peripherally inserted central catheter (PICC): a prospective cohort study. Int J Nurs Stud. (2015) 52(3):677–85. doi: 10.1016/j.ijnurstu.2014.12.002
31. Gupta N, Gandhi D, Sharma S, Goyal P, Choudhary G, Li S. Tunneled and routine peripherally inserted central catheters placement in adult and pediatric population: review, technical feasibility, and troubleshooting. Quant Imaging Med Surg. (2021) 11(4):1619–27. doi: 10.21037/qims-20-694
32. Kagan E, Salgado CD, Banks AL, Marculescu CE, Cantey JR. Peripherally inserted central catheter-associated bloodstream infection: risk factors and the role of antibiotic-impregnated catheters for prevention. Am J Infect Control. (2019) 47(2):191–5. doi: 10.1016/j.ajic.2018.07.006
33. Corti F, Brambilla M, Manglaviti S, Di Vico L, Pisanu MN, Facchinetti C, et al. Comparison of outcomes of central venous catheters in patients with solid and hematologic neoplasms: an Italian real-world analysis. Tumori. (2021) 107(1):17–25. doi: 10.1177/0300891620931172
34. Lingegowda D, Gehani A, Sen S, Mukhopadhyay S, Ghosh P. Centrally inserted tunnelled peripherally inserted central catheter: off-label use for venous access in oncology patients. J Vasc Access. (2020) 21(5):773–7. doi: 10.1177/1129729820909028
35. Peng ML. Application of tunnel PICC in catheterization of advanced tumor patients. Health Guide. (2024) 1:148–50.
Keywords: PICC, cancer, complications, tunneled, meta-analysis
Citation: Hong J and Mao X (2024) Complications of tunneled and non-tunneled peripherally inserted central catheter placement in chemotherapy-treated cancer patients: a meta-analysis. Front. Surg. 11:1469847. doi: 10.3389/fsurg.2024.1469847
Received: 28 July 2024; Accepted: 26 September 2024;
Published: 15 October 2024.
Edited by:
Saroj Das, Imperial Consultants, United KingdomReviewed by:
Mojahid Najem, Bedford Hospital NHS Trust, United KingdomMauro Pittiruti, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright: © 2024 Hong and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodan Mao, MTg3NTgxMTc5MTJAMTYzLmNvbQ==
 Jiana Hong
Jiana Hong Xiaodan Mao
Xiaodan Mao