- 1Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Nuclear Medicine Department, Tianjin Medical University Clinical Cardiovascular Institute, TEDA International Cardiovascular Hospital, Tianjin, China
- 3Department of Cardiovascular Surgery, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
We report a case of a patient with dilated cardiomyopathy who experienced recurrent ventricular tachycardia (VT) and multiple defibrillations following CRT-D implantation. Due to worsening cardiac function, the patient required surgical implantation of a left ventricular assist device (LVAD) as a bridge to heart transplantation. During the procedure, we used the Ensite three-dimensional mapping system to perform activation and substrate mapping of the VT targets, followed by endocardial and epicardial cryoballoon ablation of clinical VT. Subsequently, during LVAD implantation, dual cryoballoon ablation was applied around the surgical incision site to prevent VT associated with the surgical wound and the implanted device. At the 1-year follow-up, the patient had no recurrence of the original clinical VT and no new ventricular arrhythmias were observed.
Introduction
A 40-year-old male patient was admitted for the first time on January 29, 2021, 12 days after cardiopulmonary resuscitation, due to recurrent chest tightness and shortness of breath over the past 10 years. Cardiac Magnetic Resonance Imaging and echocardiography revealed global cardiac enlargement and significantly reduced ejection fraction (Left Atrium systolic dimension (LAS) 52 mm, Left ventricularend diastolic dimension (LVD) 85 mm, Right Atrium systolic dimension (RAS) 50 mm, Right Ventricular diastolic dimension (RVD) 42 mm, Ejection Fraction (EF) 22%). He was diagnosed with non-ischemic dilated cardiomyopathy. A 24-h Holter monitor performed after admission indicated 3,455 episodes of multifocal premature ventricular contractions and three episodes of nonsustained ventricular tachycardia (VT). Following comprehensive evaluation, cardiac resynchronization therapy-defibrillator (CRT-D) implant was implanted on February 18, 2021.
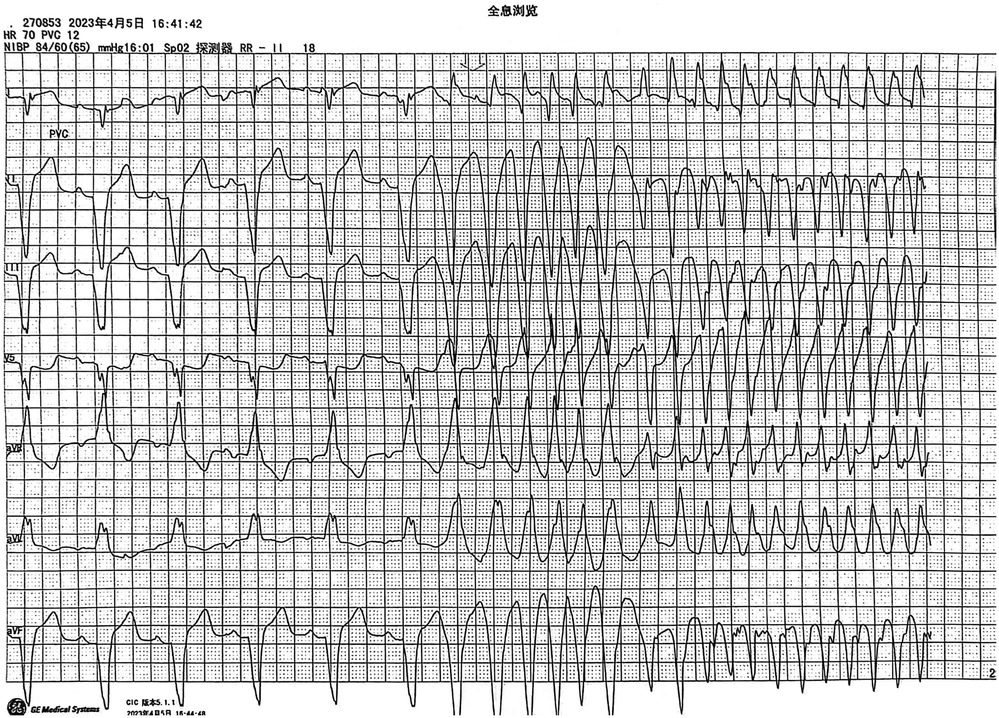
Monitoring captured episodes of VT, identified as monomorphic VT, likely scar-related arrhythmia. Due to the absence of chest leads on the monitor, only limb lead electrocardiograms were available for analysis. Based on the electrocardiogram (ECG), the VT was preliminarily determined to originate from the lower ventricle. The biphasic waveform in lead I suggested that the arrhythmia might have originated from the septal region. The VT episodes occurred spontaneously and were terminated by the CRT-D device. Given the patient's severely impaired cardiac function, with an EF of only 22%, and the accompanying hemodynamic instability, initial management prioritized medication and CRT-D implantation over VT ablation.
Despite oral amiodarone and metoprolol sustained-release tablets, the patient continued to experience VT episodes, with 30 defibrillator discharges between April 4, 2022, and April 25, 2023. After multiple hospital evaluations, heart transplantation was considered, and the patient was readmitted on April 25, 2023, for planned left ventricular assist device (LVAD) implantation.Considering the patient's recurrent VT episodes despite medication, ventricular tachycardia ablation was performed under pre-visualized guidance simultaneously with the LVAD implantation.
Epicardial mapping (Ensite, St. Jude Medical) was performed through a subxiphoid approach without cardiopulmonary bypass. During sinus rhythm, a large low-voltage area was observed in the left ventricle (Figures 2B, C). The induced VT (Figure 2A) was similar to the patient's symptomatic episodes (Figure 1). Due to the non-sustained nature of the VT, short bursts of ventricular stimulation were used to induce VT for mapping. The earliest activation points during VT coincided with the low-voltage scar areas observed during sinus rhythm, with significant fragmented potentials preceding the surface QRS complex (Figures 2D, E, F).
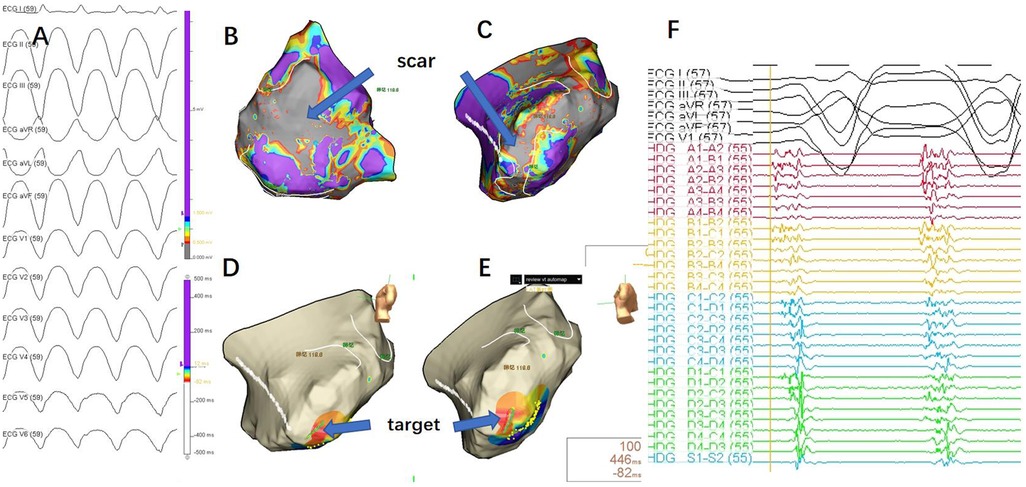
Figure 2. (A) 12-lead ECG during ventricular tachycardia (VT) episode.Due to the requirement for an anterior thoracotomy, the precordial leads were placed on the iliac crest. As a result, the precordial lead reference differed from the VT morphology typically observed during the patient's daily episodes, but the limb lead morphology was consistent with the patient's daily VT episodes. (B) Epicardial substrate map during sinus rhythm (posterior-anterior view), with the scar area shown in gray, indicating no electrical activity. (C) Epicardial substrate map during sinus rhythm (left lateral view), with the scar area shown in gray, indicating no electrical activity. (D, E) Mapping during VT, with the earliest activation point indicated by the blue arrow. (F) Mapping target potential, showing fragmented waves in high-density mapping, significantly preceding the QRS complex in the surface ECG.
Considering the need for LVAD implantation and to prevent scar-related reentrant arrhythmias at the inflow cannula and left ventricular apex anastomosis site, ablation was necessary at the incision site. Given the limited treatment area per application with radiofrequency ablation, cryoballoon ablation (Arctic Front Advance Cryoablation, Medtronic Inc) was chosen to shorten the procedure time and ensure safety. Guided by three-dimensional mapping, the target areas were delineated, and scar-related VAs were ablated using cryoballoon technology (Figure 3A), including the left ventricular apex inflow cannula anastomosis site (Figure 3B).
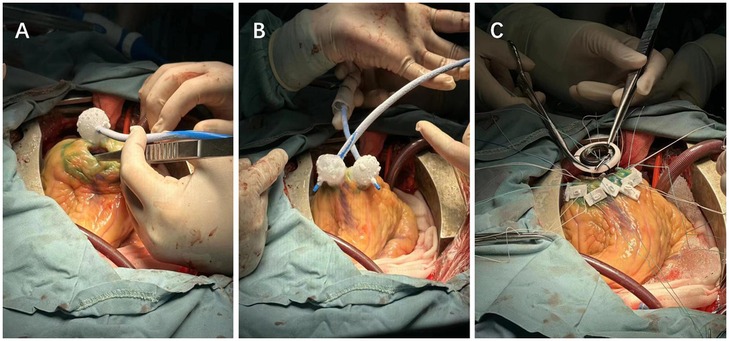
Figure 3. (A) Cryoablation of scar-related ventricular tachycardia. (B) Alternating ablation of the LVAD device incision site using dual cryoballoons. (C) Suturing after the completion of LVAD implantation and ablation.
To further reduce operative time, dual cryoballoons were used alternately and continuously to ablate the incision area (Figure 3B). This combined strategy of three-dimensional mapping and cryoablation also allowed verification of the effectiveness of cryoablation.
Outcom and follow up
The patient remained in sinus rhythm after discontinuing cardiopulmonary bypass and closing the chest cavity. Given the patient's heart failure, they have been consistently taking sacubitril/valsartan and metoprolol extended-release tablets to manage the condition.
After the surgery in May 2023, the patient has been receiving phone follow-ups every 3 months. If the patient experiences a shock sensation, they are instructed to seek medical attention immediately. If no shock sensation occurs during the phone follow-up, CRTD programming is performed every six months. During the follow-up period, the programming device indicated no episodes of ventricular tachycardia. In March 2024, the patient was readmitted due to a lung infection, and the CRTD programming during this hospitalization also showed no ventricular arrhythmias. The next CRTD programming is scheduled for September. The patient is currently still awaiting a heart transplant.
Discussion
The LVAD plays an increasingly important role in the treatment of patients with end-stage heart failure with reduced ejection fraction. While LVADs effectively bridge patients to heart transplantation and improve survival rates, ventricular arrhythmias (VAs) are common in patients with LVADs (1–4), and conservative treatments are often ineffective.
VAs may be related to the underlying heart disease or the LVAD implantation itself. The LVAD inflow cannula is anastomosed to the left ventricular apex, and extracorporeal circulation is established through the right atrial appendage. If mitral and tricuspid valve repairs are required, an incision in the anterior atrial wall is made. These sites are potential substrates for scar-related arrhythmias (4). Additionally, acute left ventricular underfilling or high pump speeds may cause the LVAD inflow cannula to contact the ventricular wall, leading to arrhythmias (5). At the cellular level, VT is associated with the β-adrenergic system and calcium regulation (5). While the latter types of VTs can be improved by adjusting LVAD parameters and oral medications, scar-related arrhythmias are typically not resolved through these measures.
Some patients may have an implantable cardioverter-defibrillator (ICD) or CRT-D before LVAD implantation. Studies have shown that preoperative VAs are predictors of post-LVAD VA occurrences (2, 6). Post-LVAD ICD implantation effectively reduces postoperative VT episodes (7), with CRT-D implantation having similar effects to ICDs (8). However, ICD or CRT-D discharges are associated with frequent rehospitalizations, posing additional risks and complicating patient management.
Catheter ablation is an effective treatment for refractory VAs, reducing ICD shocks and patient discomfort. Most VAs in LVAD patients are monomorphic and sustained, often related to pre-existing scar tissue, with fewer related to the apical cannula (9–11). To mitigate or eliminate these arrhythmias, hybrid surgical and catheter ablation techniques are promising (4). Studies have shown that catheter ablation is effective (9, 11), and localized cryoablation during LVAD implantation can also reduce postoperative VA episodes (12).
In this case report, the patient experienced VT before surgery. A three-dimensional anatomical reconstruction was performed before LVAD implantation, and the mapping system identified scar-related reentry. To reduce LVAD-related VA occurrences, intraoperative cryoablation was applied to both the existing scar-related reentry substrate and the apical cannula site.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SZ: Writing – original draft. JL: Resources, Writing – review & editing. CT: Resources, Writing – review & editing. MC: Conceptualization, Writing – review & editing. LH: Supervision, Writing – review & editing. HT: Investigation, Writing – review & editing. LL: Investigation, Writing – review & editing. XL: Conceptualization, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Financial support was obtained from the National Natural Science Foundation of China (No. 82150006) and Provincial Natural Science Foundation of Hunan (No. 2019JJ40433).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Raasch H, Jensen BC, Chang PP, Mounsey JP, Gehi AK, Chung EH, et al. Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continuous-flow left ventricular assist device implantation. Am Heart J. (2012) 164(3):373–8. doi: 10.1016/j.ahj.2012.06.018
2. Garan AR, Levin AP, Topkara V, Thomas SS, Yuzefpolskaya M, Colombo PC, et al. Early post-operative ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant. (2015) 34(12):1611–6. doi: 10.1016/j.healun.2015.05.018
3. Rosenbaum AN, Kremers WK, Duval S, Sakaguchi S, John R, Eckman PM. Arrhythmias in patients with cardiac implantable electrical devices after implantation of a left ventricular assist device. Asaio J. (2016) 62(3):274–80. doi: 10.1097/mat.0000000000000349
4. Ho G, Braun O, Adler ED, Feld GK, Pretorius VG, Birgersdotter-Green U. Management of arrhythmias and cardiac implantable electronic devices in patients with left ventricular assist devices. JACC Clin Electrophysiol. (2018) 4(7):847–59. doi: 10.1016/j.jacep.2018.04.014
5. Pettit SJ, Petrie MC, Connelly DT, Japp AG, Payne JR, Haj-Yahia S, et al. Use of implantable cardioverter defibrillators in patients with left ventricular assist devices. Eur J Heart Fail. (2012) 14(7):696–702. doi: 10.1093/eurjhf/hfs062
6. Brenyo A, Rao M, Koneru S, Hallinan W, Shah S, Massey HT, et al. Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients. J Cardiovasc Electrophysiol. (May 2012) 23(5):515–20. doi: 10.1111/j.1540-8167.2011.02223.x
7. Garan AR, Yuzefpolskaya M, Colombo PC, Morrow JP, Te-Frey R, Dano D, et al. Ventricular arrhythmias and implantable cardioverter-defibrillator therapy in patients with continuous-flow left ventricular assist devices: need for primary prevention? J Am Coll Cardiol. (2013) 61(25):2542–50. doi: 10.1016/j.jacc.2013.04.020
8. Gopinathannair R, Birks EJ, Trivedi JR, McCants KC, Sutton BS, Deam AG, et al. Impact of cardiac resynchronization therapy on clinical outcomes in patients with continuous-flow left ventricular assist devices. J Card Fail. (2015) 21(3):226–32. doi: 10.1016/j.cardfail.2014.12.006
9. Sacher F, Reichlin T, Zado ES, Field ME, Viles-Gonzalez JF, Peichl P, et al. Characteristics of ventricular tachycardia ablation in patients with continuous flow left ventricular assist devices. Circ Arrhythm Electrophysiol. (2015) 8(3):592–7. doi: 10.1161/circep.114.002394
10. Załucka L, Świerżyńska E, Orczykowski M, Dutkowski K, Szymański J, Kuriata J, et al. Ventricular arrhythmias in left ventricular assist device patients-current diagnostic and therapeutic considerations. Sensors (Basel). (2024) 24(4):1124. doi: 10.3390/s24041124
11. Cantillon DJ, Bianco C, Wazni OM, Kanj M, Smedira NG, Wilkoff BL, et al. Electrophysiologic characteristics and catheter ablation of ventricular tachyarrhythmias among patients with heart failure on ventricular assist device support. Heart Rhythm. (2012) 9(6):859–64. doi: 10.1016/j.hrthm.2012.01.018
Keywords: left ventricular assist device implantation, cryoballoon ablation, ventricular tachycardia, ventricular arrhythmias, hybrid procedures
Citation: Zhang S, Li J, Tan C, Chen M, Hu L, Tang H, Liao L and Li X (2025) Case Report: Left ventricular assist device implantation combined with cryoballoon ablation for ventricular tachycardia. Front. Surg. 11:1449007. doi: 10.3389/fsurg.2024.1449007
Received: 14 June 2024; Accepted: 16 December 2024;
Published: 7 January 2025.
Edited by:
Jacob Bergsland, Oslo University Hospital, NorwayReviewed by:
Michael Hofmann, University of Zurich, SwitzerlandKadir Caliskan, Erasmus Medical Center, Netherlands
Copyright: © 2025 Zhang, Li, Tan, Chen, Hu, Tang, Liao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuping Li, bHhweGlhbmd5YUAxNjMuY29t
 Shuang Zhang
Shuang Zhang Jianming Li
Jianming Li Changming Tan3
Changming Tan3 Mingxian Chen
Mingxian Chen Lin Hu
Lin Hu