- 1Department of Otorhinolaryngology, Jena University Hospital, Jena, Germany
- 2Department of Medical Statistics, Computer Sciences and Data Sciences, Jena University Hospital, Jena, Germany
- 3Facial-Nerve-Center, Jena University Hospital, Jena, Germany
- 4Center for Rare Diseases, Jena University Hospital, Jena, Germany
Objectives: To determine the functional outcome after facial nerve reconstruction surgery in patients with flaccid facial paralysis.
Methods: A systematic review and meta-analysis was performed on studies reporting outcomes after direct facial nerve suture (DFS), facial nerve interpositional graft suture (FIGS), hypoglossal–facial nerve suture (HFS), masseteric–facial nerve suture (MFS), and cross-face nerve suture (CFS). These studies were identified from PubMed/MEDLINE, Embase, and Web of Science databases. Two independent reviewers performed two-stage screening and data extraction. A favorable result was defined as a final House–Brackmann grade I–III and is presented as a ratio of all patients in percentage. Pooled proportions were calculated using random-effects models.
Results: From 4,932 screened records, 54 studies with 1,358 patients were included. A favorable result was achieved after DFS in 42.67% of the patients [confidence interval (CI): 26.05%–61.12%], after FIGS in 66.43% (CI: 55.99%–75.47%), after HFS in 63.89% (95% CI: 54.83%–72.05%), after MFS in 63.11% (CI: 38.53%–82.37%), and after CFS in 46.67% (CI: 24.09%–70.70%). There was no statistically significant difference between the techniques (Q = 6.56, degrees of freedom = 4, p = 0.1611).
Conclusions: The established facial nerve reconstruction techniques including the single nerve cross-transfer techniques produce satisfactory results in most of the patients with permanent flaccid facial paralysis. An international consensus on standardized outcome measures would improve the comparability of facial reanimation techniques.
Introduction
Peripheral facial palsy is the most common pathology of cranial nerves with an incidence of 20–30 patients per 100,000 people per year (1). If the facial nerve is severed, for instance by nerve trauma, tumor infiltration, or tumor resection, the mimic muscles are denervated and spontaneous recovery is impossible. The result is a flaccid facial paralysis. This results in serious consequences for the patients: Insufficient corneal lubrication can lead to corneal ulceration and ultimately to a loss of vision (2). Furthermore, oral incompetence and facial asymmetry derive from facial paralysis (3). In addition, the psychosocial impairments are burdensome. The prevalence of anxiety and depression is significantly increased compared to a healthy population (4). Therefore, it is standard of care to perform a facial nerve reconstruction, if feasible, to reanimate the facial muscles (5).
Surgical facial reanimation has been performed for over a century now. The first direct facial nerve repair was performed in 1884 by Sir Charles Alfred Ballance, a British aural surgeon, who published his experiences using this method in 1903 (6, 7). In 1895, Ballance performed the first facial-crossover nerve suture, using the accessorial nerve to reanimate the face. Neither of the surgeries succeeded, on the contrary the first patient died of sepsis (6, 7). The first successful hypoglossal–facial nerve suture (HFS), i.e., a cross-nerve suture technique, was performed by Werner Körte in 1901 (8). Since the first efforts to develop a sufficient technique to treat facial paralysis, plenty of articles have been published, showing different methods to achieve satisfactory results in facial reanimation. The most established techniques are direct facial nerve repair without a nerve graft, facial nerve interpositional graft, cross-face reanimation using the contralateral facial nerve and nerve grafts, a hypoglossal–facial nerve suture in different variations, and more recently masseteric–facial nerve suture (MFS) (Figure 1) (5, 9).
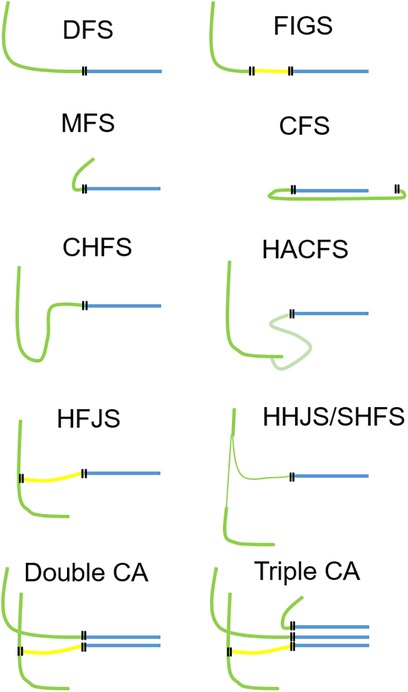
Figure 1 Overview of the most important facial nerve reconstruction techniques. Each technique can be used as a single measure, but also in combination (combined approach). Two examples for a combined approach are shown in the bottom row using two nerves for reanimation (left side) or even three nerves for reanimation (right side). Green, motor nerve used for facial reanimation; yellow, nerve graft; blue, peripheral facial nerve; each double line illustrates a suture site. DFS, direct facial nerve suture; FIGS, facial nerve interpositional graft suture; MFS, masseteric–facial nerve suture; CFS, cross-face nerve suture; CHFS, classical hypoglossal–facial nerve suture, HACFS, hypoglossal ansa cervicalis–facial nerve suture; HFJS, hypoglossal–facial jump nerve suture, HHFS, hemihypoglossal–facial nerve suture (HHFS); SHFS, split hypoglossal–facial nerve suture; CA, combined approach.
Since most of the available studies are relatively small in sample size, use only a single technique, and do not compare their methods to others, it is not clear which method for facial reanimation leads to the best results. In addition, there is a wide range of different scoring systems to report results after facial reanimation, which makes a comparison even more difficult (10).
Thus, the aim of the present study was to perform a meta-analysis to compare the results of the most used facial nerve reconstruction techniques in term of functional outcome.
Material and methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11). Ethical approval and patient informed consent were not required for a meta-analysis.
Data sources and literature search
Electronic databases (PubMed/MEDLINE, Embase, and Web of Science) were screened. The following Medical Subject Heading (MeSH) terms were used: (“facial palsy” OR “facial paralysis” OR “facial reanimation” OR “facial paresis”) AND (“hypoglossal nerve” OR “masseter nerve” OR “facial nerve” OR “nerve graft” OR “cross face” OR “accessory nerve”)”. The literature search revealed 4,932 results until the end of 2022.
Selection of studies
Two independent reviewers (FZ and OG-L) reviewed the abstracts and full texts. If they came to a different conclusion, a joint decision was made in a discussion. All studies were assessed against the general exclusion criteria: review articles, duplicate patients, absence of essential data, multiple use of the same patient dataset, and animal studies. Further exclusion criteria were as follows: non-English or non-German language; full text not available; insufficient reported data or non-extractable data; case series including less than five patients; subgroup analyses of patients from larger studies; article types including reviews, case reports, conference abstracts, letters to the editor, or book chapters. No restrictions on the publication date were applied, but peer-reviewed journal publication was a requirement for article inclusion.
Eligibility criteria
The PICOS scheme was utilized to establish the eligibility criteria for this study, as follows: Patients (P), either children or adults with acquired unilateral complete facial paralysis; Intervention (I), reconstruction of the peripheral facial nerve; Comparison (C), comparison between the different reconstruction techniques; Outcomes (O), functional outcome of the reconstruction; Study design (S), retrospective and prospective cohort studies, case–control studies, case series, and randomized clinical trials (RCTs). Studies were included when they used a nerve-to-nerve neurorrhaphy for facial reanimation without muscle flap or other nerve transposition, contained at least five patients receiving the same reanimation technique, and used the House–Brackmann (HB) grading system to report the outcome (12). Four studies that used a modification of the HB score were also included. The results had to be reported according to patient data. Studies that used multiple reanimation methods without differentiating in their reported results were excluded. In total, 54 articles were finally included in the analysis (Figure 2).

Figure 2 PRISMA flow diagram shows the selection of the included studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Data extraction
The following data were extracted from the included publications: number of patients, publication type, type of facial reconstruction surgery, HB score. The studies were pooled and sorted by the used reanimation technique into five different groups, as follows: hypoglossal–facial nerve suture, masseteric–facial nerve suture, cross-face reanimation, direct facial nerve repair with interpositional graft, and direct facial nerve repair without graft. The groups were then compared to each other to find out which technique had the best results. Results achieving an HB grade of I to III were defined as satisfactory results. The results are presented as the ratio of good results (HB grade I–III) to the total number of treated patients.
Since there are multiple different techniques using the hypoglossal nerve for reanimation, four additional subgroups were created to compare against each other. The compared techniques were the classical hypoglossal–facial nerve suture (CHFS) (end-to-end), hypoglossal–facial jump graft nerve suture (side-to-end using a jump interposition graft), hemihypoglossal–facial nerve suture (HHFS) (side-to-end without a graft), and split hypoglossal–facial nerve suture (splitting the hypoglossal nerve and using one-half to connect in an end-to-end manner to the facial nerve).
Statistics
Statistical analysis was performed in R (version 4.0.4; www.r-project.org) (13). The meta package (version 4.16-2) was used to produce pooled estimates and forest plots (14). The proportion of favorable results in the treated patients is presented in the forest plots together with a 95% Clopper–Pearson confidence interval (CI) was used in this study. Assessment of the statistical heterogeneity was performed using Cochran's Q-test. The degree of heterogeneity was also quantified using I2 and using a random-effects model according to DerSimonian and Laird. Pooled estimates are derived from this model. To investigate potential differences between the applied techniques, subgroup analyses were performed. We employed either a fixed-effect or random-effects model depending on the calculated heterogeneity. We reported Q statistics, degrees of freedom (df), and associated p-values for these comparisons.
Results
Characteristics of the studies
The analysis included 54 publications with a total of 1,358 patients (Table 1). In total 118 studies were excluded. A reconstruction of the facial nerve using an interposition graft was performed in 481 patients, a reconstruction of the facial nerve without an interposition graft, in 47 patients. For facial reanimation with a cross-nerve technique, the hypoglossal nerve was used in 778 patients, the masseteric nerve in 37 patients, and a cross-face graft in 15 patients.
Functional outcome of the different reconstruction techniques
Figure 3 gives an overview on the results of all the analyzed reconstruction techniques. The analysis showed significant heterogeneity (I2 = 61.1%, Q = 247.37, df = 62, p < 0.0001). Thus, a random-effects model was used. A direct facial nerve reconstruction achieved good results in 42.67% of the patients (CI: 26.05%–61.12%). A facial nerve reconstruction with an interpositional graft had good results in 66.43% (CI: 55.99%–75.47%). The facial reanimation using the hypoglossal nerve achieved good results in 63.89% (95% CI: 54.83%–72.05%). The use of the masseteric nerve achieved good results in 63.11% (CI: 38.53%–82.37%). Finally, a facial reanimation using a cross-face technique achieved good results in 46.67% (CI: 24.09%–70.70%). While the direct facial nerve repair exhibited the lowest proportion of good results, the random-effects model revealed no statistically significant differences between the groups (Q = 6.56, df = 4, p = 0.1611).
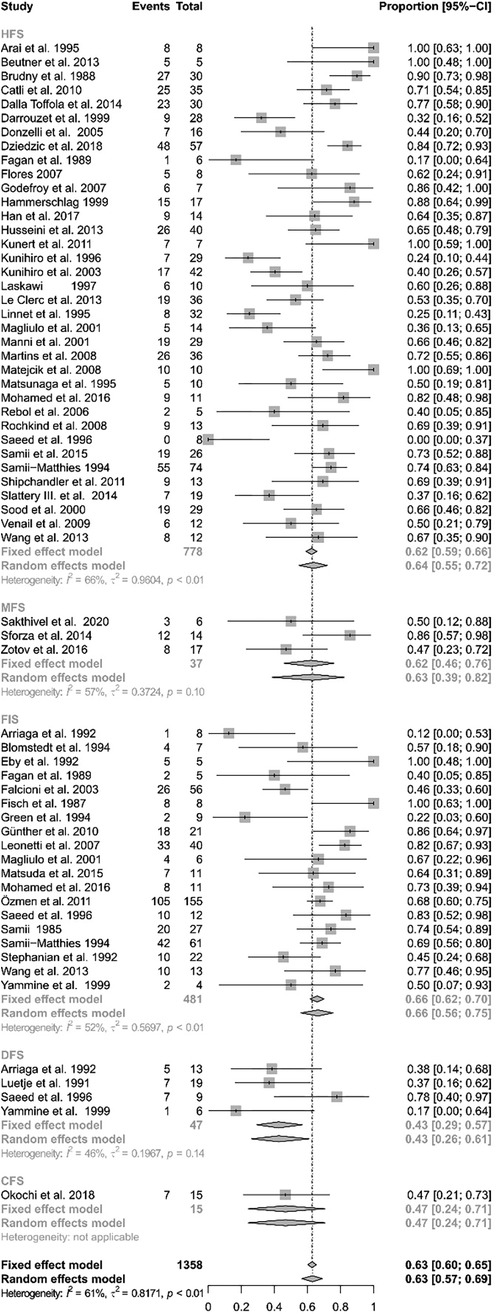
Figure 3 Forest plot illustrating the rates of successful facial reanimation for hypoglossal–facial nerve suture (HFS), masseteric–facial nerve suture (MFS), facial nerve interpositional graft suture (FIGS), direct facial nerve suture (DFS), and cross-face nerve suture (CFS). The proportion of success can reach from 0 (no patient with HB I–III as final result) to 1 (all patients reached a HB I-III as a final result).
Figure 4 shows the subanalysis on the different techniques using the hypoglossal nerve as cross-motor nerve for the peripheral facial nerve reanimation. Out of the 778 reconstructions using the hypoglossal nerve, there were 453 classical, 76 jump graft, 115 hemihypoglossal, and 102 split hypoglossal-to-facial nerve reconstructions. The specific technique was not reported for 32 patients. Here again, the analysis showed significant heterogeneity (I2 = 55.9%, Q = 184.56, df = 46, p < 0.0001) and consequently a random-effects model was used. The classical hypoglossal–facial nerve suture achieved satisfactory results in 54.90% of the patients (CI: 42.33%–66.87%). The jump-graft technique achieved satisfactory results in 60.53% (CI 40.07%–77.87%). The hemihypoglossal technique achieved satisfactory results in 66.35% (CI: 52.04%–78.18%). Finally, the reanimation using a split hypoglossal nerve achieved satisfactory results in 82.35% (CI: 73.72%–88.59%). There was a statistically significant difference between methods, when using a random-effects model (Q = 14.48, df = 3, p = 0.0023). Hence, the split hypoglossal nerve technique presented the best results among the hypoglossal nerve cross-motor techniques.
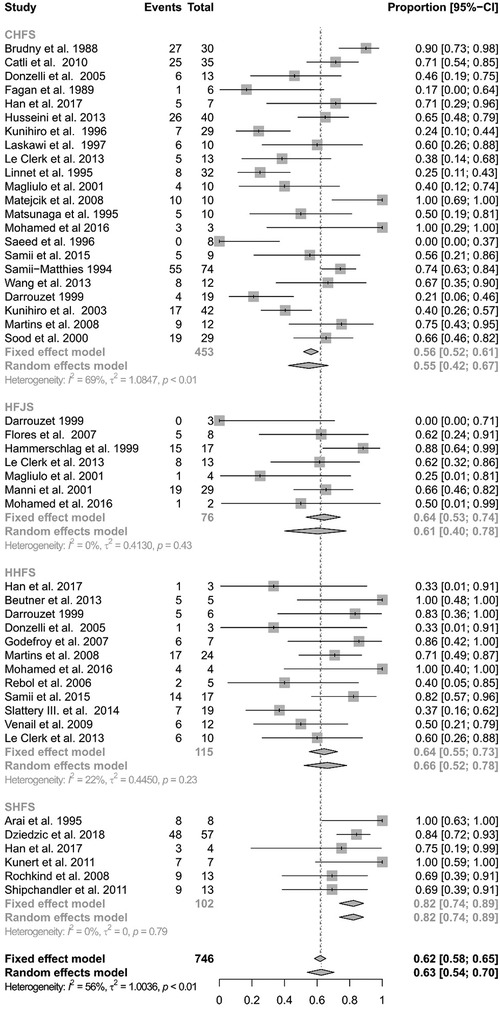
Figure 4 Forest plot illustrating the rates of successful facial reanimation for classical hypoglossal–facial nerve suture (CHFS), hypoglossal–facial jump nerve suture (HFJS), hemihypoglossal–facial nerve suture (HHFS), and split hypoglossal–facial nerve suture (SHFS). The proportion of success can reach from 0 (no patient with HB I-III as a final result) to 1 (all patients reached a HB I-III as a final result).
Discussion
This meta-analysis comparing different surgical techniques for facial nerve reconstruction for patients with permanent facial paralysis did not show a significant higher succession rate for one of the compared techniques. Numerous studies have explored surgical methods for facial reanimation, yielding results that vary and, in some cases, conflict with one another (66, 69–74). Nevertheless, a meta-analysis conducted on a large scale that compares the various approaches and techniques has not been done yet. There is one recent meta-analysis dealing only with masseteric nerve transfer and time to first movements as outcome measure, showing that such a transfer shows overall good results (3). Urban et al. also compared hypoglossal and masseteric nerve transfer for facial reanimation in a meta-analysis (75). Here, the outcome measure was oral commissure symmetry, time to reinnervation, and Sunnybrook grading. Both techniques achieved good results, but the masseteric nerve transfer overall showed better results.
Most data were available for the reconstruction using the hypoglossal nerve. The use of the hypoglossal nerve is the oldest standard cross-nerve reconstruction technique in case of long-term denervation (1, 9, 76). The cross-nerve techniques with the hypoglossal nerve, the masseteric nerve, or with branches of the contralateral facial nerve are mainly used for patients with permanent flaccid facial paralysis or in case of immediate facial nerve reconstruction if the proximal facial nerve stump is not available. The reconstruction using the hypoglossal nerve, the masseteric nerve, and the facial nerve reconstruction using an interposition graft achieved similar results. The latter is only feasible when a proximal facial nerve stump is available. A typical example is a complex defect of the extratemporal facial plexus after resection of a parotid tumor with facial nerve infiltration (77).
The facial reanimation using a direct facial nerve reconstruction or a cross-face nerve graft tended to achieve the worst results compared to the other techniques. A direct facial nerve reconstruction is typically only feasible for a sharply cut nerve, for instance in case of an iatrogenic lesion or after facial nerve trauma if immediate repair is possible. It is noteworthy that the data for the cross-face graft were extracted from a single study, in which a special emphasis was put on the reanimation of the periorbital movement, thus limiting the validity for general facial reanimation. Then, cross-face grafts are often limited to reanimation of the lower face. Lastly, a possible explanation for the worse results might be the relatively extended length of the interposition grafts, which is discussed to be a negative predictor of the results of facial reanimation (31).
Comparing the different available techniques for facial reconstruction using the hypoglossal nerve we found that the best results were achieved using a split hypoglossal technique. Due to its wider diameter compared to the facial nerve, half of the hypoglossal nerve provides a sufficient number of axons to enable facial reanimation. In addition, employing the split hypoglossal technique allows axons sprouting in their natural direction. Furthermore, the positive outcomes may be attributed to the use of a single nerve suture without the need for an interposition graft as it is necessary for the hypoglossal–jump nerve alternative (61). When using the masseteric nerve, main trunk coaptation without interpositional grafts results in faster reinnervation than in reconstruction with interpositional graft (3). If faster reinnervation correlates with functionally, better reinnervation is not proven yet.
This study has a number of limitations. There is a wide range of factors that might have influenced the result of facial reanimation procedures. Some of them include the time to reanimation, the patients’ age, graft length, the surgeon's experience, reason for palsy, post-operative rehabilitation process, and comorbidities (21, 27, 31, 78, 79). Those factors could not be assessed in the current study. Another limitation is the way the results of facial nerve were reported. The HB grading system had to be used as an outcome measure for the post-surgical facial nerve function, because there are no sufficient number of studies with more modern grading systems that would permit a meta-analysis. The HB grading system is a very gross system and not very reliable (80). Small differences or advantages of the used techniques might not be detected by the HB grading. Thus, results like spontaneous smile might not properly be displayed by the HB grading. The HB system had to be selected because most studies used it. Only in newer studies, more reliable but still subjective systems like a Sunnybrook or eFACE grading are used (75, 76). A wider use of objective automated image analysis tools for evaluation of the surgical outcome would be better (81, 82). Then, the donor site morbidity could not be analyzed. Especially, the harvest of donor nerves needed as grafts for some of the facial nerve reconstruction techniques, could lead to additional morbidity. The morbidity was often not measured at all, let alone in a standardized way. For instance, the classical HFS was abandoned in favor of the jump or split technique in many centers due to the severe morbidity, as the patients suffered greatly from the tongue palsy in the long term. Furthermore, only a limited number of studies also address the quality of life of the patients using facial-specific patient-reported outcome measures. It is recommended to use patient-reported outcome measures like the Facial disability index (FDI) or the Facial Clinimetric Evaluation Scale (FaCE) to record the patient's view of the surgical result (70, 76, 83).
Since reconstruction using a combination of multiple techniques (dual or even triple innervations) or muscle transplantations were ruled out in the present study to define the effects of a single nerve for reanimation, newer methods using multiple nerves were systematically excluded, even though they might achieve better results (84–87).
Conclusion
In conclusion, all commonly used facial nerve reconstruction techniques are a viable option for facial reanimation for patients with permanent flaccid facial paralysis. The outcome in these patients should be measured with standardized and reliable outcome parameters. Highly reliable grading systems and facial-specific quality-of-life assessment should be used on all these patients. The introduction of an objective automated image analysis tool for a comprehensive quantification of the outcome would be perfect.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FZ: Formal Analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. PS: Conceptualization, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. OG-L: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
OG-L declared that he was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Volk GF, Pantel M, Guntinas-Lichius O. Modern concepts in facial nerve reconstruction. Head Face Med. (2010) 6:25. doi: 10.1186/1746-160X-6-25
2. Biglioli F, Colombo V, Rabbiosi D, Tarabbia F, Giovanditto F, Lozza A, et al. Masseteric-facial nerve neurorrhaphy: results of a case series. J Neurosurg. (2017) 126:312–8. doi: 10.3171/2015.12.JNS14601
3. Murphey AW, Clinkscales WB, Oyer SL. Masseteric nerve transfer for facial nerve paralysis: a systematic review and meta-analysis. JAMA Facial Plast Surg. (2018) 20:104–10. doi: 10.1001/jamafacial.2017.1780
4. Pouwels S, Beurskens CH, Kleiss IJ, Ingels KJ. Assessing psychological distress in patients with facial paralysis using the hospital anxiety and depression scale. J Plast Reconstr Aesthet Surg. (2016) 69:1066–71. doi: 10.1016/j.bjps.2016.01.021
5. Guntinas-Lichius O, Genther DJ, Byrne PJ. Facial reconstruction and rehabilitation. Adv Otorhinolaryngol. (2016) 78:120–31. doi: 10.1159/000442132
6. May M, Schaitkin BM. History of facial nerve surgery. Facial Plast Surg. (2000) 16:301–7. doi: 10.1055/s-2000-15543
7. Van de Graaf RC AIFF, Nicolai JP. Sir Charles Alfred Ballance (1856–1936) and the introduction of facial nerve crossover anastomosis in 1895. J Plast Reconstr Aesthet Surg. (2009) 62:43–9. doi: 10.1016/j.bjps.2008.06.052
8. Körte W, Bernhardt M. Ein fall von nervenpfropfung: des nervus facialis auf den nervus hypoglossus. Deutsche Medizinische Wochenschrift. (1903) 29:293–5. doi: 10.1055/s-0028-1138423
9. Bianchi B, Ferri A, Sesenna E. Facial reanimation after nerve sacrifice in the treatment of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. (2012) 20:114–9. doi: 10.1097/MOO.0b013e32834fa744
10. Revenaugh PC, Smith RM, Plitt MA, Ishii L, Boahene K, Byrne PJ. Use of objective metrics in dynamic facial reanimation: a systematic review. JAMA Facial Plast Surg. (2018) 20:501–8. doi: 10.1001/jamafacial.2018.0398
11. Arya S, Kaji AH, Boermeester MA. PRISMA reporting guidelines for meta-analyses and systematic reviews. JAMA Surg. (2021) 156:789–90. doi: 10.1001/jamasurg.2021.0546
12. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. (1985) 93:146–7. doi: 10.1177/019459988509300202
13. Team RC. R: A language and environment for statistical computing. Available online at: https://www.R-project.org/ (accessed February 27, 2024).
14. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
15. Arai H, Sato K, Yanai A. Hemihypoglossal-facial nerve anastomosis in treating unilateral facial palsy after acoustic neurinoma resection. J Neurosurg. (1995) 82:51–4. doi: 10.3171/jns.1995.82.1.0051
16. Arriaga MA, Brackmann DE. Facial nerve repair techniques in cerebellopontine angle tumor surgery. Am J Otol. (1992) 13:356–9.1415500
17. Beutner D, Luers JC, Grosheva M. Hypoglossal-facial-jump-anastomosis without an interposition nerve graft. Laryngoscope. (2013) 123:2392–6. doi: 10.1002/Lary.24115
18. Blomstedt GC, Jaaskelainen JE, Pyykko I, Ishizaki H, Troupp H, Palva T. Recovery of the sutured facial nerve after removal of acoustic neuroma in patients with neurofibromatosis-2. Neurosurgery. (1994) 35:364–8; discussion 368–9. doi: 10.1227/00006123-199409000-00002
19. Brudny J, Hammerschlag PE, Cohen NL, Ransohoff J. Electromyographic rehabilitation of facial function and introduction of a facial paralysis grading scale for hypoglossal-facial nerve anastomosis. Laryngoscope. (1988) 98:405–10. doi: 10.1288/00005537-198804000-00010
20. Catli T, Bayazit YA, Gokdogan O, Goksu N. Facial reanimation with end-to-end hypoglossofacial anastomosis: 20 years’ experience. J Laryngol Otol. (2010) 124:23–5. doi: 10.1017/S0022215109991344
21. Toffola ED, Pavese C, Cecini M, Petrucci L, Ricotti S, Bejor M, et al. Hypoglossal-facial nerve anastomosis and rehabilitation in patients with complete facial palsy: cohort study of 30 patients followed up for three years. Funct Neurol. (2014) 29:183–7.25473738
22. Darrouzet V, Guerin J, Bebear JP. New technique of side-to-end hypoglossal-facial nerve attachment with translocation of the infratemporal facial nerve. J Neurosurg. (1999) 90:27–34. doi: 10.3171/jns.1999.90.1.0027
23. Donzelli R, Maiuri F, Peca C, Cavallo LM, Motta G, de Divitiis E. Microsurgical repair of the facial nerve. Zentralbl Neurochir. (2005) 66:63–9. doi: 10.1055/s-2004-836226
24. Dziedzic TA, Kunert P, Marchel A. Hemihypoglossal-facial nerve anastomosis for facial nerve reanimation: case series and technical note. World Neurosurg. (2018) 118:e460–7. doi: 10.1016/j.wneu.2018.06.217
25. Eby TL, Fisch U, Makek MS. Facial nerve management in temporal bone hemangiomas. Am J Otol. (1992) 13:223–32.1609850
26. Fagan PA, Loh KK. Results of surgery to the damaged facial nerve. J Laryngol Otol. (1989) 103:567–71. doi: 10.1017/S0022215100109351
27. Falcioni M, Taibah A, Russo A, Piccirillo E, Sanna M. Facial nerve grafting. Otol Neurotol. (2003) 24:486–9. doi: 10.1097/00129492-200305000-00022
28. Fisch U, Dobie RA, Gmur A, Felix H. Intracranial facial nerve anastomosis. Am J Otol. (1987) 8:23–9. doi: 10.1016/S0196-0709(87)80015-7
29. Flores LP. Surgical results of the hypoglossal-facial nerve jump graft technique. Acta Neurochir (Wien). (2007) 149:1205–10; discussion 1210. doi: 10.1007/s00701-007-1412-x
30. Godefroy WP, Malessy MJ, Tromp AA, van der Mey AG. Intratemporal facial nerve transfer with direct coaptation to the hypoglossal nerve. Otol Neurotol. (2007) 28:546–50. doi: 10.1097/mao.0b013e31804301b8
31. Green JD J, Shelton C, Brackmann DE. Surgical management of iatrogenic facial nerve injuries. Otolaryngol Head Neck Surg. (1994) 111:606–10. doi: 10.1177/019459989411100511
32. Günther M, Danckwardt-Lillieström N, Gudjonsson O, Nyberg G, Kinnefors A, Rask-Andersen H, et al. Surgical treatment of patients with facial neuromas—a report of 26 consecutive operations. Otol Neurotol. (2010) 31:1493–7. doi: 10.1097/MAO.0b013e3181f0c524
33. Hammerschlag PE. Facial reanimation with jump interpositional graft hypoglossal facial anastomosis and hypoglossal facial anastomosis: evolution in management of facial paralysis. Laryngoscope. (1999) 109:1–23. doi: 10.1097/00005537-199902001-00001
34. Han JH, Suh MJ, Kim JW, Cho HS, Moon IS. Facial reanimation using hypoglossal-facial nerve anastomosis after schwannoma removal. Acta Otolaryngol. (2017) 137:99–105. doi: 10.1080/00016489.2016.1212398
35. Husseini S T, Kumar DV, De Donato G, Almutair T, Sanna M. Facial reanimation after facial nerve injury using hypoglossal to facial nerve anastomosis: the gruppo otologico experience. Indian J Otolaryngol Head Neck Surg. (2013) 65:305–8. doi: 10.1007/s12070-011-0468-3
36. Kunert P, Podgorska A, Bartoszewicz R, Marchel A. Hemihypoglossal-facial nerve anastomosis for facial nerve palsy. Neurol Neurochir Pol. (2011) 45:452–60. doi: 10.1016/S0028-3843(14)60313-3
37. Kunihiro T, Kanzaki J, Yoshihara S, Satoh Y, Satoh A. Hypoglossal-facial nerve anastomosis after acoustic neuroma resection: influence of the time anastomosis on recovery of facial movement. ORL J Otorhinolaryngol Relat Spec. (1996) 58:32–5. doi: 10.1159/000276791
38. Kunihiro T, Higashino K, Kanzaki J. Classic hypoglossal-facial nerve anastomosis after acoustic neuroma resection. A review of 46 cases. ORL J Otorhinolaryngol Relat Spec. (2003) 65:1–6. doi: 10.1159/000068662
39. Laskawi R. Combination of hypoglossal-facial nerve anastomosis and botulinum-toxin injections to optimize mimic rehabilitation after removal of acoustic neurinomas. Plast Reconstr Surg. (1997) 99:1006–11. doi: 10.1097/00006534-199704000-00013
40. Le Clerc N, Herman P, Kania R, Tran H, Altabaa K, Tran Ba Huy P, et al. Comparison of 3 procedures for hypoglossal-facial anastomosis. Otol Neurotol. (2013) 34:1483–8. doi: 10.1097/MAO.0b013e31828dac62
41. Leonetti JP, Anderson DE, Marzo SJ, Origitano TC, Petruzzelli GJ. Intratemporal grafting of the facial nerve following lateral skull base tumor resection. Skull Base. (2007) 17:181–6. doi: 10.1055/s-2007-977464
42. Linnet J, Madsen FF. Hypoglosso-facial nerve anastomosis. Acta Neurochir (Wien). (1995) 133:112–5. doi: 10.1007/BF01420060
43. Luetje CM, Whittaker CK. The benefits of VII-VII neuroanastomosis in acoustic tumor surgery. Laryngoscope. (1991) 101:1273–5. doi: 10.1002/Lary.5541011203
44. Magliulo G, D'Amico R, Forino M. Results and complications of facial reanimation following cerebellopontine angle surgery. Eur Arch Otorhinolaryngol. (2001) 258:45–8. doi: 10.1007/s004059900211
45. Manni JJ, Beurskens CH, van de Velde C, Stokroos RJ. Reanimation of the paralyzed face by indirect hypoglossal-facial nerve anastomosis. Am J Surg. (2001) 182:268–73. doi: 10.1016/S0002-9610(01)00715-2
46. Martins RS, Socolovsky M, Siqueira MG, Campero A. Hemihypoglossal-facial neurorrhaphy after mastoid dissection of the facial nerve: results in 24 patients and comparison with the classic technique. Neurosurgery. (2008) 63:310–6; discussion 317. doi: 10.1227/01.NEU.0000312387.52508.2C
47. Matejcik V, Penzesova G. Our experience with surgical treatment of lesions of nervus facialis. Neurocirugia (Astur). (2008) 19:127–32. doi: 10.1016/S1130-1473(08)70236-7
48. Matsuda K, Kakibuchi M, Sotsuka Y, Kubo T, Shibata M, Hosokawa K. End-to-side “loop” graft for total facial nerve reconstruction: over 10 years experience. J Plast Reconstr Aesthet Surg. (2015) 68:1054–63. doi: 10.1016/j.bjps.2015.04.005
49. Matsunaga T, Kanzaki J, O-Uchi T, Kunihiro T, Ogata A, Inoue Y, et al. Functional and histological evaluation of the facial nerve in patients who have undergone hypoglossal-facial nerve anastomosis after removal of cerebellopontine angle tumors. ORL J Otorhinolaryngol Relat Spec. (1995) 57:153–60. doi: 10.1159/000276729
50. Mohamed A, Omi E, Honda K, Suzuki S, Ishikawa K. Outcome of different facial nerve reconstruction techniques. Braz J Otorhinolaryngol. (2016) 82:702–9. doi: 10.1016/j.bjorl.2015.12.010
51. Okochi M, Okochi H, Asai E, Sakaba T, Ueda K. Eyelid reanimation using crossface nerve graft: relationship between surgical outcome and preoperative paralysis duration. Microsurgery. (2018) 38:375–80. doi: 10.1002/micr.30264
52. Ozmen OA, Falcioni M, Lauda L, Sanna M. Outcomes of facial nerve grafting in 155 cases: predictive value of history and preoperative function. Otol Neurotol. (2011) 32:1341–6. doi: 10.1097/MAO.0b013e31822e952d
53. Rebol J, Milojkovic V, Didanovic V. Side-to-end hypoglossal-facial anastomosis via transposition of the intratemporal facial nerve. Acta Neurochir (Wien). (2006) 148:653–7; discussion 657. doi: 10.1007/s00701-006-0736-2
54. Rochkind S, Shafi M, Alon M, Salame K, Fliss DM. Facial nerve reconstruction using a split hypoglossal nerve with preservation of tongue function. J Reconstr Microsurg. (2008) 24:469–74. doi: 10.1055/s-0028-1088225
55. Saeed SR, Ramsden RT. Rehabilitation of the paralysed face: results of facial nerve surgery. J Laryngol Otol. (1996) 110:922–5. doi: 10.1017/S0022215100135364
56. Sakthivel P, Singh CA, Thakar A, Thirumeni G, Raveendran S, Sharma SC. Masseteric-facial nerve anastomosis: surgical techniques and outcomes—a pilot Indian study. Indian J Otolaryngol Head Neck Surg. (2020) 72:92–7. doi: 10.1007/s12070-019-01758-z
57. Samii M, Turel KE, Penkert G. Management of seventh and eighth nerve involvement by cerebellopontine angle tumors. Clin Neurosurg. (1985) 32:242–72.3933876
58. Samii M, Alimohamadi M, Khouzani RK, Rashid MR, Gerganov V. Comparison of direct side-to-end and end-to-end hypoglossal-facial anastomosis for facial nerve repair. World Neurosurg. (2015) 84:368–75. doi: 10.1016/j.wneu.2015.03.029
59. Samii M, Matthies C. Indication, technique and results of facial nerve reconstruction. Acta Neurochir (Wien). (1994) 130:125–39. doi: 10.1007/BF01405512
60. Sforza C, Tarabbia F, Mapelli A, Colombo V, Sidequersky FV, Rabbiosi D, et al. Facial reanimation with masseteric to facial nerve transfer: a three-dimensional longitudinal quantitative evaluation. J Plast Reconstr Aesthet Surg. (2014) 67:1378–86. doi: 10.1016/j.bjps.2014.05.039
61. Shipchandler TZ, Seth R, Alam DS. Split hypoglossal-facial nerve neurorrhaphy for treatment of the paralyzed face. Am J Otolaryngol. (2011) 32:511–6. doi: 10.1016/j.amjoto.2010.09.007
62. Slattery WH, Cassis AM, Wilkinson EP, Santos F, Berliner K. Side-to-end hypoglossal to facial anastomosis with transposition of the intratemporal facial nerve. Otol Neurotol. (2014) 35:509–13. doi: 10.1097/MAO.0b013e3182936bcf
63. Sood S, Anthony R, Homer JJ, Van Hille P, Fenwick JD. Hypoglossal-facial nerve anastomosis: assessment of clinical results and patient benefit for facial nerve palsy following acoustic neuroma excision. Clin Otolaryngol Allied Sci. (2000) 25:219–26. doi: 10.1046/j.1365-2273.2000.00348.x
64. Stephanian E, Sekhar LN, Janecka IP, Hirsch B. Facial nerve repair by interposition nerve graft: results in 22 patients. Neurosurgery. (1992) 31:73–6; discussion 77. doi: 10.1227/00006123-199207000-00010
65. Venail F, Sabatier P, Mondain M, Segniarbieux F, Leipp C, Uziel A. Outcomes and complications of direct end-to-side facial-hypoglossal nerve anastomosis according to the modified may technique. J Neurosurg. (2009) 110:786–91. doi: 10.3171/2008.9.JNS08769
66. Wang Z, Zhang Z, Huang Q, Yang J, Wu H. Long-term facial nerve function following facial reanimation after translabyrinthine vestibular schwannoma surgery: a comparison between sural grafting and VII-XII anastomosis. Exp Ther Med. (2013) 6:101–4. doi: 10.3892/etm.2013.1120
67. Yammine FG, Dufour JJ, Mohr G. Intracranial facial nerve reconstruction. J Otolaryngol. (1999) 28:158–61.10410348
68. Zotov AV, Rzayev DA, Dmitriev AB, Chernov SV, Moysak GI. Evaluation of short-term surgical outcomes in facial paralysis patients treated by trigeminal neurotization. Zh Vopr Neirokhir Im N N Burdenko. (2016) 80:31–9. doi: 10.17116/neiro201680431-39
69. Malik TH, Kelly G, Ahmed A, Saeed SR, Ramsden RT. A comparison of surgical techniques used in dynamic reanimation of the paralyzed face. Otol Neurotol. (2005) 26:284–91. doi: 10.1097/00129492-200503000-00028
70. Guntinas-Lichius O, Streppel M, Stennert E. Postoperative functional evaluation of different reanimation techniques for facial nerve repair. Am J Surg. (2006) 191:61–7. doi: 10.1016/j.amjsurg.2005.05.054
71. Hontanilla B, Marre D. Comparison of hemihypoglossal nerve versus masseteric nerve transpositions in the rehabilitation of short-term facial paralysis using the facial clima evaluating system. Plast Reconstr Surg. (2012) 130:662e–72e. doi: 10.1097/PRS.0b013e318267d5e8
72. Hontanilla B, Olivas J, Cabello A, Marre D. Cross-face nerve grafting versus masseteric-to-facial nerve transposition for reanimation of incomplete facial paralysis: a comparative study using the FACIAL CLIMA evaluating system. Plast Reconstr Surg. (2018) 142:179e–91e. doi: 10.1097/PRS.0000000000004612
73. Socolovsky M, Martins RS, di Masi G, Bonilla G, Siqueira M. Treatment of complete facial palsy in adults: comparative study between direct hemihypoglossal-facial neurorrhaphy, hemihipoglossal-facial neurorrhaphy with grafts, and masseter to facial nerve transfer. Acta Neurochir (Wien). (2016) 158:945–57; discussion 957. doi: 10.1007/s00701-016-2767-7
74. Altamami NM, Zaouche S, Vertu-Ciolino D. A comparative retrospective study: hypoglossofacial versus masseterofacial nerve anastomosis using Sunnybrook facial grading system. Eur Arch Otorhinolaryngol. (2018) 276:209–16. doi: 10.1007/s00405-018-5186-y
75. Urban MJ, Eggerstedt M, Varelas E, Epsten MJ, Beer AJ, Smith RM, et al. Hypoglossal and masseteric nerve transfer for facial reanimation: a systematic review and meta-analysis. Facial Plast Surg Aesthet Med. (2022) 24:10–7. doi: 10.1089/fpsam.2020.0523
76. Volk GF, Geitner M, Geißler K, Thielker J, Raslan A, Mothes O, et al. Functional outcome and quality of life after hypoglossal-facial jump nerve suture. Front Surg. (2020) 7:11. doi: 10.3389/fsurg.2020.00011
77. Thielker J, Wahdan A, Buentzel J, Holger Kaftan H, Boeger D, Mueller AH, et al. B. Long-term facial nerve outcome in primary parotid cancer surgery: a population-based analysis. Laryngoscope. (2021) 131:2694–700. doi: 10.1002/lary.29666
78. Terzis JK, Konofaos P. Experience with 60 adult patients with facial paralysis secondary to tumor extirpation. Plast Reconstr Surg. (2012) 130:51e–66e. doi: 10.1097/PRS.0b013e318254b149
79. Bascom DA, Schaitkin BM, May M, Klein S. Facial nerve repair: a retrospective review. Facial Plast Surg. (2000) 16:309–13. doi: 10.1055/s-2000-15545
80. Fattah AY, Gurusinghe ADR, Gavilan J, Hadlock TA, Marcus JR, Marres H, et al. Facial nerve grading instruments: systematic review of the literature and suggestion for uniformity. Plast Reconstr Surg. (2015) 135:569–79. doi: 10.1097/PRS.0000000000000905
81. Dusseldorp JR, Guarin DL, van Veen MM, Miller M, Jowett N, Hadlock TA. Automated spontaneity assessment after smile reanimation: a machine learning approach. Plast Reconstr Surg. (2022) 149:1393–402. doi: 10.1097/PRS.0000000000009167
82. Marston AP, Ziegler JP, Oyer SL. Masseteric-to-facial nerve transfer for treatment of pediatric facial paralysis: an initial report. Int J Pediatr Otorhinolaryngol. (2022) 157:111134. doi: 10.1016/j.ijporl.2022.111134
83. Steinhäuser J, Volk GF, Thielker J, Geitner M, Anna-Maria Kuttenreich AM, Klingner CM, et al. Multidisciplinary care of patients with facial palsy: treatment of 1220 patients in a German facial nerve center. J Clin Med. (2022) 11:427. doi: 10.3390/jcm11020427
84. Terzis JK, Tzafetta K. The “babysitter” procedure: minihypoglossal to facial nerve transfer and cross-facial nerve grafting. Plast Reconstr Surg. (2009) 123:865–76. doi: 10.1097/PRS.0b013e31819ba4bb
85. van Veen MM, Dijkstra PU, Werker PMN. A higher quality of life with cross-face-nerve-grafting as an adjunct to a hypoglossal-facial nerve jump graft in facial palsy treatment. J Plast Reconstr Aesthet Surg. (2017) 70:1666–74. doi: 10.1016/j.bjps.2017.06.002
86. Yoshioka N. Differential reanimation of the midface and lower face using the masseteric and hypoglossal nerves for facial paralysis. Oper Neurosurg (Hagerstown). (2018) 15:174–8. doi: 10.1093/ons/opx217
Keywords: facial nerve, hypoglossal nerve, masseteric nerve, facial reanimation, nerve suture
Citation: Zumbusch F, Schlattmann P and Guntinas-Lichius O (2024) Facial nerve reconstruction for flaccid facial paralysis: a systematic review and meta-analysis. Front. Surg. 11: 1440953. doi: 10.3389/fsurg.2024.1440953
Received: 30 May 2024; Accepted: 8 July 2024;
Published: 22 July 2024.
Edited by:
Ingo Todt, Bielefeld University, GermanyReviewed by:
Giampiero Parrinello, Azienda Ospedaliera Universitaria San Martino (IRCCS), ItalyBostjan Lanisnik, Maribor University Medical Centre, Slovenia
© 2024 Zumbusch, Schlattmann and Guntinas-Lichius. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orlando Guntinas-Lichius, b3JsYW5kby5ndW50aW5hc0BtZWQudW5pLWplbmEuZGU=
†ORCID:
Orlando Guntinas-Lichius
orcid.org/0000-0001-9671-0784
 Friedemann Zumbusch
Friedemann Zumbusch Peter Schlattmann2
Peter Schlattmann2 Orlando Guntinas-Lichius
Orlando Guntinas-Lichius