- 1Department of Neuroscience, Baylor College of Medicine, Houston, TX, United States
- 2Department of Head and Neck Surgery, Division of Surgery, University of Texas MD Anderson Cancer Center, Houston, TX, United States
Cisplatin, a commonly used chemotherapy drug, is well-established for its ototoxic effects, primarily attributed to the damage it inflicts on cochlear hair cells. However, its impact on the vestibular system remains inadequately understood. Here, we provide a comprehensive review of existing literature concerning cisplatin-induced vestibulotoxicity. Animal studies have shown that cisplatin induces a vestibular hair cell loss that is dose-dependent, with the severity of damage also varying according to the route of administration. Notably, intratympanic and systemic injections in animal models have manifested significant damage primarily to utricular hair cells, with a lesser degree of damage observed for the other vestibular end organs. The underlying mechanisms of cisplatin induced vestibular hair cell loss include apoptosis, oxidative stress, and inflammatory cytokines. Several protective agents, such as Pifithrin-α, DAPT, Ginkgolide B, and heat shock proteins, have demonstrated efficacy in inhibiting cisplatin-induced vestibular damage in preclinical studies. Human clinical findings indicate that cisplatin treatment can cause vestibular dysfunction, characterized by symptoms ranging from transient dizziness to persistent vertigo. Challenges in diagnosis, including the limited utilization of comprehensive vestibular testing for many patients, contribute to the variability in reported outcomes. Cisplatin-induced vestibulotoxicity is a significant complication of chemotherapy, necessitating further research to understand its mechanisms and to improve diagnosis and management, ultimately aiming to enhance the quality of life for cancer patients undergoing cisplatin therapy.
1 Introduction
Cisplatin, a potent chemotherapeutic agent containing platinum, stands as a cornerstone in the treatment of various solid tumors due to its remarkable antineoplastic efficacy. However, its clinical utility is limited by significant adverse effects, including nephrotoxicity, neurotoxicity, ototoxicity, and vestibulotoxicity (1).
While extensive research has focused on the ototoxic effects of cisplatin, particularly regarding auditory dysfunction, its impact on the vestibular system remains relatively understudied, creating a notable gap in our knowledge (2, 3). Studies in guinea pigs and rats have demonstrated vestibulotoxic effects, such as hair cell death and a decreased vestibulo-ocular reflex (VOR) gain (4–15). However, the translation of these findings to human clinical scenarios remains elusive. Disturbances in vestibular function with cisplatin therapy can profoundly affect patients' overall well-being. These symptoms are broad, but include balance disorders, dizziness, autonomic disorders, visual instability, and spatial disorientation, highlighting the need for a more comprehensive exploration of cisplatin-induced vestibulotoxicity (16, 17).
The present scoping review aims to characterize the known aspects of complex vestibular dysfunction caused by cisplatin, as well as lay the groundwork for future studies. The primary research questions guiding this exploration are: How do cisplatin treatment regimens adversely affect vestibular function, are these effects long-lasting, and how do vestibular healthcare professionals perceive and navigate cisplatin-induced vestibulotoxicity?
2 Methods
2.1 Inclusion criteria
The review encompasses studies on cisplatin-induced vestibulotoxicity, from animal models to human studies, ensuring a holistic understanding. A comprehensive search strategy was employed to retrieve relevant peer-reviewed reports in English from two databases, PubMed and ScienceDirect. Excluded were studies published in languages other than English and those that were not peer-reviewed. The search strategy involved a two-step approach: first, we searched for keywords covering cisplatin's ototoxic effects for both the auditory and vestibular systems, and second, we extracted the relevant representations of vestibular dysfunction from these reports. Our search strategy was implemented to ensure a thorough exploration of the literature on cisplatin ototoxicity so that all known vestibulotoxic effects could be documented, even if only briefly mentioned in a specific paper. Key search terms included “Cisplatin ototoxicity” and “Cisplatin vestibulotoxicity”, delving into its effects on the vestibular system specifically. The term “Cisplatin AND vestibular” was employed to capture studies investigating the intersection of Cisplatin and the vestibular system. Other terms associated with the vestibular system function such as vestibuloocular reflex (VOR), head impulse test (HIT), benign positional vertigo (BPV), and vestibular evoked myogenic potential (VEMP) were also used so that the search terms “inner ear”, “tinnitus”, “ vertigo”, “cisplatin AND VOR”, “cisplatin AND HIT”, “cisplatin AND gaze holding”,” “cisplatin AND BPPV”, “cisplatin AND orthostatic hypotension”, “cisplatin AND dizziness”, “cisplatin AND VEMP”, “cisplatin AND oscillopsia”, “cisplatin AND caloric”, were used to ensure a thorough search. Our search strategy aimed to uncover a broad spectrum of relevant literature that would provide a current foundation of knowledge regarding Cisplatin-induced vestibular dysfunction. A total of 38 studies were included.
2.2 Data extraction
The methodology involved extracting quantitative outcomes, qualitative insights, and clinical observations from each study to understand cisplatin-induced vestibulotoxicity. Two reviewers analyzed the studies to identify quantitative outcomes and clinical observations. A comparative analysis was then conducted to identify patterns, contradictions, and overarching themes across studies. For empirical studies, relationships between cisplatin dosage and specific vestibular anatomical or functional damage mechanisms were characterized, as well as any variability in responses and assessment methods.
2.3 Quality assessment
All studies included in the review had undergone rigorous peer review for reliability and transparency and were obtained from reputable scientific journals. We systematically evaluated each study based on its relevance, validity, and applicability. Additionally, biases and limitations within individual studies were critically considered to ensure a balanced interpretation of findings.
3 Results
3.1 Animal studies
3.1.1 Vestibular neuroanatomy
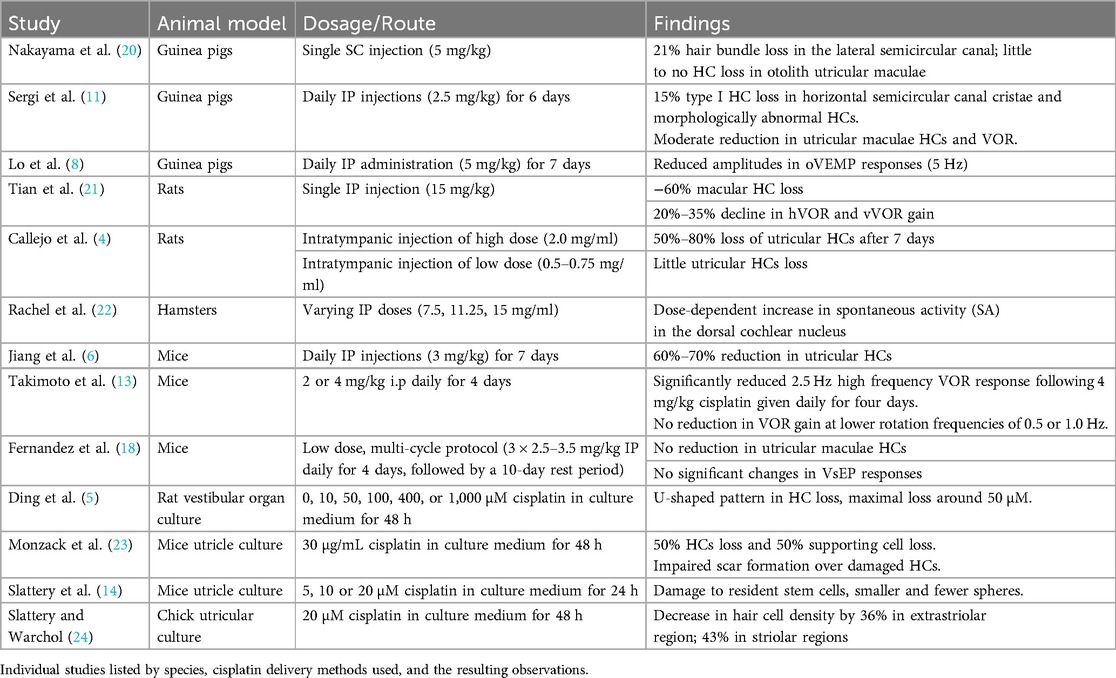
It was surprising to discover that relative few animal studies have examined cisplatin-induced vestibulotoxicity. Instead, most of the research has focused upon the ototoxic effects in the auditory system (18, 19). Of the existing literature on vestibulotoxicity, most work has focused on investigating the anatomical effects of ototoxic reactions in vestibular receptors (Table 1). Results from systemic administration of cisplatin have been mixed, with differences noted between damage resulting in the semicircular canal cristae vs. otolith maculae. Not surprisingly, differences in vestibular receptor damage are correlated to the type of cisplatin administration and the dosage used. For example, Nakayama et al. (20) reported targeted vestibular hair cell (HC) loss following a single subcutaneous (SC) injection of cisplatin. Their study in guinea pigs showed that following the administration of a single SC cisplatin injection of 5 mg/kg, the number of hair bundles in the lateral semicircular canal decreased by 21%. The decrease was observed on the central apex portion of the cristae, while the remaining areas of the cristae exhibited hair bundle numbers similar to control (20). Little to no HC loss was observed in the otolith utricular maculae. In rats, Tian et al. (21) observed a significantly higher loss of nearly 60% macular HCs following a single high dose intraperitoneal (IP) injection of 15 mg/kg cisplatin. These findings were aligned with Sergi et al.'s results when IP injections were used as a route of administration in guinea pigs. Following a daily treatment of IP 2.5 mg/kg cisplatin for six days, a moderate reduction in HCs were observed in both the horizontal semicircular canal cristae and utricular maculae (11). In the horizontal cristae, 15% of type I HCs were lost. Still other HCs appeared morphologically abnormal as defined by a loss of calyceal afferent terminals and the appearance of globular degenerating vesicles above the basal laminae (11). In mice, Jiang et al. (6) described a 60%–70% reduction in utricular HCs following daily IP injections of 3 mg/kg cisplatin for 7 days. Alternatively, Fernandez et al. (18) reported no reduction in utricular maculae HCs following a low dose, multi-cycle protocol that is similar to paradigms used to treat human gynecological cancers. These authors examined only the utricle in mice following a multi-week 3 cycle regime of 2.5–3.5 mg/kg IP given once daily for four days, followed by a 10 day rest period. It is unknown if any HC loss occurred in the semicircular canal cristae in these animals.
Although not typically employed as a clinical strategy for cancer treatment, intratympanic injections of cisplatin have also been used to examine vestibulotoxicity (Table 1). Callejo and colleagues employed intratympanic injections in rats and described that the severity of vestibular toxicity was dose dependent. These authors only examined the otolithic utricular maculae, but observed that a large loss of HC occurred following a single administration of a high dose of 1.0 or 2.0 mg/ml of cisplatin, while little hair cell loss was observed at the lowest doses of 0.5 and 0.75 mg/ml. Based upon the type of cellular marker used to identify HCs [phalloidin or myosin VIIA (MYO7A), a molecular actin binding protein], these authors described between a 50%–80% loss of utricular HCs 7 days after 2.0 mg/ml cisplatin injections. Similar findings were described three days after injection (4) (Table 1).
In addition to in vivo studies, organ culture approaches have been used to examine cisplatin vestibular ototoxicity (Table 1). Rat vestibular organ cultures were exposed to cisplatin directly by treating the vestibular explants with 0, 10, 50, 100, 400, or 1,000 μM cisplatin in a culture medium for 48 h (5). A dose-response relationship was identified where a U-shaped pattern in HC loss was described, with maximum hair cell loss around 50 μM (5). Chick utricular cultures were also studied by Slattery et al., where the utricles that were treated with 20 μM of cisplatin for 48hrs showed a decrease in hair cell density by 36% in the extrastriolar regions and by 43% in the striolar regions as compared to the control group (24). In mice, utricles cultured in 30 ug/ml cisplatin experienced a 50% loss of HCs and 50% of supporting cells within 45 h (23). These authors also noted that cisplatin impairs scar formation over damaged hair cells. They suggested that cisplatin inhibits the supporting cell phagocytic removal of dead HCs. It was noted that the accumulation of uncleared apoptotic cells may result in the release of proinflammatory cytokines, leading to secondary necrosis (23).
Cisplatin has also been shown to interfere with the transformation of stem cells into mature HC types (Table 1). Slattery et al. explanted mouse utricles, then treated them with 5, 10, or 20 µM cisplatin for 24 h (14). They next incubated the cells in suspension culture for 3, 5, and 7 days to quantify the number of spheres formed. Results showed that spheres derived from cisplatin-treated samples were smaller and significantly fewer in number compared to the control at all time points. This indicates that cisplatin exposure damages the resident stem cells of the mammalian inner ear. Moreover, it exhibits genotoxic effects even at low doses, targeting hair cells, supporting cells, and epithelial stem cells in the mouse utricle. The study's implications for sensory regeneration are profound, posing challenges for regenerative strategies (14).
A principal finding from these neuroanatomical studies examining the inner ear receptors is that cisplatin elicits a loss of hair cells (Table 1). Through the routes of administration examined so far, cisplatin produces a greater loss of auditory HCs than vestibular hair cells (11, 20). Cisplatin has been shown to gather within the cochlea over an extended period, potentially persisting indefinitely. This accumulation could elucidate its lasting and occasionally delayed impacts on the auditory system. Yet, it remains uncertain whether a comparable phenomenon occurs within the vestibular organs (25). Still, the loss of vestibular hair cells following cisplatin treatment can be profound. This is particularly true for the otolith organs, the utricle and saccule, with smaller deficits observed in the cristae of the semicircular canals (11). Variations in the degree of hair cell loss were associated with different concentrations, doses, and routes of administration. However, few of these studies have used cisplatin in a delivery routine analogous to that used in human clinical cancer treatments. For example, in the treatment of head and neck cancers, cisplatin is administered systemically over a protracted period of time. Each cycle is administered over several hours, with cycles typically spaced three weeks apart. The total number of cycles varies, and common treatment regimens often involve 2–6 cycles. Cisplatin dosage is expressed as mg/m2 and often ranges from 40 to 75 mg/m2 per cycle with a total dosage reaching up to 200–300 mg/m2 (26, 27). Therefore, it is difficult to extrapolate specific findings on vestibular damage from animal model in vitro and in vivo studies to human subjects.
3.1.2 Vestibular function deficits
Functional vestibular deficits following cisplatin administration have also been examined in animal work, albeit to a much lesser degree (Table 1). The vestibulo-ocular reflex (VOR), a benchmark for examining vestibular efficacy, has been shown to decrease with cisplatin treatment. For example, Sergi et al. (11, 12) examined the VOR in guinea pigs subjected to daily intraperitoneal administration of 2.5 mg/kg cisplatin for six consecutive days (11, 12). They reported a notable 5% reduction in both horizontal (hVOR) and vertical (vVOR) gain during mid-frequency stimulation (0.2–0.4 Hz) and a 10%–15% decrease in the vVOR at lower frequencies (below 0.1 Hz) on the third day of treatment. By the sixth day of treatment, the effects intensified with a 20%–35% decline in hVOR and vVOR gain across the frequency band tested (12). In mice, Takimoto et al. observed a significantly reduced 2.5 Hz high frequency VOR response following 4 mg/kg i.p. cisplatin given daily for four days. No reduction in VOR gain at lower rotation frequencies of 0.5 or 1.0 Hz, nor following a lower dose of 2 mg/kg i.p. cisplatin administration were noted (13). In a similar study, Lo et al. (8) treated guinea pigs with a higher dosage of 5 mg/kg cisplatin daily for seven days and found reduced amplitudes in ocular vestibular-evoked myogenic potential (oVEMP) responses (5 Hz) as compared to saline-treated controls. However, they reported no significant changes in cervical vestibular-evoked myogenic potentials (cVEMP) responses (8). Similar findings of no effect upon the vestibular evoked potential (VsEP) examining otolithic responses were observed in mice following a low dosage treatment of 3 cycles of 2.5–3.5 mg/kg × 4 days followed by a 10 day rest period in mice (18).
To date, no studies have examined vestibular afferents' neural response properties during or following cisplatin administration (Table 1). However, the impact of cisplatin on spontaneous neural activity within the hamster dorsal cochlear nucleus has been examined (22). The findings unveiled a dose-dependent increase in spontaneous activity (SA). Following treatment, the mean peak rate of SA was as follows: 28 spikes/s in the high-dose cisplatin group (15 mg/ml IP), 32 spikes/s in the intermediate dose group (11.25 mg/ml IP), and 29 spikes/s in the low dose group (7.5 mg/ml IP), compared to 14, 15, and 13 spikes/s in the respective control groups (22). It is known that some patients undergoing cisplatin therapy experience increased episodes of tinnitus (28). It is possible that the hyperactivity seen in the cochlear nuclei, which is weighted toward the medial or high frequency region of the dorsal cochlear nucleus (DCN), could underlie the elevated tinnitus. This observation suggests that further investigations are warranted into the intricate interplay between cisplatin, neural activity, and the manifestation of auditory-related complications. This neurophysiological aspect emphasizes the need for a holistic approach to mitigate the vestibular damage induced by cisplatin and the associated neural complications that can significantly impact the patient's quality of life (22).
3.1.3 Mechanisms of vestibular loss
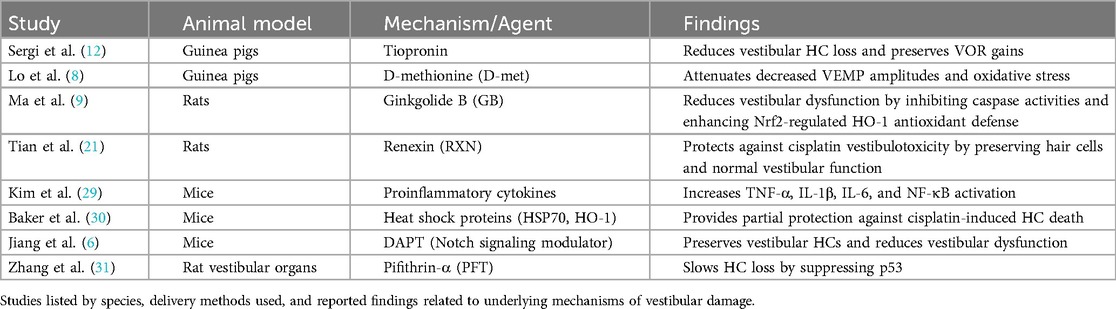
Although it is clear that cisplatin can produce vestibular hair cell damage and loss, the mechanism for such injury remains poorly understood. Several studies have attempted to address this question (Table 2). For example, it is known that many forms of HC death result from apoptosis (7). As noted above, Slattery et al. (24) found that chick utricular maculae exhibited extensive HC loss in cisplatin-treated cultures. They demonstrated that HC death was produced by apoptosis using immunolabeling activated caspase-3 (24). Following such results, it has been shown that agents that affect the apoptotic pathway may provide some protection from cisplatin-induced damage. For example, Pifithrin-α (PFT), known for suppressing p53, a crucial protein in the apoptotic cascade, slowed hair cell loss when administered in conjunction with cisplatin (31). PFT effectively suppresses the activation of caspase-1 and caspase-3, critical components in the apoptotic pathway. These findings emphasized the importance of p53 in cisplatin-induced apoptosis and positioned PFT as a potential therapeutic agent for protection against cisplatin-induced vestibulotoxicity (31). Another approach to protecting hair cells from cisplatin ototoxicity is to manipulate Notch signaling. Notch is known to induce hair cell formation through lateral inhibition and is involved in hair cell proliferation (6). In a recent study, a γ-secretase inhibitor known for modulating Notch signaling {DAPT - N-[N-(3, 5-difluorophenacetyl)-l-alanyl]-s-phenylglycinet-butyl ester}, was used together with cisplatin administration, and the effects on animal swimming test scores (a measure of vestibular function) and preservation of vestibular hair cells were examined. The results following administration of 10 mg/kg of DAPT with 3 mg/kg of cisplatin IP daily for seven days in mice showed that preserved morphologically normal hair cell bundles were present in the vestibular receptors. In contrast, mice treated with cisplatin alone had a significant reduction (−70%) in the number of hair cell bundles as well as disorganized stereocilia (6). In addition, the mice treated with cisplatin alone exhibited vestibular dysfunction manifested by an inability to keep their head above water and circling behavior in a swim test. Results from this study suggest a potential strategy for protecting against vestibular damage induced by cisplatin by inhibiting the Notch signaling pathway (6).
A separate investigation examined Ginkgolide B (GB), which serves as an inhibitor of caspase activities and mitochondrial apoptosis, as a potential agent for preserving vestibular function during cisplatin treatment (9). Rats were subjected to intraperitoneal injections of cisplatin (16 mg/kg) and GB (10 mg/kg), either alone or in combination with Zinc Protoporphyrin (ZnPP), a known inhibitor of nuclear factor erythroid 2-related factor 2 (Nrf2), or LY294002, an inhibitor of the survival and growth protein kinase B (Akt) signaling pathway implicated in Nrf2 activation (9). Compared to rats treated solely with cisplatin, those administered with cisplatin plus GB exhibited decreased head rotations during the tail-hanging test and reduced time intervals to complete a swim test. However, when ZnPP or LY294002 was co-administered with GB, the protective effects exerted by GB on vestibular function were not observed (9). Moreover, GB demonstrated inhibition of cellular and mitochondrial reactive oxygen species generation while enhancing Nrf2-regulated HO-1 antioxidant defense in cells exposed to cisplatin (9).
An extensive investigation into the protective effects of renexin (RXN), a combination of Ginkgo biloba extract and Cilostazol, against cisplatin-induced vestibulotoxicity was recently performed using rats (21) (Table 2). A single high dose of 16 mg/kg intraperitoneal cisplatin was used to induce damage. As described above, in the cisplatin-only group, the number of otolith organ hair cells was significantly reduced, with an uneven distribution and abnormal appearance compared to the control group (21). In contrast, the RXN (180 mg/kg) + cisplatin group exhibited well-preserved hair cells with normal-shaped stereocilia and a typical density level. In addition, animals were tested for behavioral vestibular function by examining head rotations and swimming ability. All experimental groups, except for the RXN (180 mg/kg) + cisplatin group, exhibited significantly increased head rotations and prolonged swimming time after five days of treatment. These findings highlighted a role for RXN's protective effect against cisplatin vestibulotoxicity (21).
Tiopronin [N-(2-mercaptopropionyl)-glycerine] is a drug with a free thiol group used in the treatment of hepatic and skin disorders and has been shown to protect against cisplatin-induced nephrotoxicity (12). The drug was also investigated for its vestibular protection by examining vestibular receptor damage and the VOR response (Table 2). It was shown that guinea pigs injected with daily cisplatin (2.5 mg/kg IP) as well as tiopronin (300 mg/kg IP) for six days had lower extent of vestibular hair cell loss in macular and semicircular canals compared to the control group (cisplatin 2.5 mg/kg IP alone). In addition, the VOR gains at 0.05–0.4 Hz were reduced in the cisplatin alone group as compared to the tiopronin + cisplatin group (12). On a histological level, the neuroepithelium of the labyrinth was better preserved (12).
Amino acid-based protectants, such as D-methionine (D-met), have also been employed as a protective agent against cisplatin-induced vestibulotoxicity (Table 2). For example, in guinea pigs, enzyme activities, lipid peroxidation, and VEMP were examined with/without D-met addition during cisplatin treatment (8). When 30 mg/kg IP D-met pre-treatment was given 30 min prior to 5 mg/kg IP cisplatin treatment daily for seven days, the decreased VEMP amplitudes observed with cisplatin alone were attenuated. The authors suggested that this effect was associated with a partial reversal in oxidative stress produced by cisplatin. They found that the cisplatin-only group had the lowest mean Na+, K + - Adenosine 5′-TriPhosphatase (ATPase), and Ca2+-ATPase and the highest lactoperoxidase (LPO) and nitrous oxide (NO) levels as compared to the D-met + cisplatin treatment animals (8).
Outside of the apoptotic pathway and oxidative stress, proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and interleukin 6 (IL-6), have been implicated in cisplatin-induced vestibular cell death (Table 2). These inflammatory markers have been observed to increase in the semicircular cristae and utricles of mice following intraperitoneal injection of 4 mg/kg cisplatin daily for four days (29). Nuclear factor kappa-B (NF-κB), a critical transcription factor involved in the expression of inflammatory cytokines, is known to be activated by TNFα and IL-1β. It was found to be elevated in both the supporting cells and hair cells of the cristae ampullae and utricles after daily administration of 4 mg/kg intraperitoneal cisplatin for four days, suggesting that cytokines would also be elevated. Moreover, cisplatin induced the nuclear translocation of NF-κB, a pivotal transcription factor in the expression of proinflammatory cytokines, by activating mitogen-activated protein kinases such as extracellular signal-regulated kinase (ERK), c-Jun N-terminal protein kinases (JNK), and a mitogen activated protein kinase (p38) (29) (Table 2).
In addition, heat shock protein-mediated protection has also been studied as a potential defense mechanism for cisplatin-induced vestibulotoxicity (30). Cultured mouse utricles that were heat shocked six hours prior to cisplatin treatment (10–60 µg/ml) had significantly greater cell survival proportional to dosage. The cellular survival was present in wild-type mice and absent in heat shock protein 70 (HSP70) knockout mice, highlighting that HSP70 is necessary for the protective effect of heat shock against cisplatin-induced hair cell death (30). However, HSP70 only provides partial protection. Heme oxygenase 1, another heat-inducible protein, was also shown to have a significant protective effect across the dose-response relationship, with macrophages playing an integral role (30). Both proteins were not found to have a synergistic or additive effect when both were expressed (30) (Table 2).
These protective factors, including RXN, tiopronin, GB and heat shock proteins, have demonstrated their efficacy in safeguarding against cochlear toxicity, highlighting a parallel between cochlear and vestibular hair cell damage and protective strategies (9, 12, 21, 30) (Table 2).
The exploration of these potential protective compounds not only guides targeted interventions for clinical applications but also sets the stage for further research, fostering the development of effective and precise strategies to alleviate the adverse effects of cisplatin on the vestibular system.
Why do cochlear hair cells appear to be more susceptible to cisplatin induced damage than vestibular hair cells? Suzuki and Kaga explored the impact of cisplatin on basement membrane anionic sites (BMAs) in guinea pigs (32). Utilizing electron microscopy to analyze temporal bones, cisplatin observed a reduction in cationic tracer density in the stria vascularis capillary wall, hinting at a reduction in BMAs. However, no change in the capillary or subepithelial basement membrane in the ampullar dark cell area was seen after treatment with cisplatin (32). No change in BMAs was observed beneath the sensory cell area of the ampulla and macula. These findings suggest that the charge barrier in the vestibular labyrinth remains functional soon after a single intravenous infusion of cisplatin (32). The authors emphasized that the establishment of a functional charge barrier in the vestibular labyrinth following cisplatin infusion allows only a few cisplatin molecules to be transported to the vestibular sensory cells and dark cells via the capillary and sub-epithelial basement membranes, potentially explaining the reduced toxicity of cisplatin on the vestibular system (32).
3.2 Human clinical findings
Opinions on the severity of vestibular deficits in the human population following cisplatin treatment vary (Table 3). Most of the work regarding possible vestibular dysfunction has been gathered from anecdotal self-reports or questionnaires (17). The most common vestibular symptoms reported following cisplatin exposure include temporary dizziness, unsteadiness, imbalance, and vertigo (2, 3, 16, 17, 33). A study on sixty-five testicular and gynecological cancer patients treated with cisplatin observed “balance symptoms” with an incidence of 17% (2, 3). The balance symptoms included vertigo, benign paroxysmal positional vertigo (BPPV), dizziness, unsteadiness, and falls; where vertigo was the most common (9.2%) (2, 3, 16). Brydøy et al. examined 1,409 testicular cancer survivors (TCS) and reported that 22% of the TCS treated with cisplatin reported persistent vertigo or dizziness (28) (Table 3).
Vestibular dysfunction has also been evaluated following cisplatin treatment using more standard assays, including head impulse test (HIT), VEMP, and rotational VOR (Table 3). For example, Hülse et al. measured VEMP responses in patients undergoing cisplatin treatment during randomized control trials at three crucial time points: before the randomized controlled trial (RCT), six weeks after, and three months after cisplatin therapy (35). These patients were given 80 mg/m2 of cisplatin at week 1 and 4 (35). At six weeks and three months following treatment, reproducible oVEMP responses were not obtainable in about a third of the patients; reproducible cVEMP responses were also unachievable in 29.3% of patients three months following RCT (35). Nonreproducible cVEMP and oVEMP suggest an impact on saccular and utricular vestibular pathways, respectively. Furthermore, a decline in HIT gain was noted. Prior to RCT, the median gain during horizontal rightward testing was 1.01 and 1.03 during leftward testing, which subsequently diminished to 0.87 and 0.85, respectively following RCT. This reduction was accompanied by an elevation in the frequency of catch-up saccades executed during each head thrust (35). These findings were observed six weeks after the RCT and persisted for three months, providing evidence of vestibular dysfunction associated with cisplatin treatment that appeared to recover over time likely due to mechanisms of central compensation (35, 39). In contrast, Prayuenyong et al. demonstrated that cancer survivors who underwent standard cisplatin therapy (100–400 mg/m2) exhibited normal results in eye movements induced by the head thrust test during HIT testing (2, 3). These authors also reported an absence of corrective saccades during the HIT response (2, 3). Similarly, Moreno and Belinchon reported no change in HIT for patients who received a median cumulative dose of 448.87 mg (36). It is worth highlighting that while HIT boasts high specificity, it may compromise sensitivity, potentially accounting for the disparities between rotational chair VOR and HIT findings.
In terms of the more traditional rotational VOR measures, Myers et al. reported that the VOR for low frequency head rotations spanning 0.01–0.16 Hz was not affected by cisplatin treatment. They observed no significant difference in VOR gain/phase values following cisplatin chemotherapy with mean cumulative dosages to 1,600 mg (37). However, a study by Kitsigianis et al. demonstrated a significant VOR deficit induced by high-frequency rotation. In this study, vestibular autorotation tests (VAT) were utilized to assess any VOR changes at high frequencies among five testicular carcinoma patients receiving six weekly treatments of 60 mg/m2 cisplatin, as well as four pulmonary carcinoma patients undergoing eight treatments of 100 mg/m2 cisplatin (34). The findings unveiled notable reductions in VOR gains and significant phase lags across head rotation frequencies ranging from 3 to 5 Hz in both groups (34) (Table 3).
In the clinical setting, the bi-thermal caloric test is considered the gold-standard measure of vestibular function. However, this tool has been seldom used to evaluate the impact of cisplatin on vestibular function in human subjects (Table 3). Kobayashi et al. observed an abnormal caloric test in half of the 10 cancer patients treated with 80–550 mg of cisplatin (38) (Table 3). Currently, no study of patients treated with cisplatin integrates the caloric test, rotational chair test, and HIT to evaluate the VOR comprehensively across different frequency ranges in the same setting.
Based upon the complexity of vestibular testing, the degree of such testing performed, and the limited literature on the subject, it is not surprising that a survey distributed among clinicians working in the audiovestibular field showed that 32% of participants believed it often causes such effects, and 52% considered it a possible outcome (17) (Table 3). This lack of consensus within the medical community adds a layer of complexity to the interpretation of vestibular symptoms. Clinicians with a biased opinion that no vestibular deficits result from cisplatin treatment may overlook subtle or complex vestibular involvement (17). It emphasizes the need for a nuanced diagnostic approach to consider a range of symptoms, from subtle balance issues to BPPV and vertigo (2, 3, 16).
The cumulative findings underscore the necessity for advanced diagnostic tools, interdisciplinary collaboration, and continued scientific research to unravel the complexities of cisplatin-induced alterations in auditory and vestibular functions across diverse patient populations. Imaging procedures such as labyrinthine enhancement on 3D black blood MR images monitoring alterations in the labyrinth induced by cisplatin exposure may allow for more precise diagnosis (40). However, these techniques remain understudied in cisplatin vestibulotoxicity, and prospective studies are needed to validate them before they can be used routinely in this condition.
4 Discussion
Here, we provide a scoping review of a comprehensive analysis of cisplatin-induced vestibulotoxicity, incorporating insights from animal studies and human clinical findings. The animal studies have illuminated various aspects of vestibular damage caused by cisplatin, including dose-dependent effects, differences in damage between intratympanic and systemic administration, and differences in damage between the semicircular canal cristae and the otolith maculae (4, 5, 8, 11, 12, 18, 23, 24). Additionally, protective factors such as Pifithrin-α, DAPT, Ginkgolide B, Tiopronin, and others have been explored, revealing potential therapeutic avenues (9, 12, 21, 30). However, it is difficult to extrapolate findings from animal studies to human subjects due to differences in delivery and dose protocols of cisplatin. Thus, it is apparent that further research in animal models is prudent particularly in adopting multi-dose cyclic treatment protocols that mimic more closely those being utilized currently in the clinic to treat various forms of human cancer.
The underlying mechanisms of vestibular hair cell loss, including apoptosis, oxidative stress, and inflammatory cytokines have been investigated, providing valuable insights into the pathophysiology of cisplatin-induced vestibulotoxicity (6, 8, 14, 24, 29, 31). However, there are challenges in translating these findings into clinical practice, highlighting the need for further research, and targeted interventions.
The clinical findings pertaining to vestibular deficits following cisplatin treatment in humans are diverse, with discrepancies in the prevalence and severity of symptoms (2, 3, 16). This underscores the pressing need for standardized vestibular assessment protocols and interdisciplinary collaboration to accurately diagnose and manage vestibular dysfunction in patients undergoing cisplatin therapy. For example, many facilities providing cancer treatment have commonly available vestibular dysfunction clinics that offer nystagmography, either electro (eVNG) or video (vVNG) for caloric, positional, or head impulse testing, as well as VEMP testing. Together, these vestibular assessment batteries require approximately 1–2 h of patient time, with subsequent interpretative findings provided to the oncology team. More sophisticated vestibular testing facilities may also have available rotational chair or dynamic posturography equipment that can further pinpoint affected vestibular dysfunction. In cases where vestibular dysfunction from cisplatin treatment is suspected, or in cases where larger cisplatin dose therapy is required, the current review suggests that a standard protocol of at least VNG or VNG combined with VEMP testing be employed so that even subtle vestibular dysfunction findings can be objectively evaluated, with subsequent vestibular rehabilitation therapies employed. This standardized protocol could mirror those for auditory testing and include baseline testing before the start of chemotherapy, testing upon completion of cisplatin therapy, and additional testing before each cycle if new vestibular symptoms emerge.
Future research directions hold immense potential, including a focus on enhancing diagnostic tools, exploring novel therapeutic targets, and improving our comprehension of the complex interplay between cisplatin and the vestibular system.
Author contributions
TF: Writing – original draft, Writing – review & editing. MN: Writing – original draft, Writing – review & editing. JD: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Supported by funding through NIDCD R01 DC 019515.
Conflict of interest
MN reports stock ownership in 3M, Amgen, Kimberly-Clark, Johnson & Johnson, Medtronic, Nestle, and Pfizer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Barabas K, Milner R, Lurie D, Adin C. Cisplatin: a review of toxicities and therapeutic applications. Vet Comp Oncol. (2008) 6(1):1–18. doi: 10.1111/j.1476-5829.2007.00142.x
2. Prayuenyong P, Baguley DM, Kros CJ, Steyger PS. Preferential cochleotoxicity of cisplatin. Front Neurosci. (2021) 15:695268. doi: 10.3389/fnins.2021.695268
3. Prayuenyong P, Kasbekar AV, Hall DA, Hennig I, Anand A, Baguley DM. Imbalance associated with cisplatin chemotherapy in adult cancer survivors: a clinical study. Otol Neurotol. (2021) 42(6):e730–4. doi: 10.1097/MAO.0000000000003079
4. Callejo A, Durochat A, Bressieux S, Saleur A, Chabbert C, Domènech Juan I, et al. Dose-dependent cochlear and vestibular toxicity of trans-tympanic cisplatin in the rat. Neurotoxicology. (2017) 60:1–9. doi: 10.1016/j.neuro.2017.02.007
5. Ding D, Jiang H, Zhang J, Xu X, Qi W, Shi H, et al. Cisplatin-induced vestibular hair cell lesion-less damage at high doses. J Otol. (2018) 13(4):115–21. doi: 10.1016/j.joto.2018.08.002
6. Jiang W, Yu J, Cao M, Xiao B, Li F, Chen C, et al. Notch signaling pathway plays a critical role in chemotherapeutic drug-induced vestibular injury. Biosci Trends. (2022) 16(5):363–6. doi: 10.5582/bst.2022.01394
7. Li L, Nevill G, Forge A. Two modes of hair cell loss from the vestibular sensory epithelia of the Guinea pig inner ear. J Comp Neurol. (1995) 355:405–17. doi: 10.1002/cne.903550307
8. Lo WC, Chang CM, Liao LJ, Wang CT, Young YH, Chang YL, et al. Assessment of D-methionine protecting cisplatin-induced otolith toxicity by vestibular-evoked myogenic potential tests, ATPase activities and oxidative state in Guinea pigs. Neurotoxicol Teratol. (2015) 51:12–20. doi: 10.1016/j.ntt.2015.07.004
9. Ma W, Hu J, Cheng Y, Wang J, Zhang X, Xu M. Ginkgolide B protects against cisplatin-induced ototoxicity: enhancement of akt-Nrf2-HO-1 signaling and reduction of NADPH oxidase. Cancer Chemother Pharmacol. (2015) 75(5):949–59. doi: 10.1007/s00280-015-2716-9
10. Roccio M, Edge ASB. Inner ear organoids: new tools to understand neurosensory cell development, degeneration and regeneration. Development. (2019) 146:1–12. doi: 10.1242/dev.177188
11. Sergi B, Ferraresi A, Troiani D, Paludetti G, Fetoni AR. Cisplatin ototoxicity in the Guinea pig: vestibular and cochlear damage. Hear Res. (2003) 182(1–2):56–64. doi: 10.1016/s0378-5955(03)00142-4
12. Sergi B, Fetoni AR, Ferraresi A, Troiani D, Azzena GB, Paludetti G, et al. The role of antioxidants in protection from ototoxic drugs. Acta Otolaryngol Suppl. (2004) (552)124:42–5. doi: 10.1080/03655230410017111
13. Takimoto Y, Imai T, Kondo M, Hanada Y, Uno A, Ishida Y, et al. Cisplatininduced toxicity decreases the mouse vestibulo-ocular reflex. Toxicol Lett. (2016) 262:49–54. doi: 10.1016/j.toxlet.2016.09.009
14. Slattery EL, Oshima K, Heller S, Warchol ME. Cisplatin exposure damages resident stem cells of the mammalian inner ear. Dev Dyn. (2014) 243(10):1328–37. doi: 10.1002/dvdy.24150
15. Talach T, Rottenberg J, Gal B, Kostrica R, Jurajda M, Kocak I, et al. Genetic risk factors of cisplatin induced ototoxicity in adult patients. Neoplasma. (2016) 63(2):263–8. doi: 10.4149/212_140820N391
16. Prayuenyong P, Taylor JA, Pearson SE, Gomez R, Patel PM, Hall DA, et al. Vestibulotoxicity associated with platinum-based chemotherapy in survivors of cancer: a scoping review. Front Oncol. (2018) 8:363. doi: 10.3389/fonc.2018.00363
17. Prayuenyong P, Kasbekar AV, Hall DA, Baguley DM. Audiovestibular clinician experiences and opinions about cisplatin vestibulotoxicity. Eur Arch Otorhinolaryngol. (2020) 277(12):3283–93. doi: 10.1007/s00405-020-06033-4
18. Fernandez K, Wafa T, Fitzgeralk TS, Cunningham LL. An optimized, clinically relevant mouse model of cisplatin-induced ototoxicity. Hearing Res. (2019) 375:66–74. doi: 10.1016/j.heares.2019.02.006
19. Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. (2007) 226(1–2):157–67. doi: 10.1016/j.heares.2006.09.015
20. Nakayama M, Riggs LC, Matz GJ. Quantitative study of vestibulotoxicity induced by gentamicin or cisplatin in the guinea pig. Laryngoscope. (1996) 106(2 Pt 1):162–7. doi: 10.1097/00005537-199602000-00011
21. Tian CJ, Kim YJ, Kim SW, Lim HJ, Kim YS, Choung YH. A combination of cilostazol and ginkgo biloba extract protects against cisplatin-induced cochleo-vestibular dysfunction by inhibiting the mitochondrial apoptotic and ERK pathways. Cell Death Dis. (2013) 4(2):e509. doi: 10.1038/cddis.2013.33
22. Rachel JD, Kaltenbach JA, Janisse J. Increases in spontaneous neural activity in the hamster dorsal cochlear nucleus following cisplatin treatment: a possible basis for cisplatin-induced tinnitus. Hear Res. (2002) 164(1–2):206–14. doi: 10.1016/s0378-5955(02)00287-3
23. Monzack EL, May LA, Roy S, Gale JE, Cunningham LL. Live imaging the phagocytic activity of inner ear supporting cells in response to hair cell death. Cell Death Differ. (2015) 22(12):1995–2005. doi: 10.1038/cdd.2015.48
24. Slattery EL, Warchol ME. Cisplatin ototoxicity blocks sensory regeneration in the avian inner ear. J Neurosci. (2010) 30(9):3473–81. doi: 10.1523/JNEUROSCI.4316-09.2010
25. Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. (2017) 8(1):1654. doi: 10.1038/s41467-017-01837-1
26. Okano S, Enokida T, Onoe T, Ota Y, Motegi A, Zenda S, et al. Induction TPF chemotherapy followed by CRT with fractionated administration of cisplatin in patients with unresectable locally advanced head and neck cancer. Int J Clin Oncol. (2019) 24(7):789–97. doi: 10.1007/s10147-019-01418-w
27. Teft WA, Winquist E, Nichols AC, Kuruvilla S, Richter S, Parker C, et al. Predictors of cisplatin-induced ototoxicity and survival in chemoradiation treated head and neck cancer patients. Oral Oncol. (2019) 89:72–8. doi: 10.1016/j.oraloncology.2018.12.010
28. Brydøy M, Oldenburg J, Klepp O, Bremnes RM, Wist EA, Wentzel-Larsen T, et al. Observational study of prevalence of long-term raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst. (2009) 101(24):1682–95. doi: 10.1093/jnci/djp413
29. Kim HJ, So HS, Lee JH, Park C, Lee JB, Youn MJ, et al. Role of proinflammatory cytokines in cisplatin-induced vestibular hair cell damage. Head Neck. (2008) 30(11):1445–56. doi: 10.1002/hed.20892
30. Baker TG, Roy S, Brandon CS, Kramarenko IK, Francis SP, Taleb M, et al. Heat shock protein-mediated protection against cisplatin-induced hair cell death. J Assoc Res Otolaryngol. (2015) 16(1):67–80. doi: 10.1007/s10162-014-0491-7
31. Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. (2003) 120(1):191–205. doi: 10.1016/s0306-4522(03)00286-0
32. Suzuki M, Kaga K. Effect of cisplatin on the basement membrane anionic sites in the ampulla, macula, and stria vascularis of guinea pigs. Ann Otol Rhinol Laryngol. (1997) 106(11):971–5. doi: 10.1177/000348949710601114
33. Trendowski MR, El Charif O, Dinh PC Jr, Travis LB, Dolan ME. Genetic and modifiable risk factors contributing to cisplatin-induced toxicities. Clin Cancer Res. (2019) 25(4):1147–55. doi: 10.1158/1078-0432.CCR-18-2244
34. Kitsigianis GA, O'Leary DP, Davis LL. Active head-movement analysis of cisplatin-induced vestibulotoxicity. Otolaryngol Head Neck Surg. (1988) 98(1):82–7. doi: 10.1177/019459988809800114
35. Hülse R, Stuck BA, Hörmann K, Rotter N, Nguyen J, Aderhold C, et al. Changes in vestibular function in patients with head-and-neck cancer undergoing chemoradiation. Ear Nose Throat J. (2022) 101(6):379–85. doi: 10.1177/0145561320949482
36. Moreno I, Belinchon A. Vestibulotoxicity in patients undergoing cisplatin-based cancer treatment: a phase IIIB randomized controlled clinical trial. Audiol Neurootol. (2023) 28(3):230–8. doi: 10.1159/000528435
37. Myers SF, Blakley BW, Schwan S. Is cis-platinum vestibulotoxic? Otolaryngol Head Neck Surg. (1993) 108(4):322–8. doi: 10.1177/019459989310800403
38. Kobayashi H, Ohashi N, Watanabe Y, Mizukoshi K. Clinical features of cisplatin vestibulotoxicity and hearing loss. ORL J Otorhinolaryngol Relat Spec. (1987) 49(2):67–72. doi: 10.1159/000275909
39. Newlands SD, Perachio AA. Compensation of horizontal canal related activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil. I. Type I neurons. Exp Brain Res. (1990) 82(2):359–72. doi: 10.1007/BF00231255
Keywords: vestibular, oncology, neurotology, cisplatin, ototoxicity
Citation: Fleihan T, Nader ME and Dickman JD (2024) Cisplatin vestibulotoxicity: a current review. Front. Surg. 11:1437468. doi: 10.3389/fsurg.2024.1437468
Received: 23 May 2024; Accepted: 18 September 2024;
Published: 3 October 2024.
Edited by:
Andrea Gallo, Sapienza University of Rome, ItalyCopyright: © 2024 Fleihan, Nader and Dickman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. David Dickman, ZGlja21hbkBiY20uZWR1
 Tamara Fleihan
Tamara Fleihan Marc Elie Nader
Marc Elie Nader J. David Dickman
J. David Dickman