
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 01 November 2024
Sec. Surgical Oncology
Volume 11 - 2024 | https://doi.org/10.3389/fsurg.2024.1430344
Background: Fumarate hydratase-deficient renal cell carcinoma (FH-RCC) is a rare subtype of kidney tumor. Most of them are solitary lesions, making preoperative diagnosis difficult, aggressive, and with poor prognosis. They may metastasize even at an early stage, however, there is currently no optimal diagnostic and therapeutic approach for metastatic FH-RCC.
Methods: We report the case of a 34-year-old male patient with renal tumor and liver metastasis, who underwent open tumor resection of the right kidney combined with resection of liver metastases. Postoperative pathology was confirmed, followed by targeted therapy.
Results: Postoperative pathological results confirmed FH-RCC, targeted therapy was performed after surgery. No tumor recurrence was observed during the follow-up of almost 16 months.
Conclusion: FH-RCC patients with liver metastasis can achieve a good prognosis through early resection of primary tumor and metastatic lesions combined with targeted therapy.
FH-RCC is a rare autosomal dominant genetic disease that occurs primarily in young and middle-aged people. The incidence of FH-RCC remains unclear and is mainly reported in individual cases. About 300 FH-RCC families have been reported in the literature (1). The improvement of diagnostic techniques has led to increased incidence rates. FH-RCC is more prone to recurrence and metastasis than other renal malignancies. The most common sites of metastasis are lymph nodes in the chest and abdomen, bone and liver and the prognosis is poor. We report a case of FH-RCC with liver metastasis, succeeding a good response with combined treatment, allowing an in-depth understanding of this rare tumor.
The reporting of this study conforms to CARE guidelines (2) and informed consent was obtained from the patient. A 34-year-old man was admitted to the hospital due to right low back pain for 5 days. The patient had no hematuria. There was no special medical history or clear family history of the tumor. Physical examination revealed a right upper abdominal bulge, a mass under the right costal margin, and percussion pain in the right renal region. Urinary system computed tomography (CT) showed: (1) Renal tumor was found in the right kidney, with a size of 11.9 × 16.5 cm. (2) Hypodense S3 and S4 liver lesions (Figures 1A,B). Magnetic resonance imaging: mixed signal shadows of T1WI and T2W1 were observed in the right renal parenchyma with a size of 10.4 × 16.5 cm, and nodular T2W1 signal shadows with a size of about 1.0 cm were observed in the left hepatic lobe (Figures 1C,D). Bilateral renal Emission Computed Tomography (ECT): left kidney GFR 60.9 mL/min, right kidney GFR 26.1 mL/min. Whole body bone imaging and chest CT showed no obvious abnormality.
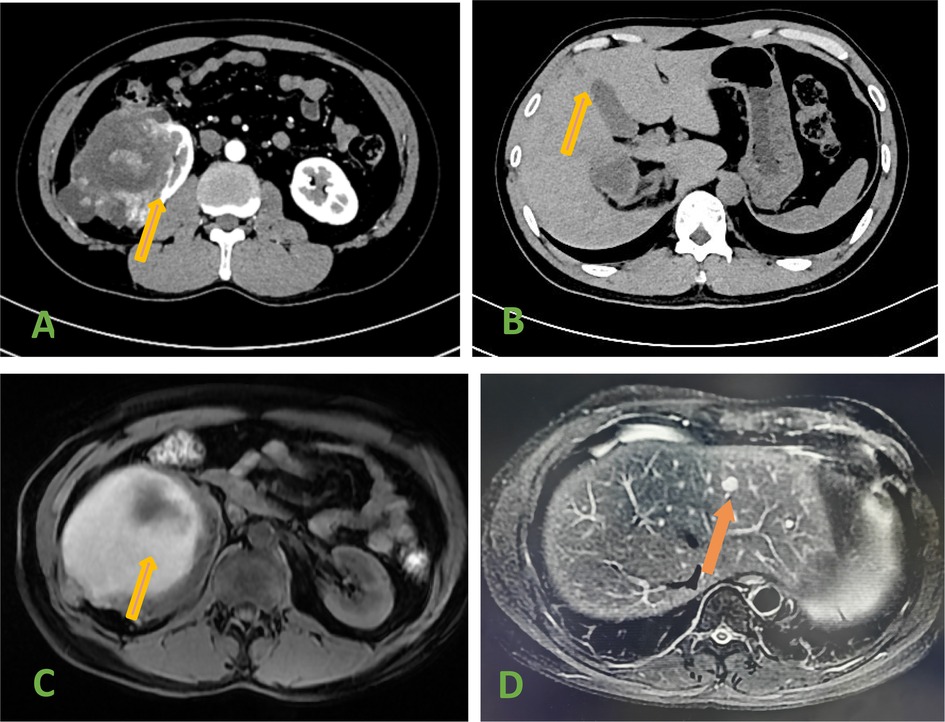
Figure 1. (A) Ct scan showed a tumor in the right kidney. (B) CT scan showed hepatic metastases. (C) MRI showed a tumor in the right kidney. (D) MRI Showed hepatic metastases (arrow).
Open tumor resection of the right kidney and hepatic metastasis was performed under general anesthesia. A subcostal incision was made, and the tumor was located in the upper pole of the right kidney, locally adherent to the surrounding tissue. The surface vessels were dilated. The right renal artery, vein, and ureter were ligated. The right kidney was separated and completely resected. Hepatobiliary surgeons assisted in the complete resection of liver metastases located in liver S3 and S4 segments. The blood loss was approximately 200 mL. After intensive postoperative care, the catheter was removed in 2 days and the abdominal drainage tube was removed in 3 days. The patient was discharged in 5 days.
Postoperative pathological results were the following: the surgically removed specimen was 16.0 cm × 11.0 cm in size, presented a cystic and solid pattern. The section showed a grayish-white color and contained a grayish-yellow fluid. Necrotic tissue was also present. The liver metastases were grayish white, with a size of 1.5 × 0.8 cm. Microscopically, right renal cell carcinoma, ISUP/WHO3 grade, and liver metastases were seen (Figures 2A,B). Immunohistochemical staining results showed: CK7 (−), CD10 (−), CAIX (−), Pax-8 (+), TFE3 (−), Vim (+), CD117 (−), Ki-67 (hot spot 20%), P504S (+), GATA-3 (−), HMB45 (−), Melan-A (+), SDHB (+), FH (−). Normal renal tissue, blood vessels, inflammatory cells, or other stromal cells were used as positive internal controls. If the internal control was positive, but FH staining was completely absent in the cytoplasm of the tumor cells, it was considered to be true FH-negative, and this kind of renal cell carcinoma was classified as FH-RCC. (FH-antibody, MAB-1014, Fuzhou Maixin biological Co. Ltd.). Right renal tumor showed FH-deficient renal cell carcinoma. Liver metastases also showed metastatic FH-deficient renal cell carcinoma (Figures 2C,D). 2-Succino cysteine (2SC) staining showed a strong positivity in the cytoplasm of neoplastic cells. Immunohistochemical staining of 2SC (Anti-2SC antibody, CRB2005017e/6773, Beijing Bole Biological Technology Co. Ltd.) was positive in both right renal tumor and liver metastases (Figures 2E,F). All antibody kits were purchased from Beijing Zhongshan Jinqiao Co.
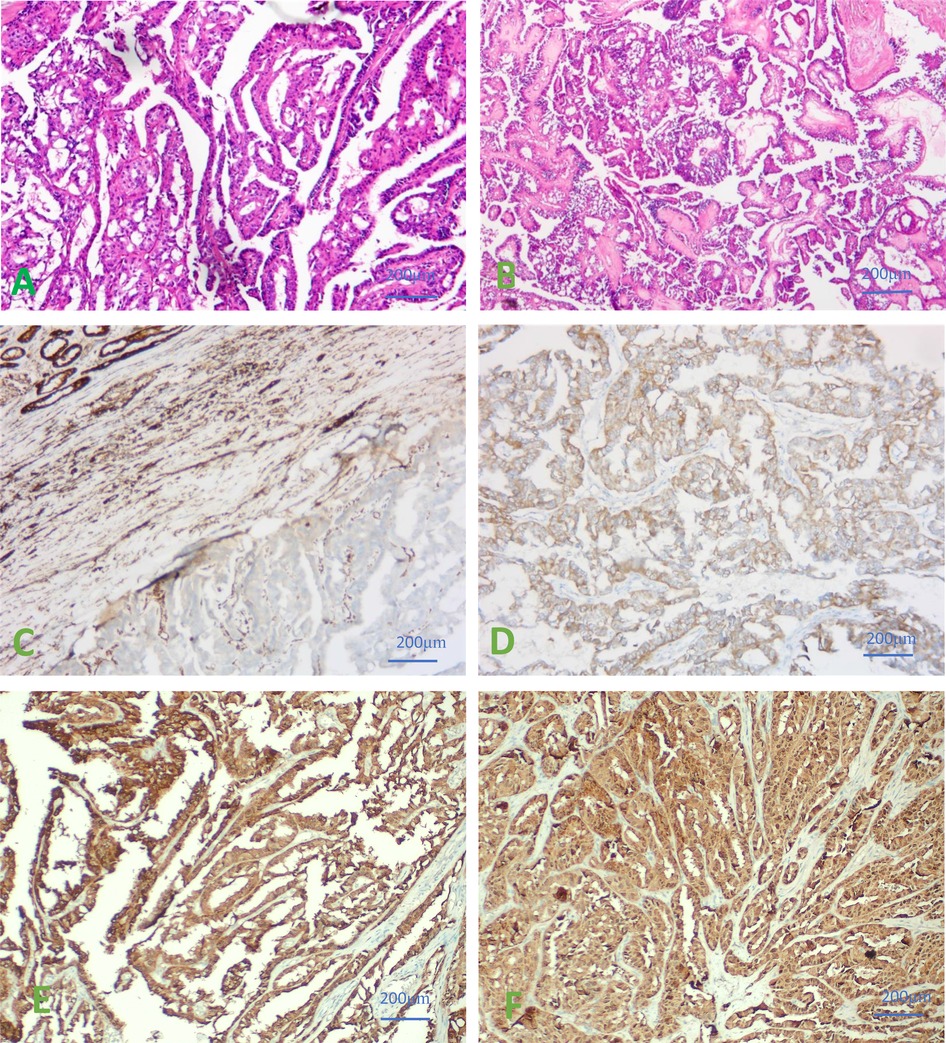
Figure 2. (A) Hematoxylin-Eosin (HE) staining of right renal cell carcinoma was seen microscopically. (B) HE staining of liver metastases were observed under microscope. (C) IHC results of negative FH staining in renal tumor cells. (D) IHC results of negative FH staining in liver metastases. (E) IHC staining of 2SC showed a strong positivity in the cytoplasm of neoplastic cells in renal tumor cells. (F) IHC staining of 2SC showed a strong positivity in the cytoplasm of neoplastic cells in Liver metastases. ×400.
Postoperative adjuvant treatment with bevacizumab and erlotinib (bevacizumab, 10 mg/kg, soluble in 0.9% NS 250 mL intravenous infusion, 1 time/14 days; erlotinib, 150 mg once a day, taken orally 2 h after meal), a total of 6 cycles. The patient had decreased appetite and leukopenia, and the symptoms were improved after symptomatic treatment. During regular reexamination of chest and abdominal CT, hematuria analysis, renal function, and follow-up for almost 16 months, no tumor recurrence was found.
FH-RCC is a rare subtype of renal cell carcinoma characterized by mutations and functional defects in the FH gene. The clinical manifestations are highly invasive biological behaviors, with early local progression and distant metastasis prone to occur. Abdominal or thoracic lymph nodes and bone and liver are the most common sites of metastasis (3), and are connected to poor prognosis. Large eosinophilic nucleoli and peripheral halos are major histological features in the tumoral tissue (4). The complete absence of FH immunohistochemical staining in the cytoplasm of the cancer cells is helpful for the diagnosis of FH-RCC. 2SC is produced by protein threonylation in patients with FH-RCC (5). In cancerous tissues, the results of IHC staining for FH and 2SC are clinically valuable in the diagnosis of FH-RCC (6, 7), as the absence of FH and high expression of 2SC in the cytoplasm of neoplastic cells support the diagnosis of FH-RCC (8). However, the diagnosis of FH-RCC further requires molecular testing and systematic investigation of the family history. In this case, immunohistochemical results of both primary and metastatic sites supported FH-RCC. We also advised the patient to inform and ensure the screening of relatives during disease management.
The clinical management of FH-RCC is challenging. In the past, most cases were treated with primary tumor resection and lack of comprehensive treatment strategy. We believe that performing a surgical intervention on time is of great prognostic significance, and wide resection margin surgery and retroperitoneal lymph node dissection are recommended (9). Data on systemic therapy of FH-RCC are mostly limited to case reports. The analysis of a Phase II clinical trial evaluating the efficacy and safety of bevacizumab in combination with erlotinib in the treatment of FH-RCC (NCT01130519) showed an objective response rate of 65% and a median progression-free survival of 24.2 months. Based on the results of this trial, in 2018, the National Comprehensive Cancer Network (NCCN) issued guidelines recommending bevacizumab in combination with erlotinib for the treatment of patients with recurrent or advanced FH-RCC (10). Compared with papillary RCC, FH-RCC shows higher incidence rates in younger patients, a higher proportion of radical nephrectomy, and the likelihood of receiving systemic therapy (11). We recommend considering the immunotherapy integration to provide a more comprehensive treatment regimen. Recent studies (12) have confirmed genomic and transcriptomic characteristics of FH-RCC metastases, revealing its early evolutionary trajectory and bringing new hope for treatment. In this case, the patient had developed liver metastases at the time of diagnosis. Nephrectomy and liver metastasis resection were performed, and a combination of bevacizumab and erlotinib was followed. No tumor recurrence was found during a 16-month follow-up with regular review of chest and abdominal CT.
In general, the prognosis of FH-RCC is poor. Recently, remarkable advances have been achieved in surgery, targeted therapy, and immunotherapy. However, concerning the pathogenesis of FH-RCC, it is urgent to explore new therapeutic agents, that may extend patients’ life expectancy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee/Institutional Review Committee of the First Affiliated Hospital of Gannan Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HC: Writing – original draft, Writing – review & editing. QZ: Resources, Writing – review & editing. FL: Investigation, Writing – review & editing. XZ: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. De Coninck V, Keller EX, Traxer O. Complications of ureteroscopy: a complete overview. World J Urol. (2020) 38(9):2147–66. doi: 10.1007/s00345-019-03012-1
2. Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan ASCARE Group. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int J Surg. (2020) 84:226–30. doi: 10.1016/j.ijsu.2020.10.034
3. Tepeler A, Resorlu B, Armagan A, Unsal A. Categorization of intraoperative ureteroscopy complications using modified Satava classification system. World J Urol. (2014) 32(1):131–6. doi: 10.1007/s00345-013-1054-y
4. Bai Y, Wei H, Ji A, Zhang Q, Wang S, Peng Y, et al. Reconstruction of full-length ureter defects by laparoscopic bladder flap forming. Sci Rep. (2021) 11(1):3970. doi: 10.1038/s41598-021-83518-0
5. Bardella C, El-Bahrawy M, Tomlinson IP, Pollard PJ. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol. (2011) 225(1):4–11. doi: 10.1002/path.2932
6. Rare Kidney Cancer Collaborative Group, Genitourinary Cancer Committee, China Anti-Cancer Association. Consensus on clinical diagnosis and treatment of fumarate hydratase-deficient renal cell carcinoma. Zhonghua Wai Ke Za Zhi. (2022) 60(11):961–8. Chinese. doi: 10.3760/cma.j.cn112139-20220729-00328
7. Yu YF, He SM, Wu YC, Li YY, Yang F, He Q, et al. Clinicopathological features and prognosis of fumarate hydratase deficient renal cell carcinoma. Beijing Da Xue Xue Bao Yi Xue Ban. (2021) 53(4):640–6. Chinese. doi: 10.19723/j.issn.1671-167X.2021.04.0038
8. Gupta S, Swanson AA, Chen YB, Jimenez RE. Incidence of succinate dehydrogenase and fumarate hydratase-deficient renal cell carcinoma based on immunohistochemical screening with SDHA/SDHB and FH/2SC. Hum Pathol. (2019) 91:114–22. doi: 10.1016/j.humpath.2019.07.004
9. Menko FH, Maher ER, Schmidt LS, Richard S, Linehan WM. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. (2014) 13(4):637–44. doi: 10.1007/s10689-014-9735-2
10. Motzer RJ, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, et al. Kidney cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20(1):71–90. doi: 10.6004/jnccn.2022.0001
11. Bai J, Li X, Chen J. The clinicopathologic and molecular features, and treatment outcome of fumarate hydratase-deficient renal cell carcinoma: a retrospective comparison with type 2 papillary renal cell carcinoma. Aging (Albany NY). (2024) 16(4):3631–46. doi: 10.18632/aging.205549
Keywords: FH-RCC, liver metastasis, surgery, targeted therapy, good prognosis
Citation: Chen H, Zeng Q, Liu F and Zou X (2024) Fumarate hydratase-deficient renal cell carcinoma complicated with liver metastasis: case report. Front. Surg. 11:1430344. doi: 10.3389/fsurg.2024.1430344
Received: 9 May 2024; Accepted: 21 October 2024;
Published: 1 November 2024.
Edited by:
Yewei Zhang, Nanjing Medical University, ChinaReviewed by:
Yanlong Shi, Nanjing Medical University, ChinaCopyright: © 2024 Chen, Zeng, Liu and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanmin Chen, MTM1NzY3MzcyNDBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.