- 1Department of Infection Control, People's Hospital of Dayi County, Chengdu, Sichuan Province, China
- 2Department of Urology, People's Hospital of Dayi County, Chengdu, Sichuan Province, China
- 3Department of Infection Control, Chengdu Fifth People’s Hospital, Chengdu, Sichuan Province, China
Objective: To evaluate the risk factors for postoperative incision infection in colorectal cancer, this meta-analysis aimed to identify key variables impacting infection incidence following colorectal cancer surgery.
Methods: Utilizing a meta-analytical approach, studies published from January 2015 to December 2022 were systematically collected and analyzed through the assessment of factors like body mass index, diabetes, albumin levels, malnutrition, and surgical duration.
Results: The meta-analysis of eleven high-quality studies revealed that elevated BMI, diabetes, low albumin levels, malnutrition, and extended surgical duration were associated with increased infection risk, while laparoscopic procedures showed potential for risk reduction.
Conclusions: This study underscores the significance of preoperative risk assessment and management in mitigating postoperative incision infections in colorectal cancer patients. The findings present actionable insights for clinicians to enhance patient prognoses and overall quality of life
Introduction
Colorectal cancer ranks among the malignancies with the highest incidence and mortality rates globally, particularly in developed countries (1, 2). As a significant type of gastrointestinal malignancy, colorectal cancer spreads through lymphatic and blood circulation, imposing significant physical and psychological burdens on patients (3–7). According to recent cancer statistics, colorectal cancer ranks third in incidence and fifth in mortality among all cancers in China, with an annual report of 376,000 new cases and 191,000 deaths (8–10). These figures highlight the substantial impact of colorectal cancer on individuals and society and underscore the importance of timely and effective diagnosis and treatment (11–13).
Clinically, surgical resection remains the mainstream treatment for colorectal cancer, with curative surgery being the standard treatment strategy (14–16). Although such surgery can control disease progression to some extent, numerous studies have identified postoperative incision infection as a standard and severe complication, which, in severe cases, may lead to sepsis, systemic infection, and death (17–19). Advances in medical technology have made laparoscopic surgery the preferred technique for treating colorectal cancer due to its minimally invasive nature, fewer complications, and faster postoperative recovery (20–24). However, the risk of postoperative incision infection persists, affecting patient recovery speed, increasing treatment costs, and exacerbating the burden on healthcare resources (25–27).
Patients undergoing surgery for colorectal cancer face a heightened risk of postoperative incision infection due to the necessity for prolonged fasting and bowel preparation preoperatively and the potential for contamination of the surgical area by intestinal contents during surgery (28–30). Furthermore, the invasion of pathogens directly causes these infections (31). In China, the incidence of surgical site infections reaches as high as 1.01% (32–36), with rates in specific regions and populations potentially soaring to 20%, especially in resource-constrained developing countries (14, 37–39).
Although extensive research has been conducted on the risk factors for postoperative incision infection in colorectal cancer, findings remain varied without a unified conclusion (40–42). Factors such as age, medical history, and surgical duration are considered potential influencers of infection risk, yet their relative importance and interactions still need to be fully clarified (43–45). Despite attempts to explore preventative measures, the effectiveness of prevention and treatment strategies requires further research due to issues like insufficient sample sizes and study design biases (46–48).
This study aims to provide a more precise risk assessment and effective prevention strategies for clinical application, thereby improving patient recovery quality and reducing associated medical costs through systematic analysis and meta-analysis of various risk factors for postoperative incision infection in colorectal cancer. Beyond focusing on the direct risk factors, this research also examines how optimizing preoperative preparation, improving surgical techniques, and enhancing postoperative management can effectively reduce the incidence of incision infection. The findings guide clinicians in devising individualized treatment plans, enhancing surgical safety, and improving patient quality of life. Additionally, reducing postoperative incision infections can significantly lower medical costs, alleviate the economic burden on patients and their families, and improve healthcare service quality and patient satisfaction. In summary, this study aims to create a safer and more effective surgical treatment environment for colorectal cancer patients through in-depth analysis and comprehensive evaluation.
Materials and methods
Literature source and retrieval
This study searched for risk factors associated with postoperative incision infection in colorectal cancer patients across Chinese and English databases, including VIP, Wanfang, CNKI, PubMed, EMBASE, and DSR. The search strategy involved selecting relevant literature based on inclusion and exclusion criteria. The Chinese search formula included combinations of terms for “colorectal cancer”, “rectal cancer”, or “colon cancer”, with “surgical site infection”, “incision infection”, and “risk factors”, or “influencing factors” or “related factors”. The English search strategy used “colorectal neoplasms” and “surgical wound infection” combined with “risk factors”, “influence factors”, or “dangerous factors”. Searches were tailored by combining phrases freely and, when necessary, seeking related literature—the search period spanned from January 2015 to December 2022.
Inclusion and exclusion criteria for literature
Inclusion criteria for the literature were: (1) Studies addressing risk factors or influencing factors for postoperative incision infection in patients with colorectal cancer. (2) Clinical studies in the form of case-control or cohort studies. (3) Studies involving at least 30 patients. (4) Studies involving patients aged between 18 and 80.
Exclusion criteria for the literature were: (1) Publications without clinical trials, such as reviews and case analyses. (2) Duplicated publications. (3) Clinical trial articles or documents with incomplete data. (4) Unpublished documents. (5) Studies with too small sample sizes in clinical trials. (6) Studies involving patients who were too young or too old.
Literature screening and data extraction
Preliminary Screening: A primary search was conducted using combinations of keywords on major literature platforms. Eligible publications were collected and organized using Excel for categorization and sorting, removing duplicates. Initial reviews of collected titles and abstracts were performed to eliminate documents with significant differences. A thorough reading of selected literature was conducted according to acceptance and organization standards to exclude documents that did not meet research criteria, documenting the number of publications and reasons for exclusion.
Secondary Screening: Conducted independently by two researchers based on the literature's inclusion and exclusion criteria to further select and extract collected documents, documenting the number and reasons for excluded literature.
Tertiary Screening: In cases of disagreement on inclusion between the researchers above, another researcher independently reviewed and resolved discrepancies in literature selection.
Literature quality assessment
This study conducted a literature quality assessment based on the Cochrane risk of bias tool to ensure the reliability of the research findings. Potential biases in each study, such as random sequence generation, allocation concealment, blinding, and outcome assessment, were classified into low, high, or unclear risk categories. Studies were categorized as having a high risk of selection bias if there were significant deficiencies in randomization or allocation concealment, as an unclear risk if there was insufficient information to assess the risk of bias, and as low risk if randomization and allocation concealment were appropriately conducted and blinding was adequately implemented.
Furthermore, two researchers assessed the quality of case-control or cohort studies using the Newcastle-Ottawa scale (NOS), which includes four items (4 points) for the selection of study participants, one item (2 points) for the comparability of groups, and three items (3 points) for the outcome measurement, with a total score above 9 considered high quality. The quality of cross-sectional studies was evaluated using the assessment criteria recommended by the Agency for Healthcare Research and Quality (AHRQ), comprising eleven standards such as data sources, inclusion criteria, observation period, continuity of subjects, subjective factors of assessors, and quality control. Studies scoring between 0 and 3 were considered low quality (Grade C), and those scoring between 4 and 7 were considered medium quality (Grade B).
Statistical analysis
Meta-analysis was performed using R software, selecting r values and their 95% confidence intervals (CI) as the effect size indicators. The chi-squared (X2) test was used to process control trial data from all selected literature, and heterogeneity of the collected experimental data was evaluated using the I2 statistic. If P < 0.01 and I2 > 50%, it indicates no difference in the data across the selected literature, allowing for a fixed-effect model to combine and analyze control trial data. If P > 0.01 and I2 < 50%, an investigation into the sources of data heterogeneity is required, followed by relevant subgroup interventions. If the value remains large, data correlation analysis is conducted using a random-effects model, with odds ratios (OR) used for effect statistics and 95% CI for interval estimation, excluding clinical studies cited no fewer than five times in the literature.
Additionally, sensitivity analysis was employed to assess the stability of the research outcomes, with funnel plots drawn to evaluate the potential for publication bias. The significance level for all statistical tests was set at P > 0.05.
Results
Overview of literature retrieval results based on the systematic screening process
In systematic reviews or meta-analyses, the retrieval and screening of literature are fundamental steps to ensure the quality and comprehensiveness of the research. This study employed a multi-database search strategy and stringent inclusion and exclusion criteria to conduct a comprehensive search and screening of relevant literature.
Initially, the search yielded 1,578 articles, including 468 from VIP, 301 from Wanfang, 618 from CNKI, 107 from PubMed, 51 from EMBASE, and 33 from DSR databases. After removing 1,263 duplicates, 315 articles remained for preliminary screening. Through careful reading of titles and abstracts, and based on the objectives and predefined conditions of the study, this number was further reduced to 115 articles. After a detailed full-text review, 11 articles met the study's inclusion and exclusion criteria (Figure 1A).
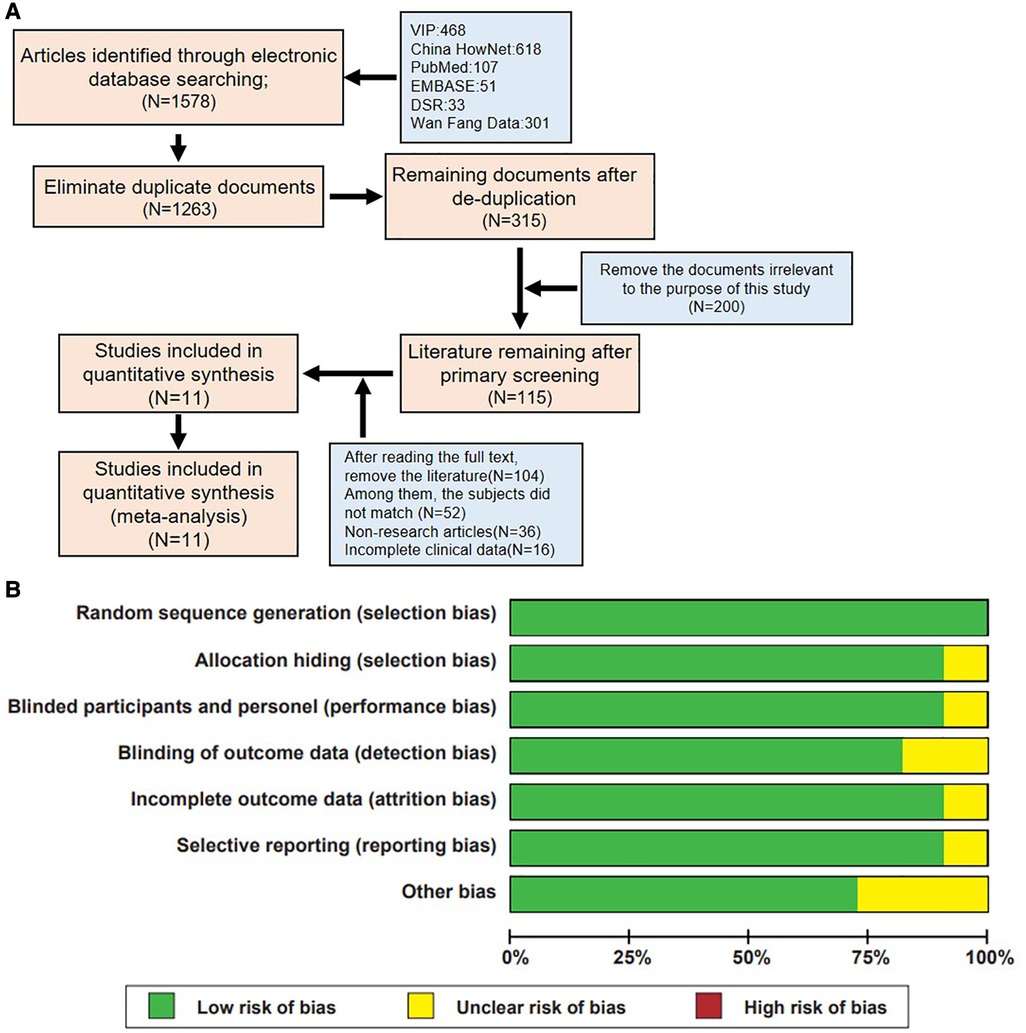
Figure 1. Literature screening process and quality assessment results of included studies. (A) Flowchart of literature inclusion. (B) Summary of bias risk assessment for included studies.
Through a rigorous literature search and screening process, this study successfully identified 11 high-quality studies from a large pool of related literature, providing a solid foundation for subsequent analysis and review. Furthermore, the quality of the included literature was evaluated based on the Cochrane risk of bias tool standards (Figure 1B), ensuring a fair and comprehensive quality review of the included studies.
Summary of high-quality literature based on NOS scores
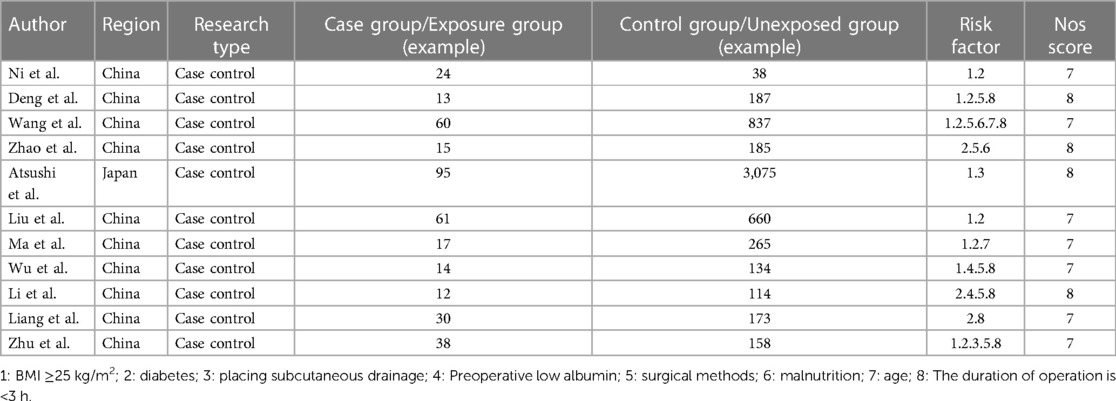
In various fields of scientific research, assessing the quality of literature and interpreting data are crucial. This study conducted an in-depth literature analysis within a specific domain, employing the NOS for literature quality assessment. All included studies scored ≥7 on the NOS, indicating they are of high quality, as detailed in Table 1 (8, 33, 37, 49).
Analysis of significant risk factors for postoperative incision infection in colorectal cancer
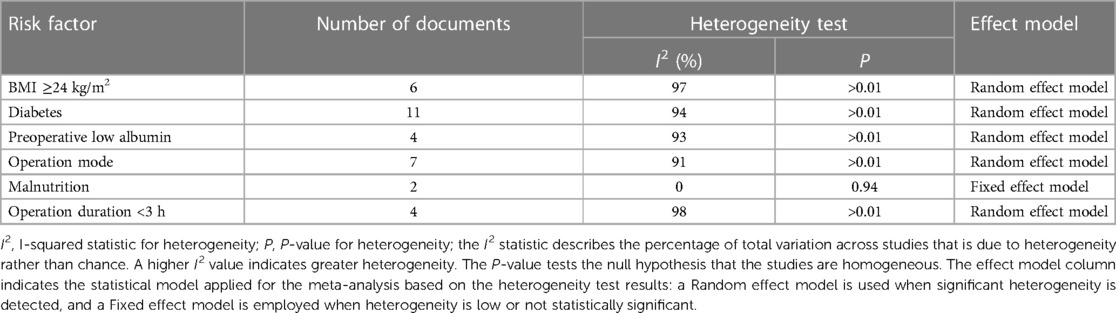
In meta-analysis research, testing for heterogeneity among risk factors is critical in evaluating differences across studies. This process aids in identifying the most suitable effect model to ensure the accuracy and reliability of the analysis results. Our study comprehensively examined six potential risk factors, conducting a detailed assessment of their heterogeneity (Table 2).
The analysis identified high body mass index (BMI), diabetes, preoperative low albumin levels, preoperative malnutrition, and surgical duration exceeding 3 h as significant risk factors for postoperative incision infection in colorectal cancer. Conversely, laparoscopic surgery emerged as a factor associated with a reduced risk of infection (Figure 2). Understanding these factors is crucial for the prevention and management of postoperative incision infection in colorectal cancer.
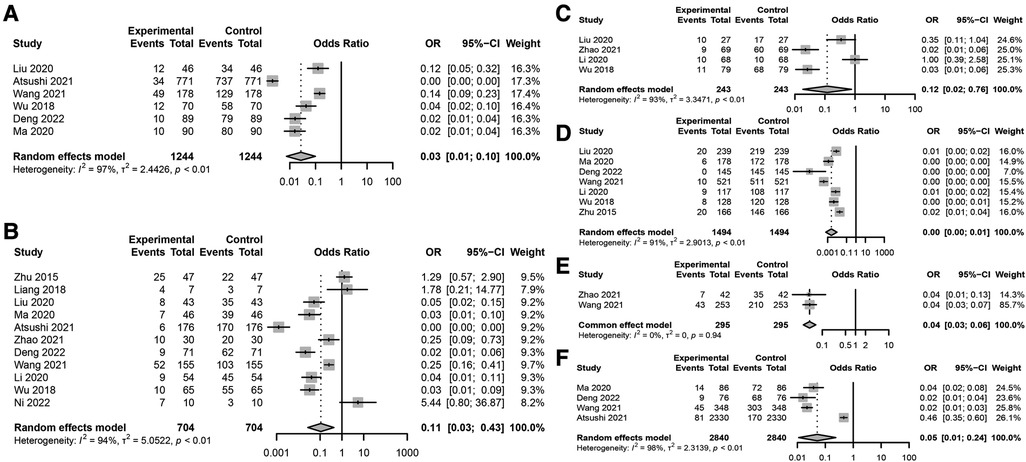
Figure 2. Key risk factors for postoperative incision infection in colorectal cancer. (A) Association between BMI ≥24 kg/m2 and postoperative incision infection in colorectal cancer. (B) Forest plot analyzing the risk of postoperative incision infection in colorectal cancer associated with diabetes. (C) Forest plot analyzing the impact of preoperative low albumin levels on the risk of postoperative incision infection in colorectal cancer. (D) Association between surgical methods and the risk of postoperative incision infection in colorectal cancer. (E) Association between preoperative malnutrition and the risk of postoperative incision infection in colorectal cancer. (F) Association between surgical duration exceeding 3 h and the risk of postoperative incision infection in colorectal cancer.
Specific meta-analysis findings include: a BMI ≥24 kg/m2 significantly increases the risk of postoperative incision infection (Figure 2A), with a combined OR of 0.03 and a 95% CI of [0.01; 0.10], indicating a significant association. However, this result showed high heterogeneity (I2 = 97%), suggesting substantial differences between included studies. Diabetes was also a significant risk factor (Figure 2B), despite high heterogeneity (I2 = 94%), with a combined OR of 0.11 and a 95% CI of [0.03; 0.43], indicating an increased risk of infection post-surgery for patients with diabetes. Preoperative low albumin levels were significantly associated with postoperative incision infection (Figure 2C), with a combined OR of 0.12 and a 95% CI of [0.02; 0.76], despite high study heterogeneity (I2 = 93%). Laparoscopic surgery appeared to be associated with a lower risk of infection (Figure 2D), with a combined OR of 0.00 [95% CI: 0.00; 0.01], even though its heterogeneity was high (I2 = 91%). Preoperative malnutrition was significantly linked to an increased risk of incision infection (Figure 2E), with a combined OR of 0.04 and a 95% CI of [0.03; 0.06], and heterogeneity testing (I2 = 0%) indicated no significant differences between studies. Surgical duration exceeding 3 h was significantly associated with an increased risk of postoperative incision infection in colorectal cancer (Figure 2F), with a combined OR of 0.05 and a 95% CI of [0.01; 0.24], and heterogeneity testing showed very high differences between studies (I2 = 98%).
Through meta-analysis, this study revealed associations between postoperative incision infection in colorectal cancer and multiple significant risk factors. Factors such as BMI ≥24 kg/m2, diabetes, preoperative low albumin levels, preoperative malnutrition, and surgical duration exceeding 3 h all demonstrated a significant increase in risk despite high heterogeneity. Meanwhile, the protective role of laparoscopic surgery warrants attention, though its heterogeneity calls for further investigation. These findings emphasize the importance of comprehensive patient assessment and management in clinical practice, particularly identifying and intervening in these risk factors preoperatively to reduce the risk of post-surgery infection.
Sensitivity analysis of risk factors for postoperative incision infection in colorectal cancer shows high stability
A sensitivity analysis of multiple risk factors for postoperative incision infections in colorectal cancer patients revealed significant impacts on the risk of incision infections by BMI ≥24 kg/m2, diabetes, low albumin levels, laparoscopic surgery, and preoperative malnutrition (Figure 3). The sensitivity analysis results of BMI ≥24 kg/m2 on the risk of postoperative incision infections remained significantly correlated even after excluding any individual study, with no substantial impact on the overall conclusion from the changes in the combined effect sizes (OR = 0.03, 95% CI: 0.01–0.10), indicating the stability of BMI ≥24 kg/m2 as a risk factor (Figure 3A). The sensitivity analysis of diabetes on the risk of postoperative incision infections also exhibited significant association even after excluding any single study, with the combined effect size (OR = 0.11, 95% CI: 0.03–0.43) confirming the robustness of diabetes as a risk factor (Figure 3B). Analysis of the sensitivity of preoperative low albumin levels on the risk of postoperative incision infections indicated a significant increase in risk, with high consistency across the study results reflected by the combined effect size (OR = 0.12, 95% CI: 0.02–0.76) (Figure 3C). The sensitivity analysis of laparoscopic surgery on the risk of postoperative incision infections revealed a significant risk reduction with this surgical approach, as evidenced by the unchanged combined effect size (OR = 0.00, 95% CI: 0.00–0.01) even after exclusion of any individual study, demonstrating the robustness of the conclusion (Figure 3D). Sensitivity analysis of preoperative malnutrition and operations exceeding 3 h on the risk of postoperative incision infections confirmed their significant increase in risk, with no substantial impact on the overall conclusion from the changes in the combined effect size and 95% confidence intervals after excluding any individual study, verifying their consistency and stability as important risk factors (Figures 3E,F). Through these sensitivity analyses, we affirmed the significant impact of BMI ≥24 kg/m2, diabetes, preoperative low albumin levels, preoperative malnutrition, operations exceeding 3 h, and laparoscopic surgery on the risk of postoperative incision infections in colorectal cancer patients. These findings underscore the importance of comprehensively assessing and managing these risk factors in clinical practice to ensure that no single study decisively influences the overall conclusion, even in the presence of high heterogeneity, where the changes in combined effect size and 95% confidence intervals remain insufficient to significantly affect the overall conclusion.
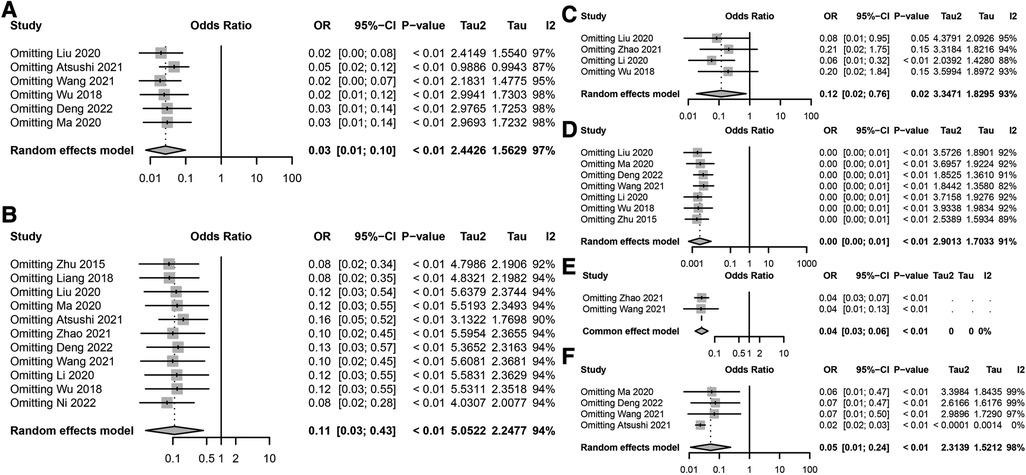
Figure 3. Sensitivity analysis of risk factors for postoperative incision infection in colorectal cancer. (A) Sensitivity analysis results for the association between BMI ≥24 kg/m2 and the risk of postoperative incision infection in colorectal cancer. (B) Sensitivity analysis results for the association between diabetes and the risk of postoperative incision infection in colorectal cancer. (C) Sensitivity analysis results for the association between preoperative low albumin levels and the risk of postoperative incision infection in colorectal cancer. (D) Sensitivity analysis results for the association between surgical methods and the risk of postoperative incision infection in colorectal cancer. (E) Sensitivity analysis results for the association between preoperative nutritional status and the risk of postoperative incision infection in colorectal cancer. (F) Sensitivity analysis results for the association between surgical duration exceeding 3 h and the risk of postoperative incision infection in colorectal cancer.
Assessment of publication bias strengthens the credibility of research on risk factors for postoperative infection in colorectal cancer
In a series of meta-analyses on risk factors for postoperative infection in colorectal cancer, funnel plots were utilized to assess publication bias (Figure 4). The analysis of these funnel plots revealed an excellent symmetry between the effect sizes and their standard errors for most studies, indicating a low risk of publication bias. While some studies deviated from the expected symmetric distribution, potentially reflecting heterogeneity among studies or the impact of specific study conditions, no evident one-sided skew or gaps were observed. This further supports the robustness and credibility of the meta-analysis results.
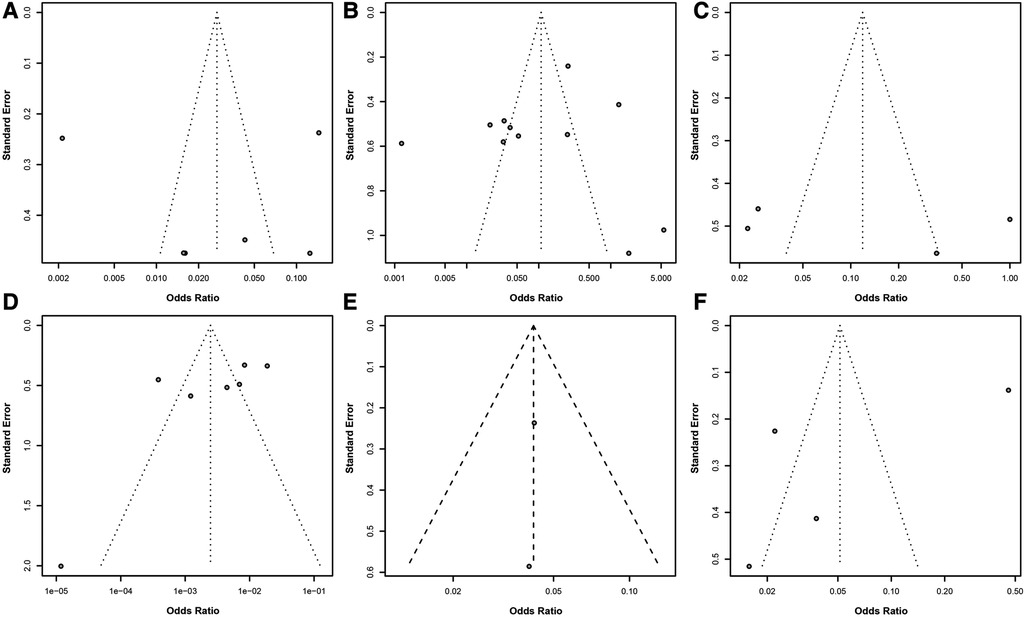
Figure 4. Assessment of publication bias in meta-analysis of risk factors for postoperative infection in colorectal cancer. (A) Funnel plot for BMI ≥24 kg/m2 as a risk factor. (B) Funnel plot for diabetes as a risk factor. (C) Funnel plot for preoperative low albumin levels as a risk factor. (D) Funnel plot for surgical methods as a risk factor. (E) Funnel plot for preoperative nutritional status as a risk factor. (F) Funnel plot for surgical duration exceeding 3 h as a risk factor. Each point in the funnel plot represents an estimate of the effect size and its precision for a study.
In summary, despite some heterogeneity among studies, the overall evidence suggests that the analysis linking these risk factors to the risk of postoperative infection in colorectal cancer is robust and highly credible. This provides critical guidance for clinicians in preoperative assessment and risk management, contributing to improved prevention and management of postoperative infections.
Discussion
In recent years, changes in dietary patterns and lifestyle habits have led to colorectal cancer becoming a common malignancy within the gastrointestinal tract, with its incidence rate gradually increasing. Annually, approximately 1.2 million new cases and 600,000 deaths are attributed to this disease (50–52). Moreover, the age of onset has been trending younger (53). Colorectal cancer ranks fourth in incidence and second in mortality among all types of cancer worldwide, posing a serious threat to patient's health and safety (54–56). Currently, the preferred treatment for colorectal cancer patients is radical surgery to remove the lesion (57–59). Laparoscopic surgery, which allows for the visualization of surrounding tissues, nerves, blood vessels, and ureters, helps minimize damage to surrounding tissues and has shown significant clinical outcomes (60). However, patients with colorectal cancer are susceptible to the adverse effects of their condition, leading to poor physical health and reducing their capacity to undergo surgery (5, 38, 61). The high bacterial content and complex microbiota within the human colorectal cavity also increase the risk of postoperative incision infection (62, 63). During surgery, the spillage of intestinal contents can lead to the displacement and colonization of intestinal pathogens, resulting in a high rate of postoperative incision infections. Incision infections are common complications in clinical surgery and, in severe cases, can lead to systemic infections and sepsis, severely impacting postoperative recovery (64–66). Literature reports the rate of incision infections following colorectal surgery ranging from 2.7% to 26.0% (67–69). Colorectal cancer, being a debilitating disease, leads to a decline in patients' immune function, making them more prone to postoperative incision infections (70).
This study, through a systematic literature review and meta-analysis, delved into several potential risk factors for postoperative incision infection in colorectal cancer, including a BMI of ≥24 kg/m2, diabetes, preoperative low albumin levels, the method of laparoscopic surgery, preoperative malnutrition, and surgical duration exceeding 3 h. It identified that a BMI of ≥24 kg/m2, preoperative low albumin levels, preoperative malnutrition, and extended surgical duration are significant risk factors for postoperative incision infection, with diabetes also being a crucial risk factor. In contrast, laparoscopic surgery methods appear to be associated with a lower risk of infection (Figure 5). Our findings reveal a significant correlation between these factors and the risk of postoperative incision infection in colorectal cancer, offering essential insights for clinicians in preoperative assessment and postoperative management.
Colorectal cancer patients with a BMI greater than 24 kg/m2 are considered overweight. Overweight individuals have thick subcutaneous fat, which affects surgical visibility and is detrimental to surgical procedures. This can lead to increased surgical complexity, prolonged operation times, and subsequently raise the risk of postoperative wound infections. High fat content in overweight patients can inhibit the proliferation of immune cells, further increasing the risk of wound infections. Patients with a higher postoperative weight are more prone to abdominal fat liquefaction. The increased weight in patients is associated with hypertrophy of fat tissue and inadequate blood supply. Additionally, these patients commonly have chronic conditions such as hypertension and diabetes, which weaken their immune function and raise the probability of postoperative wound infections. The length of the incision is closely linked to the occurrence of postoperative infections. Overweight individuals have an increased likelihood of developing diabetes, altering their immune cell function and inflammatory response, thus making them more susceptible to surgical wound infections. Therefore, stringent perioperative infection prevention measures should be taken for patients with high BMI, and appropriate plans should be implemented before surgery to prevent postoperative infections. Previous studies have shown that a higher BMI in colorectal cancer patients increases the risk of surgical site infections. Hirao et al. found that when BMI is equal to or greater than 25 kg/m2, the incidence of incision infections significantly rises (OR = 2.28, 95% CI: 1.05–7.52). This finding is consistent with the results of this study. Research by Chen Yan and colleagues demonstrates that overweight patients experience compromised surgical visibility and operation due to the influence of subcutaneous fat. To achieve better visibility during surgery, incisions may need to be extended, increasing exposure and access. Postoperatively, incisions are more susceptible to liquefaction and necrosis, resulting in slower healing and an increased rate of surgical wound infections.
As societal lifestyles change and populations age, the incidence of diabetes is on the rise, leading to an increase in colorectal cancer patients with diabetes. These patients are more susceptible to postoperative incision infections due to immune system dysregulation and suppressed immune functions (40, 71, 72). Studies have shown that diabetes disrupts glucose metabolism, reduces glycolytic capacity, and weakens neutrophil migration, phagocytosis, and bactericidal functions (73). Protein synthesis decreases while degradation accelerates, reducing immunoglobulins' production, complements, and chemotactic factors, thereby diminishing immune function (74–76). The immune response in diabetic patients is relatively lower, and surgical trauma exacerbates glucose metabolism disorder. In a hyperglycemic environment, inflammation cell migration to the surgical site is hindered, further lowering the body's immunity and increasing infection risks, consistent with findings by Wukich et al. (77). The rate of incision infection in diabetic patients is significantly higher than in non-diabetic patients. The abnormal glucose metabolism in patients with diabetes impairs the normal function of inflammatory factors, facilitating pathogen colonization and growth in a high-glucose microenvironment, thus diminishing the patient's infection resistance.
Diabetic patients have microcirculation disorders, leading to a higher risk of anastomotic leakage post-surgery and potential abdominal infections. Diabetes-induced vascular plaque formation causes the narrowing of blood vessels, reducing tissue oxygenation, which can lead to tissue hypoxia, affecting oxidative-mediated microbial killing mechanisms and tissue oxygenation, and delaying tissue healing. Postoperative malnutrition is more likely in diabetic patients, adversely affecting recovery. Furthermore, wound healing in diabetic patients is slower. In healthy individuals, the metabolic level of glucose in diabetic patients is lower than usual, resulting in lower protein synthesis capacity and poorer cellular tissue repair abilities. Severe patients have impaired inflammatory cell function, affecting leukocyte phagocytosis. Immune function is below average, with fewer fibroblasts, hindering granulation tissue formation at the wound site, delaying wound healing, and even causing local edema. Surgical trauma can lead to postoperative stress-induced hyperglycemia, conducive to bacterial growth; a high glucose environment in the blood promotes bacterial colonization. Numerous studies have confirmed the impact of diabetes and perioperative hyperglycemia on surgical site infection. Hyperglycemia provides conditions for bacterial growth, and exudate in a high-glucose environment facilitates bacterial growth, reducing the body's immunity and leading to postoperative incision infections. Immune response functions are relatively lower in colorectal cancer patients with a history of diabetes. Post-laparoscopic surgery, surgical trauma further disrupts glucose metabolism, promoting inflammatory cell migration to the incision site, weakening immunity, and increasing the risk of postoperative incision infections. Persistent hyperglycemia in diabetic patients fosters bacterial growth, thereby increasing the rate of surgical site infections. Glucose metabolism disorder leads to a decreased pathogen clearance capacity, impaired immune function, and reduced infection resistance. Therefore, for colorectal cancer patients with diabetes, perioperative blood glucose management should be strengthened, aiming to keep blood glucose levels between 5.6–11.2 mmol/L, minimizing glucose fluctuations and thereby reducing the incidence of postoperative abdominal infections following colorectal cancer resection surgery.
Albumin levels directly reflect the nutritional status of the body (78–80). Low albumin levels indicate a higher risk of malnutrition, compromising immune function and increasing incision infection risk (81–83). Albumin, a significant component of human plasma proteins, is crucial in maintaining internal homeostasis (84). Low albumin levels reduce a patient's immunity, leading to drug absorption and metabolic disorders and complicating wound healing (85, 86). Therefore, clinical nutritional support should be intensified for such patients to boost their resistance, emphasizing the importance of preoperative nutritional interventions to enhance patient resilience (87, 88).
The most common complication following abdominal surgery is surgical site infections (SSIs), leading to increased postoperative pain, suffering, and economic burden, as patients require prolonged hospital stays, face readmissions, sepsis, and possibly death. This complication is associated with adverse economic consequences, increased morbidity, extended postoperative hospitalization, readmissions, sepsis, and mortality (89). Additionally, sepsis is a severe postoperative complication that can occur following colorectal surgery. It is typically associated with bacterial infections at the surgical site, which can lead to systemic inflammatory response syndrome (SIRS) and potentially progress to severe sepsis or septic shock if not promptly managed. Patients undergoing colorectal surgery are particularly vulnerable to sepsis due to the high bacterial load in the colon and potential intraoperative contamination. Early recognition and prompt management of sepsis are crucial for improving patient outcomes (89).
Surgical methods include traditional open surgery and laparoscopic surgery (90). Studies have shown that traditional open surgery, with its extensive trauma and significant blood loss, complicates postoperative recovery (91–93). Laparoscopic surgery, a significant advancement in modern science, has emerged as a new option for curative resection of colorectal cancer (67, 94, 95). It allows for precise observation of the surrounding tissue of the lesion, thus minimizing damage (96).
Open surgery requires an extended incision to ensure an excellent surgical field of view (97, 98). The larger incision, exposed to air for an extended period during surgery, significantly increases the risk of infection and may impact wound healing (99). Laparoscopic surgery facilitates precise observation of the lesion's surrounding tissues, nerves, blood vessels, and ureters, minimizing damage (100). With the advancement of laparoscopic techniques, pain post-colorectal cancer surgery has significantly reduced, and the recovery time has considerably shortened (101–103). The incision length in laparoscopic surgery is notably shorter than in open surgery, reducing skin integrity damage, bacterial displacement within the skin, and challenges in incision healing (104–107). Additionally, laparoscopic surgery, with its minimal tissue damage and smaller incisions, facilitates postoperative recovery, encouraging early patient mobilization to support wound healing and lower postoperative incision infection rates (105–107). Some studies have found that laparoscopic surgery minimally impacts human immune function and injury, making postoperative incision infections less likely (108, 109). Therefore, the choice of surgical method is particularly crucial, with a preference for laparoscopic surgery when possible (110, 111). Research indicates that in a single-center randomized controlled trial, the postoperative incision infection rate for patients undergoing laparoscopic surgery for colorectal cancer was 4.9% (47/961), significantly lower than the open surgery group (9.6%, 95/986) (112–115). This difference may be due to laparoscopic surgery reducing the direct contact between organs and environmental pathogens. Additionally, laparoscopic surgery avoids factors like peritonitis, increased intestinal permeability, and intestinal edema that are prone to surgical site infections, thereby lowering the rate of postoperative incision infections (116–118).
Nutritional status is a primary concern in the perioperative management of colorectal cancer patients (119, 120). Malnutrition lowers cellular and humoral immune responses, and correcting malnutrition can reduce the incidence of perioperative complications by up to 10% (121–123). The occurrence rate of perioperative complications is as high as 10% (124–126). Although no universal definition for diagnosing malnutrition, it typically encompasses conditions related to inadequate food intake, weight loss, and a low BMI (127–129). The European Society for Clinical Nutrition and Metabolism (ESPEN) defines malnutrition to include at least one of the following criteria: a weight loss of more than 10% of the original weight within six months, a BMI lower than 18.5 kg/m2, serum albumin less than 35 g/L, in the absence of liver or kidney dysfunction (130–135). Fujimichi et al. reported that malnutrition is an independent risk factor for postoperative incision infection in colorectal cancer patients (OR = 2.52, 95%, p = 0.01) (42, 136, 137). Furthermore, a registry study at the Hokeland University Hospital in Norway showed that among 1,194 patients undergoing surgical treatment, those at nutritional risk were more likely to develop incision infections, with a positive correlation between the incidence of incision infections and nutritional risk (OR = 1.81, p = 0.047) (138–141). ESPEN recommends that severely malnourished patients scheduled for major gastrointestinal surgery should receive preoperative nutritional support for 10–14 days. Enteral nutrition should be the first choice if there are no contraindications (142–145). Enhancing perioperative nutrition and supportive care is crucial for malnourished patients, ensuring sufficient energy and nutrient intake to prevent perioperative incision infections. Malnourished colorectal cancer patients often have electrolyte imbalances, anemia, and lower immunity, increasing the risk of postoperative incision infections. Patients should receive enteral nutrition as soon as gastrointestinal recovery permits, maintaining the intestinal barrier and immune barrier, reducing endotoxin absorption and intestinal flora displacement, thereby providing a conducive internal environment for wound healing (49, 146–150).
This study's findings indicate that a surgical duration exceeding three hours is a risk factor for surgical site infections in patients with colorectal cancer, aligning with Katsuno's research. It has been shown that the risk of postoperative incision infection in colorectal cancer increases with the length of the surgery (151–154). Extended surgical times are often associated with increased blood loss, potentially leading to tissue hypoxia (155–157). Longer surgeries inevitably carry a higher risk of bleeding and increased blood loss, reducing the body's resistance and inducing infection (158). The longer the surgery, the more energy the patient expends, raising the risk of exogenous infection and, thereby, the risk of postoperative incision infection (159–161). Prolonged surgical duration also means the sterile environment within the abdomen is exposed to air for a longer time (162–164). Even in an operation meeting standard requirements, air cleanliness decreases with extended surgical times, increasing the probability of local bacterial contamination (165–167). The wound's exposure to air also increases, leading to a higher bacterial count at the incision site and increasing the possibility of tissue cell destruction. Longer surgeries, extended exposure to tissue traction, and prolonged use of surgical energy devices can damage tissues. Moreover, extended anesthesia can adversely affect the patient's immune function. The body's immunity diminishes as anesthesia duration and intraoperative blood loss increase. The length of the surgery is not only related to the patient's physical condition but also largely depends on the surgeon's skill and proficiency in the operation. Thus, an increased rate of postoperative incision infection indicates that more complex, challenging, and traumatic surgeries with longer durations lead to higher infection rates (168, 169). Therefore, enhancing the surgical skills and intraoperative proficiency of surgeons, reducing surgical trauma, shortening surgical duration, and lowering the incidence of incision infections is paramount. Zheng Hui's multivariate analysis of 2,308 patients showed that surgical duration (OR = 1.007, 95% CI: 1.002–1.012) is an independent risk factor for incision infection (170). Thus, effectively controlling surgical duration can significantly reduce the incidence of incision infections (171–173).
In addition to risk assessment, advancements in deep learning algorithms hold significant potential for improving the accuracy and efficiency of colorectal cancer (CRC) diagnosis. Deep learning models can analyze histopathology images with high precision, aiding in the classification and diagnosis of CRC. These algorithms can learn complex patterns from large datasets, potentially outperforming traditional diagnostic methods. Further investigation into the clinical implementation of these algorithms could enhance CRC detection's accuracy and efficacy (174, 175).
Based on the analysis of various risk factors, future clinical practices can implement the following strategies to prevent postoperative incision infections in patients with colorectal cancer:
(1) Preoperative: Implement infection prevention measures and avoid scheduling surgeries during summer. For diabetic patients, intensify monitoring and control of blood glucose levels and use insulin judiciously. Surgery should proceed only when blood glucose levels are within normal ranges. For elderly patients, complications should be vigilantly monitored and actively managed preoperatively. Additionally, patients' nutritional status should be assessed, and timely nutritional support should be provided to those with low serum albumin to ensure balanced daily nutrient intake and optimal preoperative nutrition.
(2) Intraoperative: Adhere to standard sterile procedures, minimize electrosurgical use in patients with thick adipose layers, and adjust the electrosurgical power as necessary. Inactive fatty tissue should be rinsed with saline during incision closure. Moreover, surgical preparations should be meticulously planned, requiring close cooperation among medical staff to enhance procedural proficiency and actively manage surgical duration. When appropriate, consider laparoscopic surgery for its reduced patient trauma and lower postoperative incision infection rates, taking into account the patient's specific health status and condition.
(3) Postoperative: Monitor changes in patient vitals, replenish energy promptly as needed, and encourage high-fiber and protein-rich foods to boost nutrition and maintain electrolyte balance. Pay attention to changes in the nature, volume, and color of drainage fluid, replace drainage bags timely to prevent incision-related infections, and regularly change wound dressings to prevent bacterial growth and infection.
Despite our study's rigorous design and execution, it has limitations. First, the significant heterogeneity among studies may affect the robustness of our conclusions, although sensitivity analysis and publication bias assessment have been conducted to ensure the reliability of the results. Second, the quality of included studies varies, and despite rigorous evaluation using the Cochrane risk of bias tool and NOS scoring system, the impact of low-quality studies must be partially ruled out. Additionally, language and database search limitations might have led to selection bias due to potentially relevant studies needing to be included.
Future research should further explore the causal relationships between these risk factors and postoperative incision infections in colorectal cancer and how they interact with other potential risk factors. Moreover, as medical technology advances, new surgical techniques and postoperative management strategies may influence the risk of postoperative incision infections in colorectal cancer. Therefore, ongoing research and updated meta-analyses must ensure our conclusions reflect the latest scientific evidence.
In summary, this study provides critical insights into the risk factors for postoperative incision infection in colorectal cancer, emphasizing the importance of comprehensive assessment and management of these risk factors in clinical practice. By early identification and intervention of these risk factors, the incidence of postoperative incision infections in colorectal cancer patients can potentially be reduced, thereby improving patient prognosis and quality of life. Future research should aim to explore additional potential risk factors and evaluate the effectiveness of various prevention and management strategies to optimize postoperative care for colorectal cancer patients further.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LJ: Investigation, Methodology, Writing – original draft. HZ: Data curation, Methodology, Writing – original draft, Writing – review & editing. JL: Investigation, Methodology, Supervision, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). (2021) 41(11):1137–51. doi: 10.1002/cac2.12220
2. Hang D, Shen H. Sex hormone and colorectal cancer: the knowns and unknowns. Cancer Epidemiol Biomarkers Prev. (2021) 30(7):1302–4. doi: 10.1158/1055-9965.EPI-21-0472
3. Ohno Y, Mazaki J, Udo R, Tago T, Kasahara K, Enomoto M, et al. Preliminary evaluation of a novel artificial intelligence-based prediction model for surgical site infection in colon cancer. Cancer Diagn Progn. (2022) 2(6):691–6. doi: 10.21873/cdp.10161
4. Cheong CM, Golder AM, Horgan PG, Roxburgh CSD, McMillan DC. Relationship between pre-operative glycated haemoglobin and surgical site infection in patients undergoing elective colon cancer surgery. Oncol Lett. (2022) 24(3):296. doi: 10.3892/ol.2022.13416
5. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. (2021) 325(7):669–85. doi: 10.1001/jama.2021.0106
6. Shah SC, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. (2022) 162(3):715–730.e3. doi: 10.1053/j.gastro.2021.10.035
7. Sedlak JC, Yilmaz ÖH, Roper J. Metabolism and colorectal cancer. Annu Rev Pathol. (2023) 18:467–92. doi: 10.1146/annurev-pathmechdis-031521-041113
8. Ikeda A, Fukunaga Y, Akiyoshi T, Nagayama S, Nagasaki T, Yamaguchi T, et al. Wound infection in colorectal cancer resections through a laparoscopic approach: a single-center prospective observational study of over 3,000 cases. Discov Oncol. (2021) 12(1):2. doi: 10.1007/s12672-021-00396-8
9. Xu S, Liu K, Chen X, Yao H. The safety and efficacy of laparoscopic surgery versus laparoscopic NOSE for sigmoid and rectal cancer. Surg Endosc. (2022) 36(1):222–35. doi: 10.1007/s00464-020-08260-6
10. Timotewos G, Solomon A, Mathewos A, Addissie A, Bogale S, Wondemagegnehu T, et al. First data from a population based cancer registry in Ethiopia. Cancer Epidemiol. (2018) 53:93–8. doi: 10.1016/j.canep.2018.01.008
11. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34(1):10–32. doi: 10.1016/j.annonc.2022.10.003
12. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33(10):1005–20. doi: 10.1016/j.annonc.2022.07.004
13. Zhou H, Liu Z, Wang Y, Wen X, Amador EH, Yuan L, et al. Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal Transduct Target Ther. (2022) 7(1):70. doi: 10.1038/s41392-022-00922-2
14. Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, et al. Recent advances in the treatment of colorectal cancer: a review. J Nippon Med Sch. (2022) 89(3):246–54. doi: 10.1272/jnms.JNMS.2022_89-310
15. Bonney GK, Chew CA, Lodge P, Hubbard J, Halazun KJ, Trunecka P, et al. Liver transplantation for non-resectable colorectal liver metastases: the international hepato-pancreato-biliary association consensus guidelines. Lancet Gastroenterol Hepatol. (2021) 6(11):933–46. doi: 10.1016/S2468-1253(21)00219-3. Erratum in: Lancet Gastroenterol Hepatol. 2021 6(11):e7. doi: 10.1016/S2468-1253(21)00345-9.34506756
16. Falz R, Bischoff C, Thieme R, Lässing J, Mehdorn M, Stelzner S, et al. Effects and duration of exercise-based prehabilitation in surgical therapy of colon and rectal cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. (2022) 148(9):2187–213. doi: 10.1007/s00432-022-04088-w
17. Huang W, Wei ZQ, Qiu YH, Tang G, Sun H. Effects of wound infection on prognosis after laparoscopic abdominoperineal resection of rectal cancer. Front Oncol. (2023) 12:1036241. doi: 10.3389/fonc.2022.1036241
18. Biscione A, Corrado G, Quagliozzi L, Federico A, Franco R, Franza L, et al. Healthcare associated infections in gynecologic oncology: clinical and economic impact. Int J Gynecol Cancer. (2023) 33(2):278–84. doi: 10.1136/ijgc-2022-003847
19. Pattou M, Fuks D, Guilbaud T, Le Floch B, Lelièvre O, Tribillon E, et al. Predictive value of C-reactive protein for postoperative liver-specific surgical site infections. Surgery. (2024) 175(5):1337–45. doi: 10.1016/j.surg.2024.01.030
20. Sharon CE, Grinberg S, Straker RJ 3rd, Mahmoud NN, Kelz RR, Miura JT, et al. Trends in infectious complications after partial colectomy for colon cancer over a decade: a national cohort study. Surgery. (2022) 172(6):1622–8. doi: 10.1016/j.surg.2022.09.011
21. Li YS, Meng FC, Lin JK. Procedural and post-operative complications associated with laparoscopic versus open abdominal surgery for right-sided colonic cancer resection: a systematic review and meta-analysis. Medicine (Baltimore). (2020) 99(40):e22431. doi: 10.1097/MD.0000000000022431
22. Wang L, Xu H, Zhang X, Zhang Y, Shi L, Wang M. Effect of carbon nanoparticle tracer combined with laparoscopy in the treatment of colon cancer. J Nanosci Nanotechnol. (2020) 20(10):6007–12. doi: 10.1166/jnn.2020.18598
23. Zhang H, Huang X, Qu C, Bian C, Xue H. Comparison between laparoscopic and endoscopic resections for gastric submucosal tumors. Saudi J Gastroenterol. (2019) 25(4):245–50. doi: 10.4103/sjg.SJG_412_18
24. Yang X, Zhang G, Jiang L, Zhang H, Liu Z, Liu J, et al. Laparoscopic sphincter-saving surgery for low rectal cancer through marker meeting approach. Ann Transl Med. (2018) 6(16):324. doi: 10.21037/atm.2018.08.02
25. Lender O, Göbölös L, Bajwa G, Bhatnagar G. Sternal wound infections after sternotomy: risk factors, prevention and management. J Wound Care. (2022) 31(Sup6):S22–30. doi: 10.12968/jowc.2022.31.Sup6.S22
26. Samuel AR, Hakami L, Campbell C, DeGeorge BR Jr, Black J, Stranix JT. Abdominal panniculectomy: identifying complications and potential risk factors. J Plast Reconstr Aesthet Surg. (2022) 75(9):3534–40. doi: 10.1016/j.bjps.2022.04.061
27. Roberts DJ, Nagpal SK, Stelfox HT, Brandys T, Corrales-Medina V, Dubois L, et al. Risk factors for surgical site infection after lower limb revascularization surgery in adults with peripheral artery disease: protocol for a systematic review and meta-analysis. JMIR Res Protoc. (2021) 10(9):e28759. doi: 10.2196/28759
28. Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, et al. Type 2 diabetes and cancer: an Umbrella review of observational and Mendelian randomization studies. Cancer Epidemiol Biomarkers Prev. (2021) 30(6):1218–28. doi: 10.1158/1055-9965.EPI-20-1245
29. Liu T, Wang Y, Wang X, Liu C, Zhang Q, Song M, et al. Habitually skipping breakfast is associated with the risk of gastrointestinal cancers: evidence from the Kailuan cohort study. J Gen Intern Med. (2023) 38(11):2527–36. doi: 10.1007/s11606-023-08094-7
30. Liu X, Peng S, Tang G, Xu G, Xie Y, Shen D, et al. Fasting-mimicking diet synergizes with ferroptosis against quiescent, chemotherapy-resistant cells. EBioMedicine. (2023) 90:104496. doi: 10.1016/j.ebiom.2023.104496
31. Wei Z, Liu G, Jia R, Zhang W, Li L, Zhang Y, et al. Targeting secretory leukocyte protease inhibitor (SLPI) inhibits colorectal cancer cell growth, migration and invasion via downregulation of AKT. PeerJ. (2020) 8:e9400. doi: 10.7717/peerj.9400
32. Lee SY, Yeom SS, Kim CH, Kim HR. Effect of preoperative immunonutrition on outcomes of colon cancer surgery: study protocol for a randomized controlled trial. Trials. (2020) 21(1):628. doi: 10.1186/s13063-020-04544-3
33. Zhuang J, Zheng W, Yang S, Ye J. Modified subcutaneous suction drainage to prevent incisional surgical site infections after radical colorectal surgery. Transl Cancer Res. (2020) 9(2):910–7. doi: 10.21037/tcr.2019.12.32
34. Allen G. Evidence appraisal of Sadahiro S, Suzuki T, Tanaka A, et al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery. 2014;155(3):493–503. AORN J. (2014) 100(1):107–11. doi: 10.1016/j.aorn.2014.05.005
35. Han C, Chen W, Ye XL, Cheng F, Wang XY, Liu AB, et al. Risk factors analysis of surgical site infections in postoperative colorectal cancer: a nine-year retrospective study. BMC Surg. (2023) 23(1):320. doi: 10.1186/s12893-023-02231-z
36. Reudink M, Slooter CD, Janssen L, Lieverse AG, Roumen RMH, Slooter GD. Metabolic syndrome; associations with adverse outcome after colorectal surgery. A systematic review and meta-analysis. Ann Med Surg (Lond). (2021) 71:102997. doi: 10.1016/j.amsu.2021.102997
37. Tanaka A, Sadahiro S, Suzuki T, Okada K, Saito G. Randomized controlled trial comparing subcuticular absorbable suture with conventional interrupted suture for wound closure at elective operation of colon cancer. Surgery. (2014) 155(3):486–92. doi: 10.1016/j.surg.2013.10.016
38. Mahmoud NN. Colorectal cancer: preoperative evaluation and staging. Surg Oncol Clin N Am. (2022) 31(2):127–41. doi: 10.1016/j.soc.2021.12.001
39. Mitsala A, Tsalikidis C, Pitiakoudis M, Simopoulos C, Tsaroucha AK. Artificial intelligence in colorectal cancer screening, diagnosis and treatment. A new era. Curr Oncol. (2021) 28(3):1581–607. doi: 10.3390/curroncol28030149
40. Páramo-Zunzunegui J, Alonso-García M, Rodríguez-Villar D, Drewniak-Jakubowska J, Calvo-Espino P, Cuberes-Montserrat R, et al. Incidence of surgical infection and risk factors in colorectal surgery—a prospective cohort study. Cir Cir. (2021) 89(2):156–62. English. doi: 10.24875/CIRU.20000205
41. Furukawa K, Onda S, Taniai T, Hamura R, Yanagaki M, Tsunematsu M, et al. Risk factors and overcoming strategies of surgical site infection after hepatectomy for colorectal liver metastases. Anticancer Res. (2021) 41(11):5651–6. doi: 10.21873/anticanres.15381
42. Christina NM, Tjahyanto T, Lie JG, Santoso TA, Albertus H, Octavianus D, et al. Hypoalbuminemia and colorectal cancer patients: any correlation?: a systematic review and meta-analysis. Medicine (Baltimore). (2023) 102(8):e32938. doi: 10.1097/MD.0000000000032938
43. Ali G, Shaukat A, Masood S, Akram B, Ghaffar A, Gondal KM. A profile of colorectal tumors presenting as emergency. J Coll Physicians Surg Pak. (2021) 31(1):74–8. doi: 10.29271/jcpsp.2021.01.74
44. Matsumoto A, Shinohara H, Suzuki H. Laparoscopic and open surgery in patients with transverse colon cancer: short-term and oncological outcomes. BJS Open. (2021) 5(5):zrab078. doi: 10.1093/bjsopen/zrab078
45. Ayandipo OO, Afuwape OO, Ojo AB, Egbuchulem IK, Irabor DO. Perioperative morbidity and mortality after emergency and elective colon and proximal rectal surgery in ibadan. Ann Ib Postgrad Med. (2020) 18(1):24–30. PMID: 33623490, PMCID: PMC7893302.33623490
46. Dmitrieva-Posocco O, Wong AC, Lundgren P, Golos AM, Descamps HC, Dohnalová L, et al. β-hydroxybutyrate suppresses colorectal cancer. Nature. (2022) 605(7908):160–5. doi: 10.1038/s41586-022-04649-6
47. Fernandez-Rozadilla C, Timofeeva M, Chen Z, Law P, Thomas M, Schmit S, et al. Deciphering colorectal cancer genetics through multi-omic analysis of 100,204 cases and 154,587 controls of European and east Asian ancestries. Nat Genet. (2023) 55(1):89–99. doi: 10.1038/s41588-022-01222-9. Erratum in: Nat Genet. 2023 55(3):519–520. doi: 10.1038/s41588-023-01334-w.36539618
48. Liu Y, Baba Y, Ishimoto T, Gu X, Zhang J, Nomoto D, et al. Gut microbiome in gastrointestinal cancer: a friend or foe? Int J Biol Sci. (2022) 18(10):4101–17. doi: 10.7150/ijbs.69331
49. Sadahiro S, Suzuki T, Tanaka A, Okada K, Kamata H, Ozaki T, et al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery. (2014) 155(3):493–503. doi: 10.1016/j.surg.2013.06.002
50. Ben-Aharon I, van Laarhoven HWM, Fontana E, Obermannova R, Nilsson M, Lordick F. Early-onset cancer in the gastrointestinal tract is on the rise-evidence and implications. Cancer Discov. (2023) 13(3):538–51. doi: 10.1158/2159-8290.CD-22-1038
51. Gondal TA, Chaudhary N, Bajwa H, Rauf A, Le D, Ahmed S. Anal cancer: the past, present and future. Curr Oncol. (2023) 30(3):3232–50. doi: 10.3390/curroncol30030246
52. Eng C, Ciombor KK, Cho M, Dorth JA, Rajdev LN, Horowitz DP, et al. Anal cancer: emerging standards in a rare disease. J Clin Oncol. (2022) 40(24):2774–88. doi: 10.1200/JCO.21.02566
53. Alharbi SH, Alshammari KI, Alanazi KK, Ahmed HG. Patterns and grades of presentation of colon cancer in northern Saudi Arabia. Prz Gastroenterol. (2021) 16(3):235–9. doi: 10.5114/pg.2021.104168
54. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). (2021) 134(7):783–91. doi: 10.1097/CM9.0000000000001474
55. Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. (2023) 72(2):338–44. doi: 10.1136/gutjnl-2022-327736
56. Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: a comparison among China, Europe, and northern America. Cancer Lett. (2021) 522:255–68. doi: 10.1016/j.canlet.2021.09.034
57. Kozlowski L, Malyszko J. Acute kidney injury prevalence in patients with colorectal cancer undergoing surgery with curative intent. Contemp Oncol (Pozn). (2022) 26(3):187–90. doi: 10.5114/wo.2021.111057
58. Peponis T, Stafford C, Cusack J, Cauley C, Goldstone R, Berger D, et al. The growing trend for no primary surgery in colorectal cancer. Colorectal Dis. (2021) 23(10):2659–70. doi: 10.1111/codi.15828
59. Altintas MM, Kaya S, Kocaoglu AE, Mulkut F. Does preoperative anaemia have an effect on the perioperative period in colorectal cancer surgery? Niger J Clin Pract. (2022) 25(7):1102–6. doi: 10.4103/njcp.njcp_1664_21
60. Mangano A, Gheza F, Giulianotti PC. Iatrogenic spleen injury during minimally invasive left colonic flexure mobilization: the quest for evidence-based results. Minerva Chir. (2018) 73(5):512–9. doi: 10.23736/S0026-4733.18.07737-4
61. Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. (2022) 7(3):262–74. doi: 10.1016/S2468-1253(21)00426-X
62. Constantin M, Petrescu L, Mătanie C, Vrancianu CO, Niculescu AG, Andronic O, et al. The vermiform appendix and its pathologies. Cancers (Basel). (2023) 15(15):3872. doi: 10.3390/cancers15153872
63. Azcutia V, Kelm M, Kim S, Luissint AC, Flemming S, Abernathy-Close L, et al. Distinct stimulus-dependent neutrophil dynamics revealed by real-time imaging of intestinal mucosa after acute injury. PNAS Nexus. (2022) 1(5):249. doi: 10.1093/pnasnexus/pgac249
64. Chiarello MM, Fransvea P, Cariati M, Adams NJ, Bianchi V, Brisinda G. Anastomotic leakage in colorectal cancer surgery. Surg Oncol. (2022) 40:101708. doi: 10.1016/j.suronc.2022.101708
65. Sartelli M, Coccolini F, Kluger Y, Agastra E, Abu-Zidan FM, Abbas AES, et al. WSES/GAIS/SIS-E/WSIS/AAST global clinical pathways for patients with intra-abdominal infections. World J Emerg Surg. (2021) 16(1):49. doi: 10.1186/s13017-021-00387-8
66. Tarasconi A, Perrone G, Davies J, Coimbra R, Moore E, Azzaroli F, et al. Anorectal emergencies: wSES-AAST guidelines. World J Emerg Surg. (2021) 16(1):48. doi: 10.1186/s13017-021-00384-x
67. Wei R, Crook C, Bamford R. Abdominoperineal resection. 2023 Feb 27. In: Wei R, Crook C, Bamford R, editors. StatPearls. Treasure Island (FL): StatPearls Publishing (2024). Available online at: https://pubmed.ncbi.nlm.nih.gov/34662082/ (Accessed March 24, 2024).
68. EuroSurg Collaborative. Intraperitoneal drain placement and outcomes after elective colorectal surgery: international matched, prospective, cohort study. Br J Surg. (2022) 109(6):520–9. doi: 10.1093/bjs/znac069
69. Siragusa L, Pellino G, Sensi B, Panis Y, Bellato V, Khan J, et al. Ambulatory laparoscopic colectomies: a systematic review. Colorectal Dis. (2023) 25(6):1102–15. doi: 10.1111/codi.16511
70. Bignell M, Chave H, Branagan G. Outcome of surgery for recurrent anal cancer: results from a tertiary referral centre. Colorectal Dis. (2018) 20(9):771–7. doi: 10.1111/codi.14098
71. Cai W, Wang L, Wang W, Zhou T. Systematic review and meta-analysis of the risk factors of surgical site infection in patients with colorectal cancer. Transl Cancer Res. (2022) 11(4):857–71. doi: 10.21037/tcr-22-627
72. Panos G, Mulita F, Akinosoglou K, Liolis E, Kaplanis C, Tchabashvili L, et al. Risk of surgical site infections after colorectal surgery and the most frequent pathogens isolated: a prospective single-centre observational study. Med Glas (Zenica). (2021) 18(2):438–43. doi: 10.17392/1348-21
73. Chung JS, Kwak HD, Ju JK. Thirty-day readmission after elective colorectal surgery for colon cancer: a single-center cohort study. Ann Coloproctol. (2020) 36(3):186–91. doi: 10.3393/ac.2019.11.04
74. Kautzky-Willer A, Winhofer Y, Kiss H, Falcone V, Berger A, Lechleitner M, et al. Gestationsdiabetes (GDM) (update 2023) [gestational diabetes mellitus (update 2023)]. Wien Klin Wochenschr. (2023) 135(Suppl 1):115–28. German. doi: 10.1007/s00508-023-02181-9
75. Joshi RD, Dhakal CK. Predicting type 2 diabetes using logistic regression and machine learning approaches. Int J Environ Res Public Health. (2021) 18(14):7346. doi: 10.3390/ijerph18147346
76. Parker ED, Lin J, Mahoney T, Ume N, Yang G, Gabbay RA, et al. Economic costs of diabetes in the U.S. In 2022. Diabetes Care. (2024) 47(1):26–43. doi: 10.2337/dci23-0085
77. Truong DH, Bedimo R, Malone M, Wukich DK, Oz OK, Killeen AL, et al. Meta-analysis: outcomes of surgical and medical management of diabetic foot osteomyelitis. Open Forum Infect Dis. (2022) 9(9):ofac407. doi: 10.1093/ofid/ofac407
78. Wang J, Chang E, Jiang Y. Effects of vitamin C stimulation on rehabilitation of dysphagia after stroke: a randomized trial. Eur J Phys Rehabil Med. (2022) 58(4):558–64. doi: 10.23736/S1973-9087.22.07337-3
79. Chien SC, Chandramouli C, Lo CI, Lin CF, Sung KT, Huang WH, et al. Associations of obesity and malnutrition with cardiac remodeling and cardiovascular outcomes in Asian adults: a cohort study. PLoS Med. (2021) 18(6):e1003661. doi: 10.1371/journal.pmed.1003661 Erratum in: PLoS Med. 2021 18(9):e1003784. doi: 10.1371/journal.pmed.1003784.34061848
80. Wasserstein MP, Schuchman EH. Acid sphingomyelinase deficiency. 2006 Dec 7 [updated 2023 Apr 27]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al., editors. GeneReviews®. Seattle (WA): University of Washington (1993–2024). Available online at: https://pubmed.ncbi.nlm.nih.gov/20301544/ (Accessed March 24, 2024).
81. Liu QX, Tang DY, Xiang X, He JQ. Associations between nutritional and immune status and clinicopathologic factors in patients with tuberculosis: a comprehensive analysis. Front Cell Infect Microbiol. (2022) 12:1013751. doi: 10.3389/fcimb.2022.1013751
82. Jia X, Yu XL, Lu B, Shang YY, Shen LF, Li YL, et al. Malnutrition and infection lead to poor prognosis and heavy financial burden of patients with chronic heart failure. Front Cardiovasc Med. (2022) 9:1045262. doi: 10.3389/fcvm.2022.1045262
83. Gao W, Wei L, Zhao J, Yang X, Han Y, Liu Y, et al. The measurement of 25-hydroxyvitamin-D in chronic HBV patients using LC-MS/MS. Clin Lab. (2022) 68(7). doi: 10.7754/Clin.Lab.2021.211034
84. Zeng Y, Zhang A, Yang X, Xing C, Zhai J, Wang Y, et al. Internal exposure potential of water-soluble organic molecules in urban PM2.5 evaluated by non-covalent adductome of human serum albumin. Environ Int. (2024) 184:108492. doi: 10.1016/j.envint.2024.108492
85. Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci. (2021) 22(9):4496. doi: 10.3390/ijms22094496
86. Hareedy MS, Tawfik KM. Systemic isotretinoin has an impact on hemoglobin, ferritin, urea, ceruloplasmin, albumin, uric acid levels, and neutrophil to lymphocyte ratio in acne patients. J Cosmet Dermatol. (2022) 21(11):6191–8. doi: 10.1111/jocd.15199
87. Sun JL, Xing SY. Short-term outcome of laparoscopic surgery versus open surgery on colon carcinoma: a meta-analysis. Math Biosci Eng. (2019) 16(5):4645–59. doi: 10.3934/mbe.2019233
88. Sun MY, Zheng T, Chen J, Zhan ZW, Wang ZL, Chen W, et al. Technological innovation and clinical application of direct percutaneous computed tomography-guided enterostomy vs other enterostomy techniques. J Chin Med Assoc. (2022) 85(10):1011–6. doi: 10.1097/JCMA.0000000000000793
89. Mulita F, Liolis E, Akinosoglou K, Tchabashvili L, Maroulis I, Kaplanis C, et al. Postoperative sepsis after colorectal surgery: a prospective single-center observational study and review of the literature. Prz Gastroenterol. (2022) 17(1):47–51. doi: 10.5114/pg.2021.106083
90. Chen JC, Huang CY, Wang JC, Zhang YJ, Xu L, Chen MS, et al. Robot-assisted laparoscopic partial hepatic caudate lobectomy. Minim Invasive Ther Allied Technol. (2019) 28(5):292–7. doi: 10.1080/13645706.2018.1521434
91. Williamson T, Song SE. Robotic surgery techniques to improve traditional laparoscopy. JSLS. (2022) 26(2):e2022.00002. doi: 10.4293/JSLS.2022.00002
92. Wang M, Li D, Chen R, Huang X, Li J, Liu Y, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. (2021) 6(6):438–47. doi: 10.1016/S2468-1253(21)00054-6
93. Seeras K, Philip K, Baldwin D, Prakash S. Laparoscopic gastric bypass. 2023 Sep 4. In: Seeras K, Philip K, Baldwin D, Prakash S, editors. StatPearls. Treasure Island (FL): StatPearls Publishing (2024). Available online at: https://pubmed.ncbi.nlm.nih.gov/30085510/ (Accessed March 24, 2024).
94. Chen Y, Xi D, Zhang Q. Laparoscopic radical resection versus routine surgery for colorectal cancer. Comput Math Methods Med. (2022) 2022:4899555. doi: 10.1155/2022/4899555 Retraction in: Comput Math Methods Med. 2023 2023:9790203. doi: 10.1155/2023/9790203.36238486
95. Liu B, Yao C, Li H. Laparoscopic radical resection of colorectal cancer in the treatment of elderly colorectal cancer and its effect on gastrointestinal function. Front Surg. (2022) 9:840461. doi: 10.3389/fsurg.2022.840461
96. Nothnick WB, Graham A. Dissecting the miR-451a-mif pathway in endometriosis pathophysiology using a syngeneic mouse model: temporal expression of lesion mif receptors, Cd74 and Cxcr4. Biomedicines. (2022) 10(7):1699. doi: 10.3390/biomedicines10071699
97. van Amsterdam B, Clarkson MJ, Stoyanov D. Gesture recognition in robotic surgery: a review. IEEE Trans Biomed Eng. (2021) 68(6):2021–35. doi: 10.1109/TBME.2021.3054828
98. Xiong GX, Tobert D, Fogel H, Cha T, Schwab J, Shin J, et al. Open epidural blood patch to augment durotomy repair in lumbar spine surgery: surgical technique and cohort study. Spine J. (2021) 21(12):2010–8. doi: 10.1016/j.spinee.2021.06.011
99. Tekin SB, Demir IH, Bozgeyik B, Mert A. How does tranexamic acid affect blood transfusion and bleeding amount in pelvis-acetabulum fractures treated with open reduction and internal fixation? Ulus Travma Acil Cerrahi Derg. (2022) 28(9):1323–7. doi: 10.14744/tjtes.2021.45843
100. Salati SA, Alfehaid M, Alsuwaydani S, AlSulaim L. Spilled gallstones after laparoscopic cholecystectomy: a systematic review. Pol Przegl Chir. (2022) 95(2):1–20. doi: 10.5604/01.3001.0015.8571
101. Zhao S, Zhang L, Gao F, Wu M, Zheng J, Bai L, et al. Transanal drainage tube use for preventing anastomotic leakage after laparoscopic low anterior resection in patients with rectal cancer: a randomized clinical trial. JAMA Surg. (2021) 156(12):1151–8. doi: 10.1001/jamasurg.2021.4568
102. Köhler F, Hendricks A, Kastner C, Müller S, Boerner K, Wagner JC, et al. Laparoscopic appendectomy versus antibiotic treatment for acute appendicitis-a systematic review. Int J Colorectal Dis. (2021) 36(10):2283–6. doi: 10.1007/s00384-021-03927-5
103. Lee JE, Park HJ, Chung YJ, Ahn HJ, Sim WS, Lee JY. Analgesic effect of dexmedetomidine in colorectal cancer patients undergoing laparoscopic surgery. Saudi Med J. (2022) 43(10):1096–102. doi: 10.15537/smj.2022.43.10.20220526
104. Du M, Liu B, Li M, Cao J, Liu D, Wang Z, et al. Multicenter surveillance study of surgical site infection and its risk factors in radical resection of colon or rectal carcinoma. BMC Infect Dis. (2019) 19(1):411. doi: 10.1186/s12879-019-4064-6
105. Gilna GP, Saberi RA, Baez AC, Ribieras AJ, Cioci AC, Urrechaga EM, et al. Nationwide outcomes and readmission after pediatric laparoscopic and open fundoplication. J Laparoendosc Adv Surg Tech A. (2021) 31(12):1389–96. doi: 10.1089/lap.2021.0345
106. Liang H, Zhu Z, Zhang C, Zhang H, Zhang C. A safe and feasible technique: laparoscopic manual binding technique for intracorporeal anastomosis in totally laparoscopic anterior resection of high-mid rectal cancer. Surg Endosc. (2021) 35(4):1927–30. doi: 10.1007/s00464-021-08294-4
107. Bell-Allen N, Swift K, Sontag NJ, O'Rourke N. Ventral hernia repair with a hybrid laparoscopic technique. ANZ J Surg. (2022) 92(10):2529–33. doi: 10.1111/ans.17508
108. Albers KI, Polat F, Helder L, Panhuizen IF, Snoeck MMJ, Polle SBW, et al. Quality of recovery and innate immune homeostasis in patients undergoing low-pressure versus standard-pressure pneumoperitoneum during laparoscopic colorectal surgery (RECOVER): a randomized controlled trial. Ann Surg. (2022) 276(6):e664–73. doi: 10.1097/SLA.0000000000005491
109. Yu R, Ge J, Lei Y. Effects of different nursing modes on immune function and renal function in patients with renal Calculus undergoing percutaneous nephrolithotomy. Arch Esp Urol. (2023) 76(9):703–10. doi: 10.56434/j.arch.esp.urol.20237609.86
110. Bass GA, Kaplan LJ, Forssten MP, Walsh TN, Cao Y, Mohseni S, et al. Techniques for mesoappendix transection and appendix resection: insights from the ESTES SnapAppy study. Eur J Trauma Emerg Surg. (2023) 49(1):17–32. doi: 10.1007/s00068-022-02191-8
111. Gkolfakis P, Papaefthymiou A, Facciorusso A, Tziatzios G, Ramai D, Dritsas S, et al. Comparison between enteroscopy-, laparoscopy- and endoscopic ultrasound-assisted endoscopic retrograde cholangio-pancreatography in patients with surgically altered anatomy: a systematic review and meta-analysis. Life (Basel). (2022) 12(10):1646. doi: 10.3390/life12101646
112. He J, Wang Z, Zhang S. Correlation analysis of IL-4, IL-10 and APN levels with postoperative infection of colorectal cancer. Oncol Lett. (2019) 17(2):1603–8. doi: 10.3892/ol.2018.9798
113. Amri R, Dinaux AM, Kunitake H, Bordeianou LG, Berger DL. Risk stratification for surgical site infections in colon cancer. JAMA Surg. (2017) 152(7):686–90. doi: 10.1001/jamasurg.2017.0505
114. Warps AK, Zwanenburg ES, Dekker JWT, Tollenaar RAEM, Bemelman WA, Hompes R, et al. Laparoscopic versus open colorectal surgery in the emergency setting: a systematic review and meta-analysis. Ann Surg Open. (2021) 2(3):e097. doi: 10.1097/AS9.0000000000000097
115. Sun R, Zhang Y, Feng B, Su X, Sun Y, Xu L, et al. Intracorporeal anastomosis versus extracorporeal anastomosis in laparoscopic right colectomy: an observational cohort study. World J Surg. (2023) 47(3):785–95. doi: 10.1007/s00268-022-06834-0
116. Albo D. Targeting surgical site infection-reducing bundles selectively to at-risk colon cancer surgery populations: achieving value in a MACRA world? JAMA Surg. (2017) 152(7):690. doi: 10.1001/jamasurg.2017.0506
117. Alias D, Ruiz-Tovar J, Moreno A, Manso B, Diaz G, Duran M, et al. Effect of subcutaneous Sterile vitamin E ointment on incisional surgical site infection after elective laparoscopic colorectal cancer surgery. Surg Infect (Larchmt). (2017) 18(3):287–92. doi: 10.1089/sur.2016.199
118. Gossetti F, D'Amore L, Annesi E, Bruzzone P, Bambi L, Grimaldi MR, et al. Mesh-related visceral complications following inguinal hernia repair: an emerging topic. Hernia. (2019) 23(4):699–708. doi: 10.1007/s10029-019-01905-z
119. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. (2022) 92(6):1382–7. doi: 10.1111/ans.17599
120. Inoue H, Arita T, Kuriu Y, Shimizu H, Kiuchi J, Yamamoto Y, et al. Emergency management of obstructive colorectal cancer—a retrospective study of efficacy and safety in self-expanding metallic stents and trans-anal tubes. In Vivo. (2021) 35(4):2289–96. doi: 10.21873/invivo.12502
121. Seraphin G, Rieger S, Hewison M, Capobianco E, Lisse TS. The impact of vitamin D on cancer: a mini review. J Steroid Biochem Mol Biol. (2023) 231:106308. doi: 10.1016/j.jsbmb.2023.106308
122. Wang M, Yu M, Kong WJ, Cui M, Gao F. Association between intestinal neoplasms and celiac disease: a review. World J Gastrointest Oncol. (2021) 13(9):1017–28. doi: 10.4251/wjgo.v13.i9.1017
123. Chao X, Lei Z, Hongqin L, Ziwei W, Dechuan L, Weidong D, et al. Faeces from malnourished colorectal cancer patients accelerate cancer progression. Clin Nutr. (2022) 41(3):632–44. doi: 10.1016/j.clnu.2022.01.001
124. van Stein RM, Aalbers AGJ, Sonke GS, van Driel WJ. Hyperthermic intraperitoneal chemotherapy for ovarian and colorectal cancer: a review. JAMA Oncol. (2021) 7(8):1231–8. doi: 10.1001/jamaoncol.2021.0580
125. Molenaar CJL, Minnella EM, Coca-Martinez M, Ten Cate DWG, Regis M, Awasthi R, et al. Effect of multimodal prehabilitation on reducing postoperative complications and enhancing functional capacity following colorectal cancer surgery: the PREHAB randomized clinical trial. JAMA Surg. (2023) 158(6):572–81. doi: 10.1001/jamasurg.2023.0198 Erratum in: JAMA Surg. 2023 158(6):675. doi: 10.1001/jamasurg.2023.1553.36988937
126. CReST Collaborative Group. Colorectal endoscopic stenting trial (CReST) for obstructing left-sided colorectal cancer: randomized clinical trial. Br J Surg. (2022) 109(11):1073–80. doi: 10.1093/bjs/znac141
127. Garay MB, Carbajal-Maldonado ÁL, Rodriguez-Ortiz-DE-Rozas R, Guilabert L, DE-Madaria E. Post-surgical exocrine pancreatic insufficiency. Minerva Surg. (2023) 78(6):671–83. doi: 10.23736/S2724-5691.23.10125-0
128. de van der Schueren MAE, Borkent JW, Spaans GW, Nijhof A, Manders M. GLIM In nursing homes; practical implications. Clin Nutr. (2022) 41(11):2442–5. doi: 10.1016/j.clnu.2022.09.003
129. Mikkelsen S, Geisler L, Holst M. Malnutrition measured by unintended weight loss among patients in general practice. Nutrition. (2022) 96:111554. doi: 10.1016/j.nut.2021.111554
130. Nakamura T, Sato T, Takayama Y, Naito M, Yamanashi T, Miura H, et al. Risk factors for surgical site infection after laparoscopic surgery for colon cancer. Surg Infect (Larchmt). (2016) 17(4):454–8. doi: 10.1089/sur.2015.205
131. Suzuki T, Sadahiro S, Tanaka A, Okada K, Saito G, Miyakita H, et al. Usefulness of preoperative mechanical bowel preparation in patients with colon cancer who undergo elective surgery: a prospective randomized trial using oral antibiotics. Dig Surg. (2020) 37(3):192–8. doi: 10.1159/000500020
132. Nakamura T, Takayama Y, Sato T, Watanabe M. Risk factors for wound infection after laparoscopic surgery for colon cancer. Surg Laparosc Endosc Percutan Tech. (2020) 30(1):45–8. doi: 10.1097/SLE.0000000000000735
133. Puri P, Dhiman RK, Taneja S, Tandon P, Merli M, Anand AC, et al. Nutrition in chronic liver disease: consensus statement of the Indian national association for study of the liver. J Clin Exp Hepatol. (2021) 11(1):97–143. doi: 10.1016/j.jceh.2020.09.003
134. Piccoli GB, Cederholm T, Avesani CM, Bakker SJL, Bellizzi V, Cuerda C, et al. Nutritional status and the risk of malnutrition in older adults with chronic kidney disease—implications for low protein intake and nutritional care: a critical review endorsed by ERN-ERA and ESPEN. Clin Nutr. (2023) 42(4):443–57. doi: 10.1016/j.clnu.2023.01.018
135. Muscaritoli M, Imbimbo G, Jager-Wittenaar H, Cederholm T, Rothenberg E, di Girolamo FG, et al. Disease-related malnutrition with inflammation and cachexia. Clin Nutr. (2023) 42(8):1475–9. doi: 10.1016/j.clnu.2023.05.013
136. Tajima Y, Ishida H, Yamamoto A, Chika N, Onozawa H, Matsuzawa T, et al. Comparison of the risk of surgical site infection and feasibility of surgery between sennoside versus polyethylene glycol as a mechanical bowel preparation of elective colon cancer surgery: a randomized controlled trial. Surg Today. (2016) 46(6):735–40. doi: 10.1007/s00595-015-1239-7
137. Benedek Z, Coroş MF. The impact of sarcopenia on the postoperative outcome in colorectal cancer surgery. Med Pharm Rep. (2023) 96(1):20–7. doi: 10.15386/mpr-2483
138. Ojima H, Sohda M, Ando H, Sano A, Fukai Y, Ogawa A, et al. Relationship between functional end-to-end anastomosis for colon cancer and surgical site infections. Surg Today. (2015) 45(12):1489–92. doi: 10.1007/s00595-015-1110-x
139. Global Cardiovascular Risk Consortium, Magnussen C, Ojeda FM, Leong DP, Alegre-Diaz J, Amouyel P, et al. Global effect of modifiable risk factors on cardiovascular disease and mortality. N Engl J Med. (2023) 389(14):1273–85. doi: 10.1056/NEJMoa2206916
140. Bouras E, Karhunen V, Gill D, Huang J, Haycock PC, Gunter MJ, et al. Circulating inflammatory cytokines and risk of five cancers: a Mendelian randomization analysis. BMC Med. (2022) 20(1):3. doi: 10.1186/s12916-021-02193-0
141. Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. (2022) 54(4):412–36. doi: 10.1038/s41588-022-01024-z
142. Kogo H, Yamamoto K, Yoshida H. A case of transverse colon cancer with a large liver abscess that could be treated with a radical operation after infection control. Int J Surg Case Rep. (2020) 77:182–6. doi: 10.1016/j.ijscr.2020.10.122
143. Jabłońska B, Mrowiec S. Nutritional support in patients with severe acute pancreatitis-current standards. Nutrients. (2021) 13(5):1498. doi: 10.3390/nu13051498
144. Adeyinka A, Rouster AS, Valentine M. Enteric feedings. 2022 Dec 26. In: Adeyinka A, Rouster AS, Valentine M, editors. StatPearls. Treasure Island (FL): StatPearls Publishing (2024). Available online at: https://pubmed.ncbi.nlm.nih.gov/30422471/ (Accessed March 24, 2024).
145. Lesser MNR, Lesser LI. Nutrition support therapy. Am Fam Physician. (2021) 104(6):580–8. PMID: 34913658.34913658
146. Jin L, Zhang X, Deng L, Wu W, Shao W, Yin L, et al. Analysis of risk factors for concurrent pulmonary infection after operation for colon cancer. J BUON. (2019) 24(2):436–44. PMID: 31127988.31127988
147. Ortiz H, Armendariz P, Kreisler E, Garcia-Granero E, Espin-Basany E, Roig JV, et al. Influence of rescrubbing before laparotomy closure on abdominal wound infection after colorectal cancer surgery: results of a multicenter randomized clinical trial. Arch Surg. (2012) 147(7):614–20. doi: 10.1001/archsurg.2012.150
148. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr. (2021) 40(7):4745–61. doi: 10.1016/j.clnu.2021.03.031
149. Wobith M, Weimann A. Oral nutritional supplements and enteral nutrition in patients with gastrointestinal surgery. Nutrients. (2021) 13(8):2655. doi: 10.3390/nu13082655
150. Bischoff SC, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, et al. Guía ESPEN: nutrición clínica en la enfermedad inflamatoria intestinal [ESPEN guideline: clinical nutrition in inflammatory bowel disease]. Nutr Hosp. (2022) 39(3):678–703. Spanish. doi: 10.20960/nh.03857
151. Lee SY, Lee J, Park HM, Kim CH, Kim HR. Impact of preoperative immunonutrition on the outcomes of colon cancer surgery: results from a randomized controlled trial. Ann Surg. (2023) 277(3):381–6. doi: 10.1097/SLA.0000000000005140
152. Wang R, Dai W, Gong J, Huang M, Hu T, Li H, et al. Development of a novel combined nomogram model integrating deep learning-pathomics, radiomics and immunoscore to predict postoperative outcome of colorectal cancer lung metastasis patients. J Hematol Oncol. (2022) 15(1):11. doi: 10.1186/s13045-022-01225-3
153. Feng L, Liu Z, Li C, Li Z, Lou X, Shao L, et al. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicentre observational study. Lancet Digit Health. (2022) 4(1):e8–e17. doi: 10.1016/S2589-7500(21)00215-6
154. Zhu J, Lian J, Xu B, Pang X, Ji S, Zhao Y, et al. Neoadjuvant immunotherapy for colorectal cancer: right regimens, right patients, right directions? Front Immunol. (2023) 14:1120684. doi: 10.3389/fimmu.2023.1120684
155. Yamada K, Imaizumi J, Kato R, Takada T, Ojima H. Streamlining robotic-assisted abdominoperineal resection. World J Surg Oncol. (2023) 21(1):392. doi: 10.1186/s12957-023-03260-x
156. Machairas N, Dorovinis P, Kykalos S, Stamopoulos P, Schizas D, Zoe G, et al. Simultaneous robotic-assisted resection of colorectal cancer and synchronous liver metastases: a systematic review. J Robot Surg. (2021) 15(6):841–8. doi: 10.1007/s11701-021-01213-8
157. Kim TY, Cho JH, Choi YS, Kim HK, Kim JG, Shim YM. Surgical strategy for primary colorectal carcinoma and synchronous pulmonary metastasis resection. J Chest Surg. (2022) 55(1):37–43. doi: 10.5090/jcs.21.118
158. Zhan Q, Jiang C. Chromoendoscopy plus mucosal resection versus conventional electrocoagulation for intestinal polyps in children: two case series. J Laparoendosc Adv Surg Tech A. (2018) 28(11):1403–7. doi: 10.1089/lap.2017.0633
159. Gao J, Wang Y, Song J, Li Z, Ren J, Wang P. Negative pressure wound therapy for surgical site infections: a systematic review and meta-analysis. J Adv Nurs. (2021) 77(10):3980–90. doi: 10.1111/jan.14876
160. Slagter JS, Outmani L, Tran KTCK, Ijzermans JNM, Minnee RC. Robot-assisted kidney transplantation as a minimally invasive approach for kidney transplant recipients: a systematic review and meta-analyses. Int J Surg. (2022) 99:106264. doi: 10.1016/j.ijsu.2022.106264
161. Theodorou C, Simpson GS, Walsh CJ. Theatre ventilation. Ann R Coll Surg Engl. (2021) 103(3):151–4. doi: 10.1308/rcsann.2020.7146
162. Niederman MS, Baron RM, Bouadma L, Calandra T, Daneman N, DeWaele J, et al. Initial antimicrobial management of sepsis. Crit Care. (2021) 25(1):307. doi: 10.1186/s13054-021-03736-w
163. Ojima T, Nakamura M, Hayata K, Kitadani J, Katsuda M, Takeuchi A, et al. Short-term outcomes of robotic gastrectomy vs laparoscopic gastrectomy for patients with gastric cancer: a randomized clinical trial. JAMA Surg. (2021) 156(10):954–63. doi: 10.1001/jamasurg.2021.3182
164. Feroz SH, Ahmed A, Muralidharan A, Thirunavukarasu P. Comparison of the efficacy of the Various treatment modalities in the management of perianal Crohn’s Fistula: a review. Cureus. (2020) 12(12):e11882. doi: 10.7759/cureus.11882
165. Solaini L, Cavaliere D, Avanzolini A, Rocco G, Ercolani G. Robotic versus laparoscopic inguinal hernia repair: an updated systematic review and meta-analysis. J Robot Surg. (2022) 16(4):775–81. doi: 10.1007/s11701-021-01312-6
166. Cuk P, Kjær MD, Mogensen CB, Nielsen MF, Pedersen AK, Ellebæk MB. Short-term outcomes in robot-assisted compared to laparoscopic colon cancer resections: a systematic review and meta-analysis. Surg Endosc. (2022) 36(1):32–46. doi: 10.1007/s00464-021-08782-7
167. Marescaux J, Seeliger B. Robotic surgery: a time of change. Updates Surg. (2023) 75(4):793–4. doi: 10.1007/s13304-023-01546-z
168. Sánchez-Velázquez P, Pera M, Jiménez-Toscano M, Mayol X, Rogés X, Lorente L, et al. Postoperative intra-abdominal infection is an independent prognostic factor of disease-free survival and disease-specific survival in patients with stage II colon cancer. Clin Transl Oncol. (2018) 20(10):1321–8. doi: 10.1007/s12094-018-1866-8
169. Allen N, Adam M, O'Regan G, Seery A, McNally C, McConkey S, et al. Outpatient parenteral antimicrobial therapy (OPAT) for aortic vascular graft infection; a five-year retrospective evaluation. BMC Infect Dis. (2021) 21(1):670. doi: 10.1186/s12879-021-06373-4
170. Gong Y, Wang X, Li N, Fu Y, Zheng H, Zheng Y, et al. A partially randomized patient preference trial to assess the quality of life and patency rate after minimally invasive cardiac surgery-coronary artery bypass grafting: design and rationale of the MICS-CABG PRPP trial. Front Cardiovasc Med. (2022) 9:804217. doi: 10.3389/fcvm.2022.804217
171. Tserenpuntsag B, Haley V, Van Antwerpen C, Doughty D, Gase KA, Hazamy PA, et al. Surgical site infection risk factors identified for patients undergoing colon procedures, New York state 2009–2010. Infect Control Hosp Epidemiol. (2014) 35(8):1006–12. doi: 10.1086/677156
172. Rossiter N. Levelling up: prioritisation of global health. Eur J Orthop Surg Traumatol. (2023) 33(3):559–63. doi: 10.1007/s00590-022-03394-w
173. Dwan K, Kirkham J, Paton RW, Morley E, Newton AW, Perry DC. Splinting for the non-operative management of developmental dysplasia of the hip (DDH) in children under six months of age. Cochrane Database Syst Rev. (2022) 10(10):CD012717. doi: 10.1002/14651858.CD012717.pub2
174. Bousis D, Verras GI, Bouchagier K, Antzoulas A, Panagiotopoulos I, Katinioti A, et al. The role of deep learning in diagnosing colorectal cancer. Prz Gastroenterol. (2023) 18(3):266–73. doi: 10.5114/pg.2023.129494
175. Chlorogiannis DD, Verras GI, Tzelepi V, Chlorogiannis A, Apostolos A, Kotis K, et al. Tissue classification and diagnosis of colorectal cancer histopathology images using deep learning algorithms. Is the time ripe for clinical practice implementation? Prz Gastroenterol. (2023) 18(4):353–67. doi: 10.5114/pg.2023.130337
Keywords: colorectal cancer, postoperative incision infection, risk factors, meta-analysis, laparoscopic surgery
Citation: Jia L, Zhao H and Liu J (2024) Meta-analysis of postoperative incision infection risk factors in colorectal cancer surgery. Front. Surg. 11:1415357. doi: 10.3389/fsurg.2024.1415357
Received: 10 April 2024; Accepted: 9 July 2024;
Published: 13 August 2024.
Edited by:
Marta Goglia, Sapienza University of Rome, ItalyReviewed by:
Francesk Mulita, General University Hospital of Patras, GreeceGabriella Distefano, Sapienza University of Rome, Italy
© 2024 Jia, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Jia, MTUwODIzNzU5NTBAMTYzLmNvbQ==
 Li Jia
Li Jia Huacai Zhao2
Huacai Zhao2 Jia Liu
Jia Liu