- Department of Surgery, Dong-A University College of Medicine, Dong-A University Medical Center, Busan, Republic of Korea
Hepatopancreaticoduodenectomy (HPD) is a controversial surgical technique for the treatment of perihilar cholangiocarcinoma. This study aimed to clarify the mortality, morbidity, and survival outcomes in patients with perihilar cholangiocarcinoma who underwent HPD at a small-volume hepatobiliary-pancreatic center. This retrospective study included 78 patients with perihilar cholangiocarcinoma who underwent HPD (n = 18) or major liver resection with bile duct resection (n = 60) at our center between October 2013 and December 2022. The primary endpoints were the in-hospital morbidity and 90-day mortality rates. The secondary endpoints included the recurrence-free and overall survival rates in both groups. Major complications (Clavien–Dindo grade ≥3) were more common in the HPD group (Group 1, 61.1%) than in the major liver resection group (Group 2; 23.3%, p = 0.03). The 1-, 3- and 5-year overall survival rates for Groups 1 and 2 were 66.7%, 41.7%, and 27.8% and 79.9%, 44.5%, and 22.7%, respectively (p = 0.89). The 1-, 3-, and 5-year recurrence-free survival rates for Groups 1 and 2 were 64.2%, 53.5%, and 35.6% and 85.3%, 46.8%, and 25.0%, respectively (p = 0.41). Although morbidity and mortality after HPD are higher than those after other surgeries, our findings suggest that HPD is a feasible treatment option for perihilar cholangiocarcinoma, even in small-volume centers. However, meticulous pre- and perioperative evaluation of the patient's overall health status, quality of life, and prospective advantages are required.
1 Introduction
Although several chemotherapy regimens have been developed for various types of bile-duct cancer, surgical resection remains the definitive cure, and surgical techniques vary according to the location of bile-duct cancer. Hepatectomy is usually performed for intrahepatic cholangiocarcinomas, and bile-duct resection (BDR) or pancreaticoduodenectomy (PD) can be performed for extrahepatic cholangiocarcinoma. However, perihilar cholangiocarcinoma, which tends to spread laterally along the hepatoduodenal ligament, cannot be completely removed using hepatectomy or PD alone. Thus, hepatopancreaticoduodenectomy (HPD), in which hepatectomy and PD are performed simultaneously, is appropriate in selected cases. Introduced by Takasaki et al. (1) in 1980, HPD integrates hepatectomy and PD, facilitating the removal of the total extrahepatic biliary system along with the adjacent liver, pancreas, and duodenum. This procedure was a significant leap in the surgical management of gallbladder cancer and has since been applied to perihilar cholangiocarcinoma with modifications to enhance its efficacy and reduce associated risks (1). While HPD offers a potential for cure in selected cases, its complexity leads to considerable morbidity and mortality, making it a controversial option in the surgical oncology (2).
Although the procedure is controversial, and its use is limited to a handful of centers with advanced hepatobiliary-pancreatic surgical skills, perioperative mortality has notably declined, and survival rates have considerably improved over the years (3–5). These studies underline the necessity of high-volume centers where specialized skills can significantly mitigate the inherent risks associated with complex surgeries. Moreover, the application of HPD in small-volume centers has been explored, providing new insights into its feasibility and the meticulous planning required to manage such high-risk procedures effectively. The retrospective nature of studies conducted in these settings highlights the variability in surgical outcomes and emphasizes the importance of patient selection and perioperative management in improving prognosis (6, 7).
This study aimed to provide new insights into the usefulness of this surgical intervention by focusing on the mortality, morbidity, and survival outcomes of patients who underwent HPD for perihilar cholangiocarcinoma in a small-volume hepatobiliary-pancreatic center over approximately a decade. Furthermore, owing to the small number of HPD cases, the outcomes were compared with those of major liver resection for perihilar cholangiocarcinoma performed during the same period to validate the findings.
2 Methods
This retrospective study included 78 patients with perihilar cholangiocarcinoma who underwent HPD (n = 18) or major liver resection with BDR (n = 60) at Dong-A University Medical Center between October 2013 and December 2022.
The definition of HPD varies across studies. In this study, we defined HPD as a surgical technique in which PD was combined with a major hepatectomy involving more than three Couinaud segments. Major hepatectomy for perihilar cholangiocarcinoma involves concomitant resection of the caudate lobe.
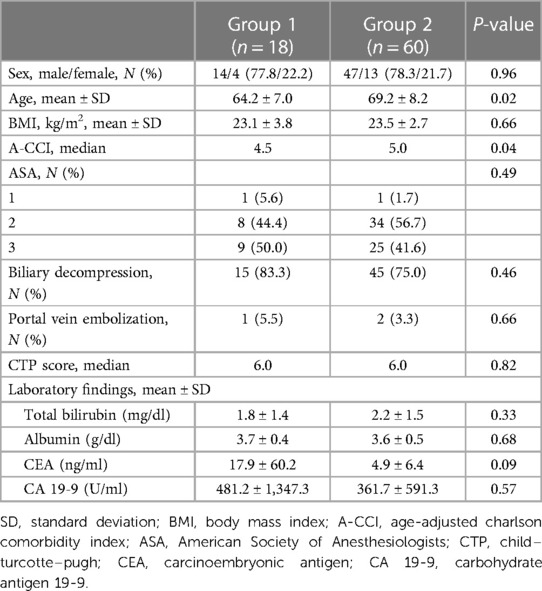
Data regarding patients’ demographic characteristics, comorbidities, American Society of Anesthesiologists score, pre- and post-operative laboratory test results, Child–Turcotte–Pugh score, operative findings, post-operative complications, age-adjusted Charlson comorbidity index (A-CCI) (8), and mortality were extracted from the hospital's medical records. The primary endpoints were the in-hospital morbidity and 90-day mortality rates. The secondary endpoints included the recurrence-free and overall survival rates in both groups.
In patients with jaundice or with a preoperative total bilirubin level >3 mg/dl, preoperative biliary decompression (percutaneous transhepatic bile drainage, endoscopic retrograde biliary drainage, or endoscopic nasobiliary drainage) was performed. Portal vein embolization (PVE) using the percutaneous portal venous approach was performed in patients at risk of post-hepatectomy liver failure (PHLF) due to low future remnant liver volume.
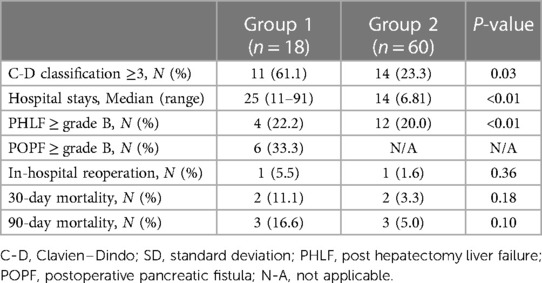
Liver failure was classified according to the International Study Group of Liver Surgery criteria (9); “no liver failure” was defined as grade A, whereas PHLF included grades B and C. Post-operative pancreatic fistula (POPF) was defined according to the International Study Group of Pancreatic Fistula criteria (10). Only grades B and C were included in the pancreatic fistula group. Post-operative complications were evaluated using the Clavien–Dindo classification (11), and major morbidity included grade III–V complications.
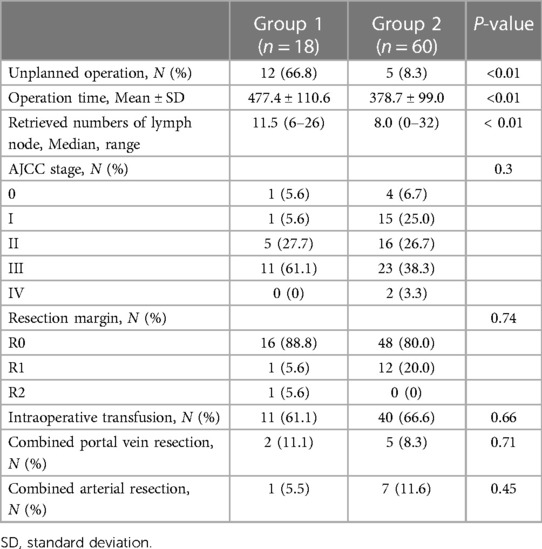
Resection margins were categorized as R0 (margin-to-tumor distance ≥1 mm), R1 (margin-to-tumor distance <1 mm), or R2 (macroscopically positive margin) (12). The tumor stage was classified according to the American Joint Committee on Cancer TNM 8th edition system (13).
This study was approved by the Institutional Review Board of Dong-A University Medical Center (No. DAUHIRB-23-140, dated August 2, 2023). The requirement for informed consent was waived because of the retrospective nature of the study.
2.1 Surgical procedure
A reversed L-shaped incision was made for laparotomy. Individual hilar dissection and extensive lymph node dissection, including lymph node 16, were performed. Additionally, a frozen distal-bile-duct biopsy was performed to determine the need for PD before proceeding with routine PD. During hepatoduodenal ligament dissection, if major vessel invasion was suspected and seemed resectable, concurrent vessel resection and anastomosis were performed. To facilitate liver resection, we used the hanging maneuver of the liver and a Cavitron Ultrasonic Surgical Aspirator (CUSA, Tyco Healthcare, Mansfield, MA, USA). Subsequently, pancreatojejunostomy, hepaticojejunostomy, duodenojejunostomy, and gastrojejunostomy reconstruction were sequentially performed. All surgical procedures were performed by an experienced hepatobiliary surgeon.
2.2 Statistics
Data were analyzed using statistical software (IBM SPSS for Windows, Version 26.0; Chicago, IL, USA). Quantitative variables are expressed as mean ± standard deviation and were compared using Student's t-test or the Wilcoxon–Mann–Whitney test. Using chi-square or Fisher's exact tests, other quantitative variables were expressed as numbers and percentages. Kaplan–Meier analysis was used to estimate the survival and recurrence rates from the time of surgery. Statistical significance was set at p < 0.05.
3 Results
3.1 Patient characteristics
During the study period, 18 (23.1%) and 60 (76.9%) patients underwent HPD (Group 1) and major liver resection with BDR (Group 2) for perihilar cholangiocarcinoma, respectively.
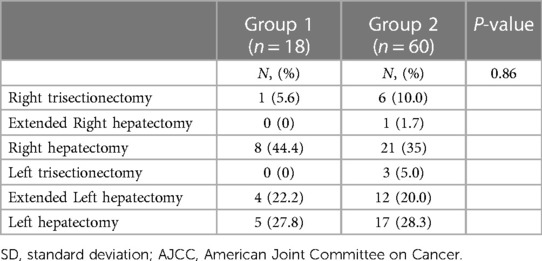
The preoperative clinical characteristics of all 78 patients are presented in Table 1, and the extent of hepatectomy for perihilar cholangiocarcinoma in each group is presented in Table 2. The mean age in the two groups was 64.2 and 69.2 years, respectively (p = 0.02). Biliary decompression and PVE to increase future remnant liver volume were performed in 15 (83.3%) and 45 (75%) and one (5.5%) and two (3.3%) patients in Groups 1 and 2, respectively.
In 12 (66.8%) cases, HPD was not planned before the surgery (Table 3), and the surgical strategy was altered based on intraoperative frozen biopsy results. The mean operation time was 477.4 ± 110.6 and 378.7 ± 99.0 min in Groups 1 and 2, respectively (p < 0.01), and a median of 11.5 and 8.0 lymph nodes were retrieved in Groups 1 and 2, respectively (p < 0.01). The cancer stage was not significantly different between the groups (p = 0.30). R0 resection was achieved in 16 (88.9%) and 48 (80%) patients in Groups 1 and 2, respectively (p = 0.74). No statistically significant intergroup differences were observed in intraoperative transfusion requirements or concurrent resection of the portal and arterial vessels.
Major complications (Clavien–Dindo Grade ≥3) were more common in Group 1 than in Group 2 (61.1% vs. 23.3%; p = 0.03) (Table 4). The most frequent major complication was abnormal fluid collection in the abdominal cavity (e.g., bile, pancreatic juice, or ascites), for which ultrasonography-guided drainage was performed. The average hospital stay was also longer in Group 1 than in Group 2 (25.0 vs. 14.0 days; p < 0.01). The incidence of PHLF (grades B and C) was not significantly different between the groups (p = 0.83). Grade B and C POPFs occurred in six patients (33.3%) in Group 1. One patient in each group required reoperation. In Group 1, the cause of reoperation was bleeding from the gastroduodenal artery due to pancreatojejunostomy leakage, and in Group 2, the cause of reoperation was wound evisceration.
The 30- and 90-day mortality rates in Groups 1 and 2 were 11.1% (n = 2) and 3.3% (n = 2) and 16.6% (n = 3) and 5% (n = 3), respectively, and the differences were not statistically significant. In Group 1, the causes of 90-day mortality were post-operative acute respiratory distress syndrome (n = 1), pancreatojejunostomy leakage (n = 1), and liver failure (n = 1). In Group 2, the causes of 90-day mortality were liver failure (n = 2) and multi-organ failure (n = 1).
3.2 Survival outcomes
The 1-, 3-, and 5-year overall survival rates in Groups 1 and 2 were 66.7%, 41.7%, and 27.8% and 79.9%, 44.5%, and 22.7%, respectively. The overall survival rates did not differ significantly between the two groups (p = 0.89; Figure 1A). The 1-, 3-, and 5-year recurrence-free survival rates in Groups 1 and 2 were 64.2%, 53.5%, and 35.6% and 85.3%, 46.8%, and 25.0%, respectively. The recurrence-free survival rates did not differ significantly between the two groups (p = 0.41; Figure 1B).
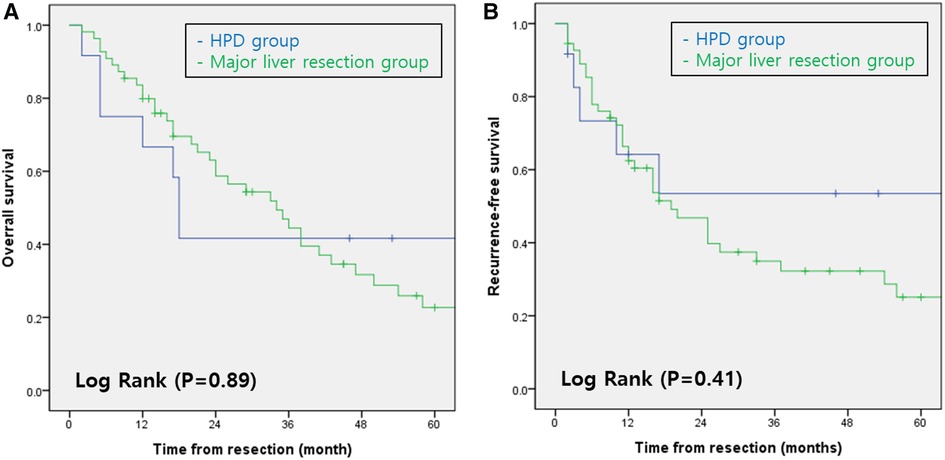
Figure 1. (A) overall survival rates of patients with perihilar cholangiocarcinoma according to type of surgery. (B) Recurrence-free survival rates of patients with perihilar cholangiocarcinoma according to type of surgery. HPD, Hepatopancreaticoduodenectomy.
The overall survival rates for perihilar cholangiocarcinoma according to R status in both groups are shown in Figure 2. The 5-year overall survival rates according to the R status in Group 1 were 50% and 0% for R0 and R1, respectively (Figure 2A). The 5-year overall survival rates according to the R status in Group 2 were 29.2% and 0% for R0 and R1, respectively (Figure 2B). Patient outcomes after R1 resection were extremely poor in both groups (p < 0.01).
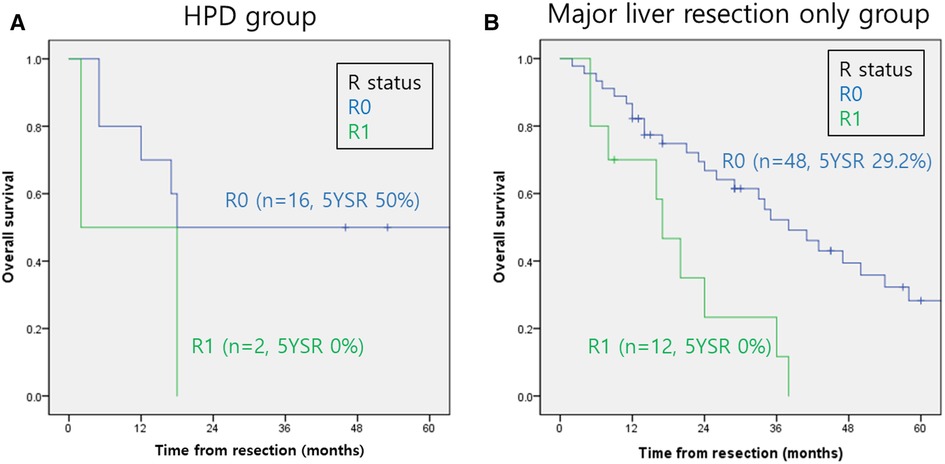
Figure 2. Overall survival rates of perihilar cholangiocarcinoma according to the R status. (A) HPD group. (B) Major liver resection only group. HPD, Hepatopancreaticoduodenectomy.
4 Discussion
In this study, comparing groups 1 and 2 validated the surgical and oncological outcomes of HPD despite the small number of cases of perihilar cholangiocarcinoma in Group 1. Theoretically, HPD, which merges major hepatectomy with PD, is the sole curative option for bile-duct carcinoma that has extensively spread horizontally, afflicting both the hepatic hilum and the intrapancreatic bile duct (6).
In this study, HPD was not anticipated preoperatively in 12 patients, which represents 66.8% of cases. This rate exceeds what has been reported in the literature, where unplanned HPD occurs in 25%–46% of cases as shown by D'Souza et al. (7) and Aoki et al. (14). The preoperative anticipation of HPD presents significant difficulties owing to the nature of tumor proliferation, which tends to follow a vertical path along the mucosal lining of the bile duct (2). Despite advancements in preoperative imaging studies, these details often remain hidden and are typically revealed intraoperatively through a frozen-section biopsy. These considerations explain why there were many unplanned surgeries and only one case of preoperative PVE in this study.
Although the occurrence of unplanned HPD in our study was higher than in prior reports, the associated complications such as PHLF (22.2%), POPF (33.3%), in-hospital morbidity (5.5%) and 90-day mortality (16.6%) aligned with previous studies (6, 7, 15). PHLF is an important predictor of mortality after perihilar cholangiocarcinoma resection. we observed PHLF in 22.2% of patients in Group 1% and 20% in Group 2, which sits within the reported variability of 4%–66% seen in similar procedures. Ebata et al. (5) and Aoki et al. (14) have similarly reported variable rates, which suggests that differing definitions of PHLF could impact the reported incidence across studies.
Furthermore, we encountered a 33.3% rate of POPF in our HPD procedures, reflecting the complex nature of the surgery. This is somewhat higher than the approximately 20% incidence for clinically significant POPF (grades B and C) following PD, as delineated by the criteria from the International Study Group of Pancreatic Fistula (16, 17).
The risk of POPF is higher in patients with soft pancreatic texture and narrow pancreatic ducts, particularly among patients who undergo HPD. POPF is associated with increased morbidity and mortality after PD. Unfortunately, we encountered one case each of mortality due to PHLF and POPF in Group 1.
Patients in Group 1 were younger and had a lower mean A-CCI than did those in Group 2. The differences in age (p = 0.02) and A-CCI (p = 0.04) could be attributed to the surgeon’s selection of healthier patients to improve the surgical outcomes of HPD, which is complex and is associated with higher morbidity and mortality. Therefore, owing to the frequency of unplanned HPD, age and A-CCI are essential factors that must be considered when deciding to proceed with HPD. The longer operation time and larger lymph node retrieval numbers in Group 1 were expected and are indicative of the complexity of the HPD. Furthermore, the higher incidence of major complications and longer mean duration of hospital stay in Group 1 (Table 4) were expected, demonstrating the increased demand for post-operative care and the potential need for more comprehensive management strategies for patients undergoing HPD.
Despite the small cohort in Group 1, the lack of significant intergroup differences in the overall and recurrence-free survival rates suggests that this surgery has a place in the treatment protocol, despite the complexity of HPD (Figure 1). The overall and recurrence-free survival rates for perihilar cholangiocarcinoma, regardless of the surgery type, are consistent with those in several other studies (14, 18–20). This validates the results of this study despite its small sample size.
Because the risk factors affecting the prognosis of perihilar cholangiocarcinoma, such as tumor invasion depth (21), lymph-node metastasis (22), and the degree of histologic differentiation and recurrence (19), are well established, they were not specifically investigated in this study. However, a clear resection margin (R0) is the most important prognostic factor affecting long-term survival rates (15, 19). In this study, the dismal prognosis following R1 resection across groups reinforced the critical need to achieve negative margins (Figure 2). Despite the small number of cases, these results suggest that HPD is a justifiable treatment for perihilar cholangiocarcinoma, as the survival outcomes did not differ significantly from those of major liver resection with BDR. This indicates that the additional risks and complexities of HPD are counterbalanced by its potential to improve survival outcomes.
The higher complication rates and longer duration of hospital stay in Group 1 highlight the need for careful patient selection and perhaps more aggressive perioperative management. When discussing treatment options and the potential risks and benefits of HPD, these findings should be communicated to patients and their families.
Owing to the retrospective nature of this study and the small-volume center data, further studies with larger sample sizes are required to confirm the findings of this study.
5 Conclusions
Although the morbidity and mortality after HPD are higher than those after other surgeries, our findings suggest that HPD is a feasible treatment option for perihilar cholangiocarcinoma, even in small-volume centers. However, meticulous pre- or perioperative evaluations of the patient's overall health status and prospective advantages are required.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by this study was approved by the Institutional Review Board of Dong-A University Medical Center (No. DAUHIRB-23-140, dated August 2, 2023). The requirement for informed consent was waived because of the retrospective nature of the study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft. EJ: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – review & editing. SK: Project administration, Supervision, Writing – review & editing. EP: Project administration, Writing – review & editing. KK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Dong-A University Research Fund. The Dong-A University research fund had no part in the design, execution, analysis, or writing of the study or the decision to submit it for publication. No other entity had a role in funding, writing, designing, analyzing, or sponsoring the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Takasaki K, Kobayashi S, Muto S. Our experience (5 cases) of extended right lobectomy combined with pancreatoduodenectomy for carcinoma of the gallbladder. Tan to Sui. (1980) 1:923–32.
2. Fancellu A, Sanna V, Deiana G, Ninniri C, Turilli D, Perra T, et al. Current role of hepatopancreatoduodenectomy for the management of gallbladder cancer and extrahepatic cholangiocarcinoma: a systematic review. World J Gastrointest Oncol. (2021) 13:625–37. doi: 10.4251/wjgo.v13.i6.625
3. Endo I, Hirahara N, Miyata H, Yamamoto H, Matsuyama R, Kumamoto T, et al. Mortality, morbidity, and failure to rescue in hepatopancreatoduodenectomy: an analysis of patients registered in the national clinical database in Japan. J Hepatobiliary Pancreat Sci. (2021) 28:305–16. doi: 10.1002/jhbp.918
4. Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Nagino M. Review of hepatopancreatoduodenectomy for biliary cancer: an extended radical approach of Japanese origin. J Hepatobiliary Pancreat Sci. (2014) 21:550–5. doi: 10.1002/jhbp.80
5. Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y, et al. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg. (2012) 256:297–305. doi: 10.1097/SLA.0b013e31826029ca
6. Shimizu A, Motoyama H, Kubota K, Notake T, Fukushima K, Ikehara T, et al. Safety and oncological benefit of hepatopancreatoduodenectomy for advanced extrahepatic cholangiocarcinoma with horizontal tumor spread: Shinshu University experience. Ann Surg Oncol. (2021) 28:2012–25. doi: 10.1245/s10434-020-09209-8
7. D’Souza MA, Valdimarsson VT, Campagnaro T, Cauchy F, Chatzizacharias NA, D’Hondt M, et al. Hepatopancreatoduodenectomy -a controversial treatment for bile duct and gallbladder cancer from a European perspective. HPB (Oxford). (2020) 22:1339–48. doi: 10.1016/j.hpb.2019.12.008
8. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
9. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the international study group of liver surgery (ISGLS). Surgery. (2011) 149:713–24. doi: 10.1016/j.surg.2010.10.001
10. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. (2017) 161:584–91. doi: 10.1016/j.surg.2016.11.014
11. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
12. Durán M, Briceño J, Padial A, Anelli FM, Sánchez-Hidalgo JM, Ayllón MD, et al. Short-term outcomes of robotic liver resection: an initial single-institution experience. World J Hepatol. (2022) 14:224–33. doi: 10.4254/wjh.v14.i1.224
13. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging: the eighth edition AJCC cancer staging manual. CA Cancer J Clin. (2017) 67:93–9. doi: 10.3322/caac.21388
14. Aoki T, Sakamoto Y, Kohno Y, Akamatsu N, Kaneko J, Sugawara Y, et al. Hepatopancreaticoduodenectomy for biliary cancer: strategies for near-zero operative mortality and acceptable long-term outcome. Ann Surg. (2018) 267:332–7. doi: 10.1097/SLA.0000000000002059
15. Kawano F, Yoshioka R, Ichida H, Mise Y, Saiura A. Essential updates 2021/2022: update in surgical strategy for perihilar cholangiocarcinoma. Ann Gastroenterol Surg. (2023) 7:848–55. doi: 10.1002/ags3.12734
16. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. (2013) 216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002
17. Mungroop TH, van Rijssen LB, van Klaveren D, Smits FJ, van Woerden V, Linnemann RJ, et al. Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg. (2019) 269:937–43. doi: 10.1097/SLA.0000000000002620
18. Liu F, Hu HJ, Ma WJ, Wang JK, Ran CD, Regmi P, et al. Is radical resection of hilar cholangiocarcinoma plus partial resection of pancreatic head justified for advanced hilar cholangiocarcinoma? ANZ J Surg. (2020) 90:1666–70. doi: 10.1111/ans.15955
19. Kang MJ, Jang JY, Chang J, Shin YC, Lee D, Kim HB, et al. Actual long-term survival outcome of 403 consecutive patients with hilar cholangiocarcinoma. World J Surg. (2016) 40:2451–9. doi: 10.1007/s00268-016-3551-9
20. DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution: thirty-one-year experience with 564 patients at a single institution. Ann Surg. (2007) 245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3
21. Oba M, Nakanishi Y, Amano T, Okamura K, Tsuchikawa T, Nakamura T, et al. Stratification of postoperative prognosis by invasive tumor thickness in perihilar cholangiocarcinoma. Ann Surg Oncol. (2021) 28:2001–9. doi: 10.1245/s10434-020-09135-9
Keywords: perihilar cholangiocarcinoma, hepatectomy, pancreatoduodenectomy, overall survival rates, recurrence-free survival rates
Citation: Shin SY, Jang EJ, Kang SH, Park EH and Kim KW (2024) Advancing treatment for perihilar cholangiocarcinoma: role of hepatopancreaticoduodenectomy in small-volume centers. Front. Surg. 11:1406508. doi: 10.3389/fsurg.2024.1406508
Received: 25 March 2024; Accepted: 30 April 2024;
Published: 14 May 2024.
Edited by:
Francesco Ardito, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Muhammer Ergenç, Marmara University, TürkiyeTevfiktolga Sahin, İnönü University, Türkiye
© 2024 Shin, Jang, Kang, Park and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kwan Woo Kim, ZDAwMjA0NUBuYXZlci5jb20=
 Sang Yun Shin
Sang Yun Shin Eun Jeong Jang
Eun Jeong Jang Kwan Woo Kim
Kwan Woo Kim