- Department of Thoracic Surgery, Xuzhou Medical University Affiliated Hospital Sihong Branch, The First People's Hospital of Sihong County, Suqian, Jiangsu, China
Lipopolysaccharide (LPS) is related to atrial fibrillation (AF). But so far, the relationship between LPS and new-onset AF (NOAF) in patients with lung cancer is unrevealed. This study was to investigate the association between LPS and NOAF in patients after lung cancer surgery. This was a single-center retrospective clinical observational study. Patients diagnosed with non-small-cell lung cancer (NSCLC) were enrolled. All patients receiving lung cancer surgery and at least 24 h electrocardiogram (ECG) examination was recorded during the hospitalization. The incidence of NOAF in this study was 34/406 (8.4%). The univariate analysis showed that NOAF was associated with age, intraoperative blood transfusion (IBT), chronic obstructive pulmonary disorder (COPD), and LPS. After adjusting risk factors, it was found that age, IBT and LPS (OR, 1.031; 95% CI: 1.001–1.042; P = 0.002) were still risk factors for NOAF. The area under curve (AUC) value was 0.709 for the LPS. When the LPS was added to the conventional model, the Net reclassification index (NRI) and integrated discrimination index (IDI) were improved significantly. Elevated LPS is associated with an increased risk of NOAF in patients after lung cancer surgery. LPS contributed to the discrimination of the NOAF risk model and improved it markedly.
1 Introduction
Cardiovascular diseases (CVDs) are one of the leading causes of death in the population worldwide (1). As one of the cardiovascular disease challenges in the 21st century, atrial fibrillation (AF) is the most common tachyarrhythmia in clinical practice, which can increase the risk of heart failure, stroke and death (2). Recent studies have shown that the prevalence of AF in cancer patients is significantly higher than in the general population. In postoperative lung cancer patients, the prevalence is as high as 8%–42% (1, 3). This is related to a variety of reasons such as cardiac injury and body stress response, in which cancer-related systemic inflammation promotes atrial remodeling and is closely associated with the development of AF (4, 5). Notably, patients with postoperative atrial fibrillation after lung cancer have a poorer prognosis and a higher mortality rate (1). In addition to this, previous studies have shown that patients with a history of cancer are less likely to be treated according to AF guideline recommendations (6, 7). Therefore, exploring more risk factors for the development of atrial fibrillation after lung cancer surgery is of clinical value.
Lipopolysaccharide (LPS), as a component of intestinal flora, has been shown to play an important role in several cardiovascular diseases (8). It is well known that inflammation is one of the central mechanisms of AF, and LPS is a potent trigger of systemic inflammation. A previous animal study showed that LPS activated toll-like receptor 4 (TLR4), which promotes the expression of inflammatory factors, leading to the development of arrhythmias (9). In elderly patients, elevated LPS could promote the development of AF by activating atrial nod-like receptor protein (NLRP)-3 inflammasome (10). Recently, Wang M et al. found that elevated LPS was associated with systemic inflammation and fibrosis biomarkers in patients undergoing AF ablation and was an independent risk factor for AF recurrence during one-year follow-up (11). Notably, LPS could also increase the risk of new-onset AF (NOAF) after cardiac surgery (12, 13). However, the relationship between LPS and new-onset atrial fibrillation (NOAF) after lung cancer surgery has not been revealed. The aim of this study was to evaluate the relationship between LPS and NOAF in postoperative patients with non-small cell lung cancer (NSCLC).
2 Methods
2.1 Study population
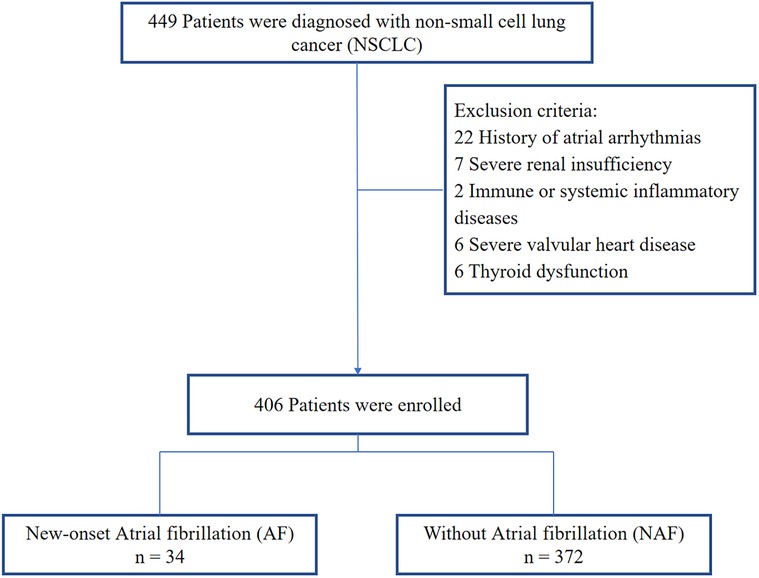
This was a single-center retrospective clinical observational study. We consecutively included patients with NSCLC diagnosed in the Xuzhou Medical University Affiliated Hospital Sihong Branch from January 2020 to December 2023 (14). The Ethics Committee of the Xuzhou Medical University Affiliated Hospital Sihong Branch reviewed this study. All methods were performed in accordance with relevant guidelines and regulations, and written consent was waived owing to the minimal patient risk. Inclusion criteria: lung cancer received surgical treatment; received at least 24 h of cardiac monitoring during hospitalization. Exclusion criteria: history of atrial arrhythmias, severe renal insufficiency, immune or systemic inflammatory diseases, severe valvular heart disease, thyroid dys function. Ultimately, 406 patients who met the eligibility criteria were enrolled (Figure 1).
2.2 Data collection and definition of NOAF
We collected all baseline clinical information of enrolled patients, including sex, age, body mass index (BMI), smoking, and past medical history. Fasting venous blood was centrifuged and stored at −80°C until biochemical assays were performed. Serum LPS levels were determined using a commercial ELISA kit (Cusabio, Wuhan, China). LPS standards purified from E. coli and blood samples were incubated for 2 h at room temperature and then dropped onto microtiter plates pre-coated with LPS-specific antibodies. After incubation, samples were read at 450 nm. Intra-assay precision was <8% and inter-assay precision was <10%. Values are expressed in pg/ml. Postoperative heart rates were recorded for all patients. NOAF was defined as at least a 30 s run of AF on electrocardiogram (ECG)/telemetry and no history of AF (15).
2.3 Statistical analysis
Statistical analysis was performed using SPSS 26.0 and R software. Kolmogorov–Smirnov method was used for normality test (16). Measures obeying normal distribution were expressed as mean ± standard deviation (SD), and statistical analysis was performed by unpaired t-test; measures not normally distributed were expressed as median (Q75, Q75), and statistical analysis was performed by Mann–Whiney U-test. Count data were expressed as frequencies or percentages and statistically analyzed using the χ2 test. Statistically significant variables were included in logistic regression analysis using stepwise forward method was used to analyze the risk factors for NOAF. Receiver operating characteristic (ROC) was used to analyze the predictive value of lipopolysaccharide for NOAF. DeLong test was used to compare the performance of ROC. The net reclassification index (NRI) was calculated based on a comparison between the model's predictions and actual observations (17). NRI and integrated discrimination index (IDI) were used to assess the additional discriminatory power of risk markers. P < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
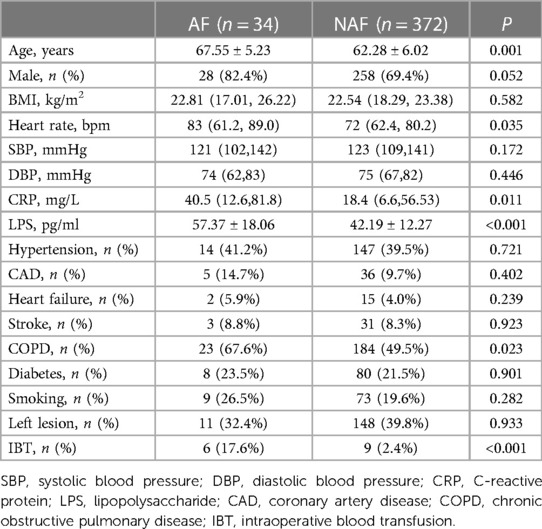
The incidence of NOAF in this study was 34/406 (8.4%). Compared with the NAF group, patients with AF were older [(67.55 ± 5.23) years vs. (62.28 ± 6.02) years; P = 0.001], with higher C-reactive protein (CRP) [40.5 (12.6, 81.8) mg/L vs. 18.4 (6.6, 56.53) mg/L; P = 0.011], heart rate [83 (61.2, 89.0) bpm vs. 72 (62.4, 80.2) bpm; P = 0.035] and LPS [(57.37 ± 18.06) ng/ml vs. (42.19 ± 12.27) ng/ml; P < 0.001] were significantly higher, with statistically significant differences. Compared with the NAF group, there were significantly higher proportions of chronic obstructive pulmonary disease (COPD) (67.6% vs. 49.5%; P = 0.023) and intraoperative blood transfusion (IBT) (17.6% vs. 2.4%; P < 0.001) in patients with AF, and the difference was statistically significant (Table 1).
3.2 Univariate and multivariate logistic regression analysis
All variables were included in univariate regression analysis, the result showed that NOAF was associated with age, COPD, IBT, and LPS. Inclusion of these variables in multivariate regression analysis using the stepwise forward method revealed that age (OR, 1.102; 95% CI: 1.035–1.180; P = 0.001), IBT (OR, 4.211; 95% CI: 1.177–11.518; P = 0.037) and LPS (OR, 1.031; 95% CI: 1.001–1.042; P = 0.002) were independent risk factors for NOAF during hospitalization in postoperative patients with NSCLC (Table 2).
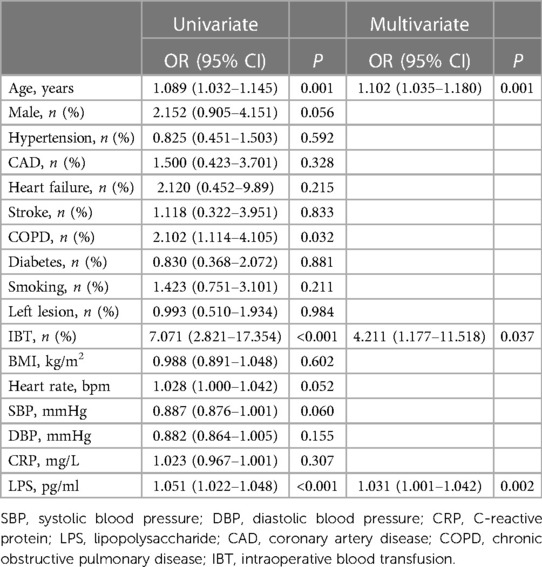
Table 2. Univariate and multivariate logistic regression analysis for new-onset atrial fibrillation.
3.3 Predictive value of lipopolysaccharide for NOAF
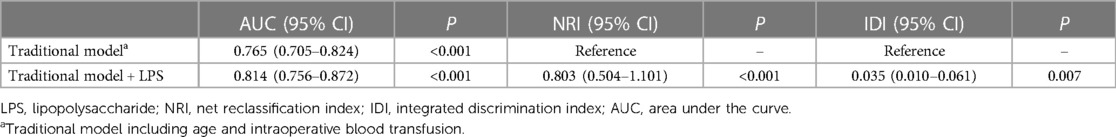
After incorporating age, IBT and LPS into the ROC, the area under the curve (AUC) values were found to be 0.733 for age, 0.626 for IBT and 0.709 for LPS (Supplementary Figures A–C). The AUC was found to be 0.765 after the joint inclusion of age and IBT in the ROC, and the AUC was 0.814 after the addition of LPS (P < 0.001) (Figure 2). DeLong test showed that the diagnostic performance of ROC integrating LPS for NOAF was significantly improved (P = 0.010). Based on the results of the multifactorial regression analysis, a traditional model including age and IBT was constructed, and significant improvements in IDI and NRI were observed when LPS was added to the traditional model (Table 3).
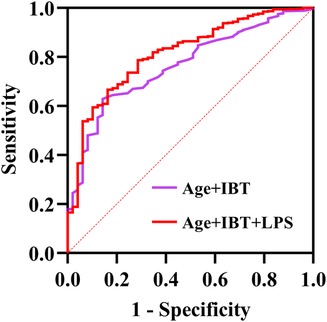
Figure 2. Receiver operating characteristics (ROC) regarding new-onset AF after surgery for non-small cell lung cancer (DeLong test P-value was 0.010). AUC, area under the curve; IBT, intraoperative blood transfusion; LPS, lipopolysaccharide.
4 Discussion
To our knowledge, this was the first study on the association between NOAF and LPS in postoperative lung cancer patients. The main findings of this study were: higher LPS was associated with the development of NOAF, and it had a better predictive value for NOAF in postoperative patients with NSCLC. In addition, integration of LPS could significantly improve on the NOAF risk model.
Atrial fibrillation (AF) is one of the most common arrhythmias after pulmonary surgery and is associated with increased mortality, hospitalization time and costs (18, 19). Its pathogenesis is complex and may be related to multiple factors. A notable feature of lung stripping is that after removal of one side of the lung, all pulmonary circulation is forced to flow through the remaining pulmonary vessels, increasing the pressure in the pulmonary veins, which may promote the development of AF. In addition, activation of inflammation can play a further role (20). Previous studies have shown that postoperative NOAF in lung cancer was strongly associated with age (21), heart rate (22), hs-CRP (23), IBT (24) and COPD (25). Consistent with these studies, we found that patients with NOAF were older, had higher hs-CRP, heart rate, and LPS, and greater proportions of COPD and IBT compared with the NAF group. In addition, age and IBT were still found to be associated with NOAF after adjustment for the above risk factors. A previous study found that IBT was a predictor of postoperative NOAF in lung cancer, which is consistent with our findings (3). A possible explanation for this is that an inflammatory immune response is generated by iron and particles in damaged erythrocytes during transfusion (20). Age had been widely recognized as a risk factor for the development of NOAF in patients after surgical procedures (21). Amar et al. (26) evaluated 527 patients who underwent thoracic surgery and they found that patients aged 60 years and older had a significantly higher risk of developing AF. In addition, we innovatively found that LPS was significantly associated with postoperative NOAF in NSCLC patients.
LPS is a cell wall component of Gram-negative bacteria that can cause several cardiovascular diseases once it crosses the intestinal mucosa and enters the body circulation (27). In a prospective study, Pastori et al. (28) found that LPS was significantly associated with major adverse cardiovascular events in patients with AF through increased platelet activation. In addition to this, the association between LPS and AF has been confirmed by studies in patients undergoing radiofrequency ablation of AF as well as in patients undergoing cardiac surgery (11–13). Consistent with these results, we found that LPS and NOAF remained significantly correlated after adjusting for possible confounders. This may be related to the following reasons. It is well known that inflammatory response and oxidative stress were strongly associated with AF, which had likewise been confirmed by many studies in postoperative lung cancer patients (29, 30). It was found that LPS was significantly associated with hs-CRP and IL-6 (11). In an animal study, LPS-TLR4 interaction was revealed to be an important mechanism leading to systemic inflammation and increasing the risk of AF (31). In addition, Menichelli et al. (32) demonstrated in the ATHERO-AF study that LPS can lead to an impaired antioxidant status of the organism. An experimental study have shown that LPS-treated bovine epithelial cells displayed a significant reduction in antioxidant enzyme activities (33). Indeed, LPS has been shown to be involved in multiple pathways leading to increased production of reactive oxygen species in different cardiac diseases (34). These studies suggest that inflammatory responses and oxidative stress may be important mechanisms by which LPS triggers AF. Electrical remodeling and structural remodeling are central mechanisms for the development and maintenance of AF. An animal experiments showed that LPS increased the expression of inflammatory cytokines and l-type calcium channel proteins and shortened the effective atrial occlusion period, thereby promoting the development of AF (30). It has also been reported that LPS, as a potential trigger, could directly alter the function of ionic currents in cardiomyocytes, which together form an arrhythmia axis (35, 36). TGF-β1 is an important marker of atrial fibrosis. Wang M et al. (11) demonstrated a positive correlation between LPS and TGF-β1, and they also found a correlation between the level of LPS and the diameter of the atria. These results may further support that LPS induced NOAF by promoting electrical remodeling and structural remodeling. The present study also found that LPS has a satisfactory predictive value for NOAF, with significant improvements in both IDI and NRI after the addition of LPS to a conventional model about NOAF. In the clinic, the fact that LPS measurements are accurate, reproducible, and available at a reasonable cost seems to provide additional information for NOAF risk assessment in postoperative lung cancer patients.
This study has some limitations. Firstly, it was a single-center retrospective study, which may have some unavoidable bias. Second, this study was conducted on non-small cell lung cancer patients, so the findings may not be applicable to all patients undergoing lung surgery. Third, only patients who received at least 24 h of ECG monitoring were included in this study, which may have introduced some bias. Fourth, although we found that LPS and NOAF were associated, the exact mechanism remains unclear, which may require more basic research to elucidate.
5 Conclusion
Elevated LPS is associated with new-onset atrial fibrillation. The addition of LPS can increase the predictability of NOAF in postoperative patients with non-small cell lung cancer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Xuzhou Medical University Affiliated Hospital Sihong Branch. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because written consent was waived owing to the minimal patient risk.
Author contributions
HX: Writing – original draft. JZ: Writing – review & editing. FY: Writing – original draft. YG: Writing – original draft.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
Suqian guiding science and technology plan project (Z202347).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1404450/full#supplementary-material
Supplementary Figure
Receiver operating characteristics (ROC) regarding new-onset AF after surgery for non-small cell lung cancer. (A) Predictive value of intraoperative blood transfusion for new-onset AF; (B) Predictive value of age for new-onset AF; (C) Predictive value of lipopolysaccharide for new-onset AF. AUC, area under the curve.
References
1. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS) [published correction appears in Eur Heart J. 2023 May 7;44(18):1621]. Eur Heart J. (2022) 43(41):4229–361. doi: 10.1093/eurheartj/ehac244
2. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. (2014) 6:213–20. doi: 10.2147/CLEP.S47385
3. Imperatori A, Mariscalco G, Riganti G, Rotolo N, Conti V, Dominioni L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. (2012) 7:4. doi: 10.1186/1749-8090-7-4
4. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. (2015) 12(10):584–96. doi: 10.1038/nrclinonc.2015.105
5. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
6. O’Neal WT, Claxton JS, Sandesara PB, MacLehose RF, Chen LY, Bengtson LGS, et al. Provider specialty, anticoagulation, and stroke risk in patients with atrial fibrillation and cancer. J Am Coll Cardiol. (2018) 72(16):1913–22. doi: 10.1016/j.jacc.2018.07.077
7. Rohrmann S, Witassek F, Erne P, Rickli H, Radovanovic D. Treatment of patients with myocardial infarction depends on history of cancer. Eur Heart J Acute Cardiovasc Care. (2018) 7(7):639–45. doi: 10.1177/2048872617729636
8. Shen X, Li L, Sun Z, Zang G, Zhang L, Shao C, et al. Gut microbiota and atherosclerosis-focusing on the plaque stability. Front Cardiovasc Med. (2021) 8:668532. doi: 10.3389/fcvm.2021.668532
9. Ghosh S, Lertwattanarak R, Garduño JDJ, Galeana JJ, Li J, Zamarripa F, et al. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol A Biol Sci Med Sci. (2015) 70:232–46. doi: 10.1093/gerona/glu067
10. Zhang Y, Zhang S, Li B, Luo Y, Gong Y, Jin X, et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc Res. (2022) 118(3):785–97. doi: 10.1093/cvr/cvab114
11. Wang M, Xiong H, Lu L, Zhu T, Jiang H. Serum lipopolysaccharide is associated with the recurrence of atrial fibrillation after radiofrequency ablation by increasing systemic inflammation and atrial fibrosis. Oxid Med Cell Longev. (2022) 2022:2405972. Published 2022 Oct 15. doi: 10.1155/2022/2405972
12. Liu H, Zhang B, Chen S, Zhang Y, Ye X, Wei Y, et al. Identification of ferroptosis-associated genes exhibiting altered expression in response to cardiopulmonary bypass during corrective surgery for pediatric tetralogy of fallot. Sci Prog. (2021) 104(4):368504211050275. doi: 10.1177/00368504211050275
13. Seo EJ, Hong J, Lee HJ, Son YJ. Perioperative risk factors for new-onset postoperative atrial fibrillation after coronary artery bypass grafting: a systematic review. BMC Cardiovasc Disord. (2021) 21:418. doi: 10.1186/s12872-021-02224-x
14. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2017) 15(4):504–35. doi: 10.6004/jnccn.2017.0050
15. Lasek-Bal A, Gąsior Z, Kowalewska-Twardela T, Urbanek T. New-onset atrial fibrillation in patients with elevated troponin I levels in the acute phase of stroke. Int J Cardiol. (2015) 195:210–1. doi: 10.1016/j.ijcard.2015.05.117
16. Habibzadeh F. Data distribution: normal or abnormal? J Korean Med Sci. (2024) 39(3):e35. Published 2024 Jan 22. doi: 10.3346/jkms.2024.39.e35
17. Grunkemeier GL, Jin R. Net reclassification index: measuring the incremental value of adding a new risk factor to an existing risk model. Ann Thorac Surg. (2015) 99(2):388–92. doi: 10.1016/j.athoracsur.2014.10.084
18. Rozencwajg S, Desthieux C, Szymkiewicz O, Ynineb Y, Fulgencio JP, Bonnet F. The risk of atrial fibrillation after pneumonectomy is not impaired by preoperative administration of dexamethasone. A cohort study. Anaesth Crit Care Pain Med. (2017) 36(3):185–89. doi: 10.1016/j.accpm.2016.04.005
19. Berry MF, D'Amico TA, Onaitis MW. Use of amiodarone after major lung resection. Ann Thorac Surg. (2014) 98(4):1199–206. doi: 10.1016/j.athoracsur.2014.05.038
20. Wang H, Wang Z, Zhou M, Chen J, Yao F, Zhao L, et al. Postoperative atrial fibrillation in pneumonectomy for primary lung cancer. J Thorac Dis. (2021) 13(2):789–802. doi: 10.21037/jtd-20-1717
21. Amar D. Postoperative atrial fibrillation: is there a need for prevention? J Thorac Cardiovasc Surg. (2016) 151(4):913–5. doi: 10.1016/j.jtcvs.2015.09.041
22. Ciszewski P, Tyczka J, Nadolski J, Roszak M, Dyszkiewicz W. Lower preoperative fluctuation of heart rate variability is an independent risk factor for postoperative atrial fibrillation in patients undergoing major pulmonary resection. Interact Cardiovasc Thorac Surg. (2013) 17(4):680–6. doi: 10.1093/icvts/ivt238
23. Olesen OJ, Vinding NE, Østergaard L, Butt JH, Gislason GH, Torp-Pedersen C, et al. C-reactive protein after coronary artery bypass graft surgery and its relationship with postoperative atrial fibrillation. Europace. (2020) 22(8):1182–88. doi: 10.1093/europace/euaa088
24. Vaporciyan AA, Correa AM, Rice DC, Roth JA, Smythe WR, Swisher SG, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg. (2004) 127(3):779–86. doi: 10.1016/j.jtcvs.2003.07.011
25. Amar D, Burt M, Reinsel RA, Leung DH. Relationship of early postoperative dysrhythmias and long-term outcome after resection of non-small cell lung cancer. Chest. (1996) 110(2):437–9. doi: 10.1378/chest.110.2.437
26. Amar D, Zhang H, Leung DH, Roistacher N, Kadish AH. Older age is the strongest predictor of postoperative atrial fibrillation. Anesthesiology. (2002) 96(2):352–6. doi: 10.1097/00000542-200202000-00021
27. Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. (2018) 9(5):416–31. doi: 10.1007/s13238-018-0549-0
28. Pastori D, Carnevale R, Nocella C, Novo M, Santulli M, Cammisotto V, et al. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: effect of adherence to mediterranean diet. J Am Heart Assoc. (2017) 6(6):e005784. doi: 10.1161/JAHA.117.005784
29. Weymann A, Popov AF, Sabashnikov A, Ali-Hasan-Al-Saegh S, Ryazanov M, Tse G, et al. Baseline and postoperative levels of C-reactive protein and interleukins as inflammatory predictors of atrial fibrillation following cardiac surgery: a systematic review and meta-analysis. Kardiol Pol. (2018) 76(2):440–51. doi: 10.5603/KP.a2017.0242
30. Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. (2018) 138(20):2227–42. doi: 10.1161/CIRCULATIONAHA.118.035202
31. Huo JY, Jiang WY, Yin T, Xu H, Lyu YT, Chen YY, et al. Intestinal barrier dysfunction exacerbates neuroinflammation via the TLR4 pathway in mice with heart failure. Front Physiol. (2021) 12:712338. doi: 10.3389/fphys.2021.712338
32. Menichelli D, Carnevale R, Nocella C, Cammisotto V, Castellani V, Bartimoccia S, et al. Circulating lipopolysaccharides and impaired antioxidant status in patients with atrial fibrillation. Data from the ATHERO-AF study. Front Cardiovasc Med. (2021) 8:779503. doi: 10.3389/fcvm.2021.779503
33. Shi H, Guo Y, Liu Y, Shi B, Guo X, Jin L, et al. The in vitro effect of lipopolysaccharide on proliferation, inflammatory factors and antioxidant enzyme activity in bovine mammary epithelial cells. Anim Nutr. (2016) 2:99–104. doi: 10.1016/j.aninu.2016.03.005
34. Ferro D, Baratta F, Pastori D, Cocomello N, Colantoni A, Angelico F, et al. New insights into the pathogenesis of non-alcoholic fatty liver disease: gutderived lipopolysaccharides and oxidative stress. Nutrients. (2020) 12:2762. doi: 10.3390/nu12092762
35. Gawałko M, Agbaedeng TA, Saljic A, Müller DN, Wilck N, Schnabel R, et al. Gut microbiota, dysbiosis and atrial fibrillation: arrhythmogenic mechanisms and potential clinical implications. Cardiovasc Res. (2022) 118:2415–27. doi: 10.1093/cvr/cvab292
Keywords: lipopolysaccharide, atrial fibrillation, non-small-cell lung cancer, surgery, new-onset AF
Citation: Xu H, Zhou J, Ye F and Gao Y (2024) Serum lipopolysaccharide associated with new-onset atrial fibrillation in patients with non-small-cell lung cancer a retrospective observational study. Front. Surg. 11:1404450. doi: 10.3389/fsurg.2024.1404450
Received: 21 March 2024; Accepted: 29 April 2024;
Published: 9 May 2024.
Edited by:
Savvas Lampridis, Imperial College London, United KingdomReviewed by:
Massimo Baudo, Lankenau Institute for Medical Research, United StatesMohamed Rahouma, NewYork-Presbyterian, United States
© 2024 Xu, Zhou, Ye and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Zhou, amllemhvdWRvY3RvckAxNjMuY29t
 Haifeng Xu
Haifeng Xu Jie Zhou
Jie Zhou