
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 24 June 2024
Sec. Colorectal and Proctological Surgery
Volume 11 - 2024 | https://doi.org/10.3389/fsurg.2024.1400264
Introduction: A mini-laparotomy for colorectal cancer (CRC) has been reported to shorten postoperative ileus (POI) and hospital stay. Interleukin-6 (IL-6) plays a role in intestinal tissue inflammation, leading to POI. This study investigated the effects of abdominal wounds and IL-6 levels on POI in patients having CRC surgery.
Materials and methods: Forty-three patients with CRC underwent bowel resection. Serum samples were collected preoperatively and at 2, 24, and 48 h after surgery for cytokine quantification by ELISA. Clinical data, including time from surgery to first passage of flatus and postoperative hospital stay, demographic and pathological data, and routine blood tests, were compared statistically with abdominal wound length and the postoperative increments of cytokines (designated as Δ).
Results: The length of the abdominal wound showed a significant correlation with clinical variables (length of operation time, time of first flatus passage, and length of postoperative hospital stay) and cytokine variables (IL-6(Δ2 h), IL-8(Δ2 h) and IL-10(Δ2 h). Linear regression analysis showed that the abdominal wound length significantly influenced the operation time, time of first flatus passage, and length of postoperative hospital stay (p < 0.001). The length of the abdominal wound showed a significant influence on the IL-6(Δ2 h) and IL-8(Δ2 h) (p < 0.001, respectively) but no influence on IL-10(Δ2 h). IL-6(Δ2 h), but not IL-8(Δ2 h), significantly influenced the time to first flatus passage and length of hospital stay (p = 0.007, p = 0.006, respectively). The mini-laparotomy approach (wound length <7 cm) led to significantly shortened operation time, time of first flatus passage, length of postoperative stay (p = 0.004, p = 0.003, p = 0.006, respectively) as well as reduced postoperative increment of IL-6(Δ2 h) (p = 0.015). The mini-laparotomy for anterior resection surgery significantly influenced operation time, time of first passage of flatus, length of postoperative stay, and IL-6(Δ2 h).
Conclusion: Our study is the first to report the complex interaction among the length of the abdominal wound, IL-6 serum level, recovery of the first passage of flatus, and postoperative hospital stay. These results suggest that smaller abdominal wounds and smaller postoperative IL-6 increments were associated with faster recovery of flatus passage and shorter hospital stays.
Postoperative ileus (POI), which occurs after major abdominal operations, such as colorectal cancer resection, is considered a physiological cessation of bowel motility during transit. The estimated duration of this temporary POI is between 24 and 48 h in the stomach, less than 24 h in the small intestine, and 48–72 h in the colon (1). However, the recovery of colonic motility can be delayed by surgical procedures involving colon resection with anastomosis compared to other types of abdominal surgery without colonic anastomosis (2). The resolution of POI has been assessed using bowel sounds, flatus passage, defecation, tolerance to meals, and myoelectric activities of smooth muscle as indicators of bowel motility recovery in different studies (3, 4). Thus, the criteria to define prolonged POI in clinical studies included delayed recovery of bowel motility (e.g., passage of flatus or defecation) and/or associated abdominal symptoms (e.g., intolerance to diet, abdominal distension, and nausea/vomiting). The duration of such criteria was defined as three to seven days in different studies (5). Clinically important, prolonged POI is associated with longer hospital stays and increased medical costs after colonic surgery (6–8).
The evidence indicates that POI mechanisms are multifactorial. Animal experiments and human studies have shown that both local inflammation of the bowel wall and inflammatory cytokines in the systemic circulation may play active roles in developing POI. Interleukin-6 (IL-6) is a proinflammatory cytokine and is among the most studied cytokines. In animal experiments, direct trauma to the bowel wall has been shown to cause a local inflammatory response in the muscularis propria, including inflammatory cell infiltration and elevated cellular expression of proinflammatory cytokines, including IL-6 (9, 10). Similar phenomena were observed in a study of human bowel tissues (11). In an animal experiment, abdominal laparotomy induced elevated serum IL-6 levels and inhibition of gastrointestinal transit in the same time course (12). In a human study, elevated serum IL-6 levels after abdominal surgery were involved in delayed recovery of gastric emptying and impaired gastric electrical activity in patients with postoperative ileus (13, 14). Although these data have indicated the possible role of IL-6 in the development of POI, there is still a lack of clinical studies focusing on the association between postoperative alterations in IL-6 and the recovery of colonic motility of transit among patients undergoing surgical resection for colorectal cancer.
To date, only a few studies have reported that IL-8 and IL-10 may have biological functions related to bowel motility. In an animal study, proinflammatory IL-8 enhanced the contractile response of the small intestine to stimulation by acetylcholine (15). IL-10 is an anti-inflammatory cytokine. In an animal model of inflammatory bowel disease, the colonic smooth muscle of IL-10 knockout mice showed decreased contractility relative to the colonic smooth muscle of wild-type IL-10 mice (16). In contrast, the elevation of serum IL-10 levels elicited by peritoneal air exposure was correlated with a decrease in gastrointestinal transit in an animal study (12). These reports suggest a potential influence of IL-8 and IL-10 on bowel motility; however, the observed functions of IL-10 are contradictory.
In recent years, an increasing number of studies have reported the clinical benefits of mini-laparotomies for colorectal cancer resection. Different authors defined a mini-laparotomy as an abdominal wound length either less than 7 or 8 cm (17–20). Regardless of the mild difference in wound length, the mini-laparotomy was significantly associated with earlier recovery of flatus passage than a conventional laparotomy (17, 20). A recent case-controlled study comparing the medical costs of a mini-laparotomy (less than 8 cm) and a conventional laparotomy in patients with colorectal cancer reported that the mini-laparotomy is more cost-effective because of fewer complications and a shorter post-operative hospital stay (19). Thus far, the biological mechanism underlying the benefits of mini-laparotomy abdominal wounds on the earlier recovery of flatus passage or shorter hospital stay remains to be investigated.
Based on the current data, we hypothesized that the length of the abdominal wound influences the postoperative systemic cytokine response (IL-6) and clinical outcomes (including recovery of colonic motility and postoperative hospital stay). The postoperative IL-6 response may also be involved in the complex interaction between abdominal wounds and clinical outcomes.
In the present study, we clarified the complex interactions between abdominal wound length, postoperative IL-6 levels, and recovery of colon motility. We demonstrated the beneficial effect of the mini-laparotomy approach in decreasing the postoperative IL-6 cytokine response and shortening the duration of recovery from flatus passage and postoperative hospital stay. In addition, we demonstrated that postoperative IL-8 and IL-10 did not influence the recovery of colon motility.
This study retrospectively analyzed prospectively collected data from 45 patients. After obtaining approval from the Institutional Review Board (MMH-I-S-154), the patients were enrolled in the study between September 2005 and February 2007. Patients with complicated conditions, including perforation, peritonitis, preexisting infection, poor nutrition, and severe comorbidities, were excluded. All enrolled patients signed an informed consent form to check their serum cytokine levels before and after surgery. Of the 45 patients, two were not included in the data analysis after entering the study because of anastomotic leakage and unexpected colostomy. The remaining 43 patients who had resectable colorectal cancer received open abdominal approach to remove the primary tumor. Among them, five patients with synchronous metastasis to liver (two cases), lung (one case), omentum (one case) and small bowel mesentery (one patient) only had resection of the primary tumor. We did not include patients who received laparoscopic approach or robotic approach for tumor resection to compare with patients having open approach. The patients underwent surgery at the Department of Surgery, Division of Colorectal Surgery, Mackay Memorial Hospital, by two surgeons who reached a consensus on the study design, surgical procedures, and postoperative care. The abdominal wound length was determined based on the surgeon's judgment according to clinical indications, including tumor size and an adequate operation field to dissect the mesentery and feeding vessels. In this study, an abdominal wound length of <7 cm was defined as a mini-laparotomy (17, 18).
The demographic and clinical variables were prospectively collected, including age, gender, body mass index (BMI), abdominal wound length, operation time, blood loss, tumor characteristics, tumor locations, anesthetic methods, postoperative analgesic methods, the first recovery of flatus passage, the postoperative hospital stay, the routine preoperative data from blood tests.
The timing of blood collection and quantification of cytokine levels in blood samples have been described previously (21). Serum samples were collected before surgery and at indicated time points. Serum levels of IL-6, IL-8, and IL-10 were measured using an ELISA kit, according to the manufacturer's instruction (R&D Systems, Minneapolis, MN, USA).
All serum samples were measured within the range of the standard curve of the ELISA kit: 0–300 pg/ml for IL-6 (kit D6050, R&D Systems); 0–2,000 pg/ml for IL 8 (kit D8000C, R&D Systems); and 0–500 pg/ml for IL-10 (kit D1000B, R&D Systems). The cytokine concentration was considered zero when the sample concentration was below the measurable limit.
To minimize the effect of individual physiological variations between patients in this study, the postoperative change (designated Δ) in cytokines was calculated by the following formula: cytokine (Δ time point) = postoperative value of a cytokine at an indicated time–the paired preoperative cytokine value from the same patient. Therefore, the individual variations among patients were normalized, and the data for cytokine preoperative baseline level, Δ2 h, Δ24 h, and Δ48 h were used for comparative analysis.
Correlations between variables were analyzed using Spearman's correlation analysis. The causation between variables was analyzed using linear regression analysis. Differences in variables between the two groups were analyzed using the Mann-Whitney U-test. The Kruskal Wallis test was used for comparison between multiple groups. Categorical variables were compared using the chi-squared test (Fisher's exact test). Values of p < 0.05 were considered statistically significant.
Table 1 shows the clinical variables and characteristics of the enrolled patients. There were 21 females and 22 males with a mean age of 60.3 ± 10.7 years old. Eighteen patients (42%) had colon cancer and 25 (58%) had rectal cancer. The mean length of the abdominal incision wound was 10.2 ± 4.8 cm. The mean operation time was 186.8 ± 88.3 min. The mean blood loss was 204.7 ± 158.8 ml. The mean BMI was 23.5 ± 3.1 (kg/m2). The mean duration from operation to first flatus passage was 3.2 ± 1.3 days, and the mean duration of postoperative hospital stay was 10.9 ± 4.5 days. All patients recovered from the surgery smoothly without any remarkable complications.
Figure 1 shows the mean levels of IL-6, IL-8, and IL-10 2, 24, and 48 h after colorectal cancer surgery. The highest postoperative levels of the three cytokines occurred at 2 h after operation (Δ2 h). The median serum cytokine levels are shown in Supplementary Figure S1.
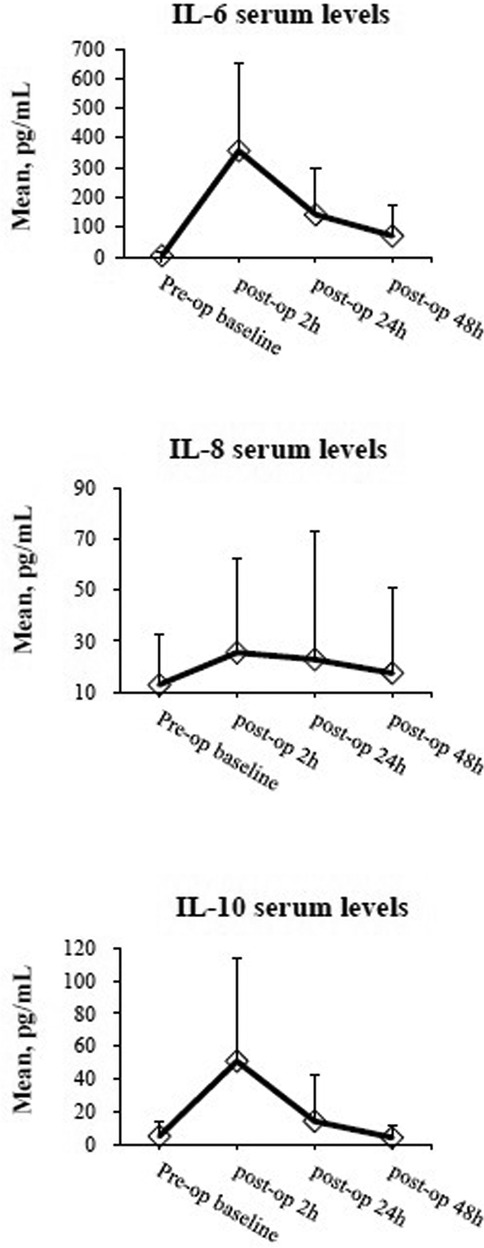
Figure 1 The mean serum levels of IL-6, IL-8, and IL-10 are shown in time course. Briefly, cytokines were quantified by ELISA assay on serum taken preoperatively at 2, 24, and 48 h after surgery. Data are expressed as mean ± standard deviation.
Table 2 shows that abdominal wound length was positively correlated with clinical outcomes, including operation time (p < 0.001), time to first passage of flatus (p < 0.001), and duration of postoperative hospital stay (p < 0.001). The length of the abdominal wound was also positively correlated with the postoperative increments of IL-6 (Δ2 h), IL-8 (Δ2 h) and IL-10(Δ2 h) (p = 0.001, p = 0.016, p = 0.003, respectively).
Linear regression analysis was performed to verify whether the abdominal wound length had a causative effect on the correlated variables (Table 3). The results showed that the length of the abdominal wound significantly influenced the operation time, recovery of the first passage of flatus, and length of postoperative hospital stay (p < 0.001, respectively). The length of the abdominal wound also showed a significant influence on the increment magnitude of IL-6 (Δ2 h) and IL-8 (Δ2 h) (p < 0.001, respectively) but no influence on the increment of IL-10(Δ2 h) (p = 0.069).
Linear regression analysis was further performed to examine whether the magnitudes of IL-6 (Δ2 h) and IL-8 (Δ2 h) have a causative influence on the recovery of first flatus passage and postoperative hospital stay. Table 4 shows that the increment magnitude of IL-6 (Δ2 h) significantly influenced the recovery of flatus passage and length of hospital stay (p = 0.007 & p = 0.006, respectively). However, IL-8 (Δ2 h) did not significantly influence these clinical outcomes.
Furthermore, linear regression analysis showed that the recovery of first flatus passage significantly influences the postoperative hospital stay (R = 1.000, unadjusted coefficient β = 1.012, p < 0.001).
By taking these analyzed results together, we summarized an interaction between abdominal wound length, postoperative increments of IL-6 (Δ2 h), and recovery of first flatus passage in Figure 2. Figure 2A estimated that a one-centimeter length of abdominal wound was associated with a 35.8 pg/ml increment of IL-6 (Δ2 h). In turn, a 1 pg/ml increment of IL-6 (Δ2 h) was associated with 0.002 days of waiting time for the first passage of flatus. Meanwhile, the abdominal wound could influence, through other pathways, the recovery of the first flatus passage, with one centimeter of wound length associated with 0.151 days of waiting time to have the first passage of flatus (Figure 2B). A waiting time of one day for the first flatus passage was associated with 1.012 days postoperative hospital stay.
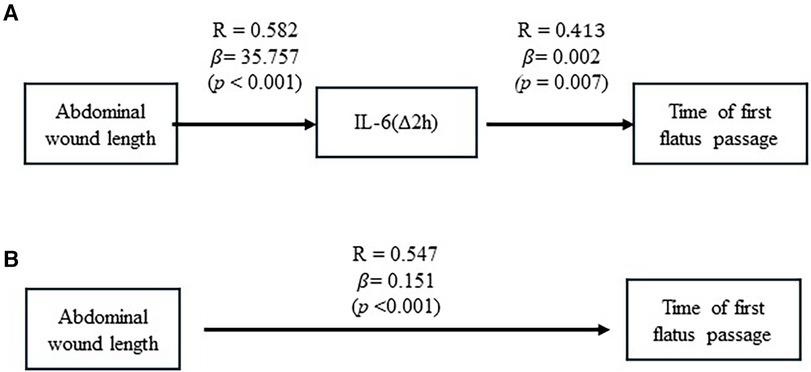
Figure 2 The causative effect of abdominal wound length on posterior IL-6(Δ2 h) increment and recovery of flatus passage. (A) Results of linear regression analysis showed the causative effect of abdominal wound length on postoperative IL-6(Δ2 h) increment and the causative effect of postoperative IL-6(Δ2 h) increment on recovery of flatus passage. (B) Results of linear regression analysis were illustrated to show the causative effect of abdominal wound length on recovery of flatus passage.
Having verified the effects of abdominal wound length and IL-6 levels on clinical outcomes, we further examined the clinical benefits of mini-laparotomy (wound size <7 cm) in our patients. In Table 5, the median abdominal wound length in the mini-laparotomy group was 6.8 cm (range: 5.8–7, IQR: 0.3), compared to a median abdominal wound of 14.5 cm (range: 7.5–24, IQR:7.0) in the non-mini-laparotomy group. The operation time, time to first passage of flatus, and length of postoperative stay were shorter in the mini-laparotomy group (p = 0.004, p = 0.003, p = 0.006, respectively). The postoperative increments of IL-6(Δ2 h) in the mini-laparotomy group were significantly less than the increment in the non-mini-laparotomy group (p = 0.015).
Clinical variables, including age, sex, cancer stage, cancer sites, amount of blood loss, BMI, and methods of postoperative analgesia, showed no statistical differences between the two groups (Table 5). The preoperative blood test results of the two groups did not show significant differences (Table 6).
One may wonder that the site of mesenteric dissection or surgery involving the small intestine may also influence the IL-6 response and clinical outcomes. Thus, we analyzed the data of patients who had surgical dissection limited to the mesentery of the inferior mesenteric artery and no surgery on the small bowel and investigated the effects of mini-laparotomy on patients who underwent anterior resection for cancer of the sigmoid and rectum. Table 7 shows that the operation time, time to first passage of flatus, and length of postoperative stay were shorter in the mini-laparotomy group (p = 0.02, p = 0.012, p = 0.006, respectively). The postoperative increments of IL-6(Δ2 h) in the mini-laparotomy group were significantly less than the increment in the non-mini-laparotomy group (p = 0.009). Clinical variables, including age, sex, cancer stage, cancer site, amount of blood loss, BMI, and methods of postoperative analgesia, showed no statistical differences between the two groups. Preoperative blood tests of CBC and biochemistry did not show significant differences between the two groups (data not shown).
We further investigated whether the characteristics of tumor and locations of tumor would influence serum levels of IL-6. We stratified patients based on stage of tumor, histological grade of cancer cells, or site of tumor, respectively, to compare the serum IL-6 levels between different patients groups. The pre-operative levels and the postoperative increments of serum IL-6 (Δ2 h, Δ24 h and Δ48 h) showed no significant differences between different patients groups stratified by stage of tumor, histologic grade, and site of tumor (Table 8).
The pre-operative level and the postoperative increments of serum IL-6 showed no significant differences between different patients groups stratified by pathologic characteristics, including depth of tumor invasion, tumor size larger or smaller than median value, status of lymph node invasion, presence or absence of vascular invasion, presence or absence of lymphatic invasion, and presence or absence of perineurial invasion (data not shown).
Our study is the first to demonstrate that abdominal wound length influences postoperative IL-6 levels and recovery of flatus passage after colorectal cancer resection. Notably, the IL-6 level at two hours after operation, in turn, influenced the recovery of flatus passage. These interactions influence the postoperative hospital stay. These findings are clinically relevant because faster recovery of bowel function and shorter postoperative hospital stays are important for enhanced recovery after surgery (ERAS).
The pathophysiology of POI after abdominal surgery has been proposed to be multifactorial and includes autonomic nerve activity, neurotransmitters, and bowel tissue inflammation. The type of anesthesia and analgesics, malnutrition, electrolyte imbalance, intra-abdominal infection, amount of blood loss, male sex, advanced age, respiratory insufficiency, and emergency surgery are risk factors for prolonged POI (1, 5). Recent studies have shown that mini-laparotomy (a smaller abdominal wound) and laparoscopic surgery are associated with earlier bowel motility (22–24). The influence of wound size on bowel motility is also supported by an animal study (25).
The ERAS protocol for colorectal surgery recommendations suggests the use of laparoscopic surgery for colorectal resection to achieve quicker recovery of bowel function and shorter hospital stay (26). In the Color II trial, laparoscopic surgery for rectal cancer, compared with conventional open surgery, yielded quicker recovery of bowel movement and shortened hospital stay (one-day reduction, respectively) (24). Hong et al. reported that laparoscopic resection of colorectal cancer shortened the time to first flatus passage by 1.2 days and hospital stay by four days compared to conventional open surgery (23). Our data and those of others have shown that mini-laparotomy surgery for colorectal cancer could shorten the time from surgery to the first flatus passage by 1–2 days and the postoperative stay by 3–5 days (18, 20). These data suggest that the mini-laparotomy and laparoscopic surgery have similar effects on earlier flatus passage and reduced postoperative hospital stay. This similar merit of mini-laparotomy and laparoscopic surgery can be explained by the smaller abdominal wounds compared with those of conventional open surgery.
Studies have reported that laparoscopic surgery for colorectal cancer, relative to open abdominal surgery, reduces the increase in serum IL-6 levels after surgery (27, 28). This reduction may be due to less trauma to the abdominal wall caused by laparoscopic surgery than by conventional open surgery (9, 11, 27, 28). Our study showed that mini-laparotomy surgery for colorectal cancer was significantly associated with decreased increment of IL-6 at 2 h after surgery [IL-6 (Δ2 h)]. The reduced increments in postoperative serum levels of IL-6, seen in both laparoscopic surgery and mini-laparotomy, can also be explained by smaller abdominal incision wounds. In our study, the decreased increment of IL-6, but not the decreased increment of IL-8, was significantly associated with earlier recovery from flatus passage and a shorter postoperative hospital stay. This finding is consistent with those of previous studies that reported an association between the inflammatory response to IL-6 and POI (9–12). This result also suggests that IL-6 influences not only the gastric emptying function but also colonic motility (13).
Using statistical analysis, our data showed a numeric correlation between wound length, recovery of the first flatus passage, and postoperative hospital stay. The results showed that one centimeter of wound length was associated with a waiting time of 0.151 days for the first flatus passage. In turn, a waiting time of one day for the first flatus passage was associated with a 1.012-day delay in the postoperative hospital stay. A similar study reported the effect of the length of the abdominal laparotomy wound on bowel function recovery. The authors estimated that an abdominal wound greater than 18 cm increased the waiting time of bowel movement by 0.5 days, compared to an abdominal wound length less than 18 cm. One centimeter increase in wound length correlated with a 2% increase in the delay of the first bowel movement (29). Our study further revealed a correlation between IL-6 increment and waiting time for the first flatus passage. A 1 pg/ml increment of IL-6(Δ2 h) was associated with 0.002 days of waiting time for the first flatus passage.
Studies have reported that surgery of the right colon has a higher chance of inducing delayed recovery of bowel function and prolonged ileus than surgery of the left-sided colon (30–32). One may wonder whether the different sites of mesenteric dissection or surgery in the small intestine influence clinical outcomes and IL-6 response. To exclude these potential confounding factors, we further analyzed the effects of mini-laparotomy in patients who underwent anterior resection in which the dissection of the mesentery was limited to the inferior mesenteric artery territory. The results suggested that the effects of mini-laparotomy remained the same with regard to a decrease in postoperative IL-6(Δ2 h) increment, a decrease in waiting time to have the first flatus passage, and a decrease in postoperative hospital stay. These results provide further evidence for the biological benefits of mini-laparotomy in the recovery of bowel function.
There have been studies reporting the alterations of serum IL-6 levels related to tumor characteristics of colorectal cancer. Concerning the association of IL-6 serum levels and tumor stage, one such study found a significant association of advanced staging of tumor and increasing IL-6 serum levels (33). On the contrary, another study did not find a significant association between tumor stage and serum levels of IL-6 (34). Tumor size and status of lymph node invasion have also been reported to be associated with serum levels of IL-6 (35). Our results did not show significant influences of these tumor characteristics on serum levels of IL-6. The discrepancy in results between our study and other studies may be related to 174 G>C polymorphism of IL-6 gene promoter (36). To further clarify this question, a prospective study with large numbers of patients to investigate the correlation between IL-6 promoter polymorphism and IL-6 serum levels is needed.
Surgical complications e.g., surgical site infection, anastomotic leakage, and sepsis, can occur after colorectal cancer resection (37, 38). These complications can cause local and systemic inflammation response, leading to elevated levels of IL-6 in the serum (39, 40). Recent studies showed that butyrylcholinesterase plasma level has emerged as a potential predictive marker for the surgical complications that occurred after colorectal cancer resection (41). Surgical complications are often associated with prolonged postoperative ileus and increased chance of morbidities (e.g., malnutrition, sepsis, and even mortality), leading to prolonged postoperative hospital stay and increased economic burden. Of note, the elevation of serum IL-6 elicited by surgical complications can cause misinterpretation of the IL-6 data in our study. To minimize the confounding effect caused by surgical complications, we have excluded patients who had surgical complications in this study. Thus the interpretation of our results should be applied only to patients who did not have surgical complications after the tumor resection surgery.
The strength of this study lies in the prospective collection of serum samples and clinical data. The high homogeneity of the patient sample, as the two surgeons who participated in this study, led to a consensus on the study design, surgical procedures, and postoperative care. Patients who developed complications or required colostomies were excluded from the final analysis. The limitations of this study are as follows: (1) the small sample size and patients enrolled only in a single hospital, (2) the physiological variations in cytokine levels between individuals, (3) the interpretation of our results only applied to patients who did not have surgical complications, and (4) lacking the information of 174 G>C polymorphism of IL-6 gene promoter. We have used the Δ values as described in the methodology in this study in the hope of minimizing the influence of physiological variations. Our study indicates the need for a prospective study enrolling large numbers of colorectal cancer patients who are willing to have IL-6 gene analysis and cytokine analysis during the treatment of tumor resection.
Our study provides novel findings to better understand the complex mechanisms of POI after surgical resection of colorectal cancer. These results suggest that smaller abdominal wounds and smaller postoperative IL-6 increments were associated with faster recovery from flatus passage and shorter hospital stays.
The datasets presented in this article are not readily available because, under the institutional requirements, only the principal investigator is allowed to access the raw data of this study. Requests to access the datasets should be directed to the principal investigator M-JC via email: mjchen@mmh.org.tw.
The studies involving humans were approved by the Institutional Review Board of MacKay Memorial Hospital (MMH-I-S-154). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
P-LT: Writing – review & editing, Data curation, Formal Analysis, Writing – original draft. J-SC: Data curation, Writing – original draft, Writing – review & editing, Methodology, Software. C-HL: Writing – original draft, Formal Analysis, Investigation. T-CH: Formal Analysis, Writing – original draft, Data curation. Y-WL: Data curation, Methodology, Writing – review & editing. M-JC: Writing – review & editing, Conceptualization, Funding acquisition, Project administration.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the MacKay Memorial Hospital grants MMH-9487 and MMH-E-113-8.
The authors wish to thank Mackay Memorial Hospital for their support. We are grateful for help from Yi-Min Liu for collecting and organizing the data and Fang-Ju Sun for help with the statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1400264/full#supplementary-material
Supplementary Figure S1
Median serum levels of IL-6, IL-8, and IL-10 are shown over time. Briefly, cytokines were quantified by ELISA in the serum collected preoperatively and at 2, 24, and 48 h after surgery.
1. Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. (2000) 87(11):1480–93. doi: 10.1046/j.1365-2168.2000.01595.x
2. Roberts JP, Benson MJ, Rogers J, Deeks JJ, Williams NS. Characterization of distal colonic motility in early postoperative period and effect of colonic anastomosis. Dig Dis Sci. (1994) 39(9):1961–7. doi: 10.1007/bf02088132
3. Waldhausen JH, Shaffrey ME, Skenderis BS 2nd, Jones RS, Schirmer BD. Gastrointestinal myoelectric and clinical patterns of recovery after laparotomy. Ann Surg. (1990) 211(6):777–84. discussion 85. doi: 10.1097/00000658-199006000-00018
4. Shibata Y, Toyoda S, Nimura Y, Miyati M. Patterns of intestinal motility recovery during the early stage following abdominal surgery: clinical and manometric study. World J Surg. (1997) 21(8):806–9. discussion 09-10. doi: 10.1007/s002689900310
5. Venara A, Neunlist M, Slim K, Barbieux J, Colas PA, Hamy A, et al. Postoperative ileus: pathophysiology, incidence, and prevention. J Visc Surg. (2016) 153(6):439–46. doi: 10.1016/j.jviscsurg.2016.08.010
6. Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm. (2009) 15(6):485–94. doi: 10.18553/jmcp.2009.15.6.485
7. Barletta JF, Senagore AJ. Reducing the burden of postoperative ileus: evaluating and implementing an evidence-based strategy. World J Surg. (2014) 38(8):1966–77. doi: 10.1007/s00268-014-2506-2
8. Asgeirsson T, El-Badawi KI, Mahmood A, Barletta J, Luchtefeld M, Senagore AJ. Postoperative ileus: it costs more than you expect. J Am Coll Surg. (2010) 210(2):228–31. doi: 10.1016/j.jamcollsurg.2009.09.028
9. Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. (1998) 228(5):652–63. doi: 10.1097/00000658-199811000-00004
10. Wehner S, Schwarz NT, Hundsdoerfer R, Hierholzer C, Tweardy DJ, Billiar TR, et al. Induction of IL-6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery. (2005) 137(4):436–46. doi: 10.1016/j.surg.2004.11.003
11. Kalff JC, Türler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, et al. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. (2003) 237(3):301–15. doi: 10.1097/01.Sla.0000055742.79045.7e
12. Tan S, Yu W, Lin Z, Chen Q, Shi J, Dong Y, et al. Peritoneal air exposure elicits an intestinal inflammation resulting in postoperative ileus. Mediators Inflamm. (2014) 2014:924296. doi: 10.1155/2014/924296
13. Lehrskov L L, Lyngbaek MP, Soederlund L, Legaard GE, Ehses JA, Heywood SE, et al. Interleukin-6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab. (2018) 27(6):1201–11.e3. doi: 10.1016/j.cmet.2018.04.008
14. Frasko R, Maruna P, Gurlich R, Trca S. Transcutaneous electrogastrography in patients with ileus. Relations to interleukin-1beta, interleukin-6, procalcitonin and C-reactive protein. Eur Surg Res. (2008) 41(2):197–202. doi: 10.1159/000134918
15. Plattner V, Leray V, Leclair MD, Aubé AC, Cherbut C, Galmiche JP. Interleukin-8 increases acetylcholine response of rat intestinal segments. Aliment Pharmacol Ther. (2001) 15(8):1227–32. doi: 10.1046/j.1365-2036.2001.01009.x
16. Park JH, Kwon JG, Kim SJ, Song DK, Lee SG, Kim ES, et al. Alterations of colonic contractility in an interleukin-10 knockout mouse model of inflammatory bowel disease. J Neurogastroenterol Motil. (2015) 21(1):51–61. doi: 10.5056/jnm14008
17. Nakagoe T, Sawai T, Tsuji T, Ayabe H. Use of minilaparotomy in the treatment of colonic cancer. Br J Surg. (2001) 88(6):831–6. doi: 10.1046/j.1365-2168.2001.01765.x
18. Takegami K, Kawaguchi Y, Nakayama H, Kubota Y, Nagawa H. Minilaparotomy approach to colon cancer. Surg Today. (2003) 33(6):414–20. doi: 10.1007/s10595-002-2534-8
19. Chiu HC, Hsieh HM, Wan CL, Tsai HL, Wang JY. Cost-effectiveness of mini-laparotomy in patients with colorectal cancers: a propensity scoring matching approach. PLoS One. (2019) 14(1):e0209970. doi: 10.1371/journal.pone.0209970
20. Ishikawa M, Nishioka M, Hanaki N, Miyauchi T, Kashiwagi Y, Miki H. Colorectal resection by a minilaparotomy approach vs. conventional operation for colon cancer. Results of a prospective randomized trial. Hepatogastroenterology. (2007) 54(79):1970–5.18251141
21. Hsu T-C, Lin C-H, Sun F-J, Chen M-J. Postoperative serum levels of interleukin-6 are affected by age in patients with colorectal cancer. Int J Gerontol. (2017) 11(2):75–9. doi: 10.1016/j.ijge.2016.06.004
22. Vather R, Josephson R, Jaung R, Robertson J, Bissett I. Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery. (2015) 157(4):764–73. doi: 10.1016/j.surg.2014.12.005
23. Hong D, Tabet J, Anvari M. Laparoscopic vs. open resection for colorectal adenocarcinoma. Dis Colon Rectum. (2001) 44(1):10–8. discussion 18-9. doi: 10.1007/bf02234812
24. van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. (2013) 14(3):210–8. doi: 10.1016/s1470-2045(13)70016-0
25. Uemura K, Tatewaki M, Harris MB, Ueno T, Mantyh CR, Pappas TN, et al. Magnitude of abdominal incision affects the duration of postoperative ileus in rats. Surgical Endoscopy and Other Interventional Techniques. (2004) 18(4):606–10. doi: 10.1007/s00464-003-8161-6
26. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS(®)) society recommendations: 2018. World J Surg. (2019) 43(3):659–95. doi: 10.1007/s00268-018-4844-y
27. Leung KL, Lai PB, Ho RL, Meng WC, Yiu RY, Lee JF, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg. (2000) 231(4):506–11. doi: 10.1097/00000658-200004000-00008
28. Kampman SL, Smalbroek BP, Dijksman LM, Smits AB. Postoperative inflammatory response in colorectal cancer surgery: a meta-analysis. Int J Colorectal Dis. (2023) 38(1):233. doi: 10.1007/s00384-023-04525-3
29. Song J, Yang Y, Guan W, Jin G, Yang Y, Chen L, et al. Association of abdominal incision length with gastrointestinal function recovery post-operatively: a multicenter registry system-based retrospective cohort study. Front Surg. (2021) 8:743069. doi: 10.3389/fsurg.2021.743069
30. Yuan L, O'Grady G, Milne T, Jaung R, Vather R, Bissett IP. Prospective comparison of return of bowel function after left versus right colectomy. ANZ J Surg. (2018) 88(4):E242–e47. doi: 10.1111/ans.13823
31. Grass F, Lovely JK, Crippa J, Ansell J, Hübner M, Mathis KL, et al. Comparison of recovery and outcome after left and right colectomy. Colorectal Dis. (2019) 21(4):481–86. doi: 10.1111/codi.14543
32. Garfinkle R, Al-Rashid F, Morin N, Ghitulescu G, Faria J, Vasilevsky CA, et al. Are right-sided colectomies for neoplastic disease at increased risk of primary postoperative ileus compared to left-sided colectomies? A coarsened exact matched analysis. Surg Endosc. (2020) 34(12):5304–11. doi: 10.1007/s00464-019-07318-4
33. Esfandi F, Mohammadzadeh Ghobadloo S, Basati G. Interleukin-6 level in patients with colorectal cancer. Cancer Lett. (2006) 244(1):76–8. doi: 10.1016/j.canlet.2005.12.003
34. Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. (1999) 85(12):2526–31. doi: 10.1002/(sici)1097-0142(19990615)85:12%3C2526::aid-cncr6%3E3.0.co;2-3
35. Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients–a summary of published results. Int J Colorectal Dis. (2010) 25(2):135–40. doi: 10.1007/s00384-009-0818-8
36. Belluco C, Olivieri F, Bonafè M, Giovagnetti S, Mammano E, Scalerta R, et al. −174 G>C polymorphism of interleukin 6 gene promoter affects interleukin 6 serum level in patients with colorectal cancer. Clin Cancer Res. (2003) 9(6):2173–6.12796383
37. Panos G, Mulita F, Akinosoglou K, Liolis E, Kaplanis C, Tchabashvili L, et al. Risk of surgical site infections after colorectal surgery and the most frequent pathogens isolated: a prospective single-centre observational study. Med Glas (Zenica. (2021) 18(2):438–43. doi: 10.17392/1348-21
38. Mulita F, Liolis E, Akinosoglou K, Tchabashvili L, Maroulis I, Kaplanis C, et al. Postoperative sepsis after colorectal surgery: a prospective single-center observational study and review of the literature. Prz Gastroenterol. (2022) 17(1):47–51. doi: 10.5114/pg.2021.106083
39. Alonso S, Pascual M, Salvans S, Mayol X, Mojal S, Gil MJ, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol. (2015) 41(2):208–14. doi: 10.1016/j.ejso.2014.10.052
40. Procházka V, Lacina L, Smetana K Jr., Svoboda M, Skřivanová K, Beňovská M, et al. Serum concentrations of proinflammatory biomarker interleukin-6 (IL-6) as a predictor of postoperative complications after elective colorectal surgery. World J Surg Oncol. (2023) 21(1):384. doi: 10.1186/s12957-023-03270-9
41. Mulita F, Verras G-I, Bouchagier K, Dafnomili V-D, Perdikaris I, Perdikaris P, et al. Butyrylcholinesterase levels as a predictive factor of septic complications development in the postoperative period of colorectal patients: univariate analysis and predictive modeling. Eur J Surg Oncol. (2023) 49(1):e15. doi: 10.1016/j.ejso.2022.11.083
Keywords: abdominal wound length, interleukin 6 (IL-6), first flatus passage, hospital stay, colorectal cancer (CRC)
Citation: Tsai P-L, Chen J-S, Lin C-H, Hsu T-C, Lin Y-W and Chen M-J (2024) Abdominal wound length influences the postoperative serum level of interleukin-6 and recovery of flatus passage among patients with colorectal cancer. Front. Surg. 11:1400264. doi: 10.3389/fsurg.2024.1400264
Received: 13 March 2024; Accepted: 10 June 2024;
Published: 24 June 2024.
Edited by:
Vito D'Andrea, Sapienza University of Rome, ItalyReviewed by:
Francesk Mulita, General University Hospital of Patras, Greece© 2024 Tsai, Chen, Lin, Hsu, Lin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Jen Chen, bWpjaGVuQG1taC5vcmcudHc= bWpjaGVuQG1tYy5lZHUudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.