
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 27 June 2024
Sec. Surgical Oncology
Volume 11 - 2024 | https://doi.org/10.3389/fsurg.2024.1393159
Introduction: We present the case of a patient with recurrent bilateral hemothorax. After misdiagnosis despite several histological samples, a pleural manifestation of epithelioid angiosarcoma was diagnosed by further immunohistological staining. Based on this situation, we aim to sensitize the reader to this rare disease.
Main concerns and important clinical findings: A 73-year-old fully conscious woman presented with dyspnea for 3 days. She was in stable general condition, pain was denied, she had a history of cigarette smoking, she had no cardiopulmonary events, and she was not receiving any anticoagulation medication. Physical examination revealed decreased breath sounds on the left side, and her hemoglobin level was 7.0 mmol/L.
Primary diagnoses, interventions, and outcomes: The initial chest x-ray showed a left-sided effusion. Hemothorax was then diagnosed. Further investigation revealed no evidence of malignancy (CT, EBUS, cytology, etc.). VATS was performed, and biopsies of pleural lesions did not reveal congruent findings for the hemothorax. Due to recurrent bilateral hemothorax with the need for erythrocyte transfusion, the patient underwent several operations, including histological sampling, without evidence of malignancy. After further processing, an additional pathological report revealed an epithelioid angiosarcoma defined by massively proliferating epithelioid cells strongly positive for ERG and CD31 and negative for CD34. The neoplastic cells coexpressed D2-40 (podoplanin). Finally, due to multiple cerebral metastases, palliative therapy was indicated.
Conclusion: Physicians and pathologists treating spontaneous hemothorax need to have broad knowledge of the possible, sometimes rare, etiologies. If the clinical course and intraoperative findings do not agree with the histopathological results, this finding must be questioned, and further immunohistochemical staining is mandatory. Thus, in the case of recurrent hemothorax, angiosarcoma of the pleura should also be considered for differential diagnosis.
Angiosarcomas account for 1%–2% of soft tissue sarcomas and arise from endothelial cells of small blood and lymphatic vessels and are commonly found in the skin, soft tissues, liver, spleen, heart and breast (1, 2). Thus, primary epithelioid angiosarcoma of the pleura is an extremely rare malignancy. Approximately 46 case reports have been published to date (3). In general, angiosarcoma often presents with benign symptoms, despite the presence of advanced disease with infiltrative growth and metastatic spread (4). In patients with a pleural origin, the most common symptoms are dyspnea, chest tightness, pain, pleural thickening, pleural effusion and recurrent hemothorax. Distinct mass formation is uncommon but has been described (5, 6). The clinical presentation may be confused with malignant mesothelioma or even benign diagnoses (7–9). The average age at presentation is 55 years, and men are more commonly affected than women are (6). Surgery is appropriate for localized tumors. However, the prognosis is poor. Although most patients die shortly after diagnosis, some authors argue that a multidisciplinary approach to treating angiosarcoma, consisting of surgery, radiotherapy and chemotherapy, may result in a positive outcome (4).
We present the unusual case of a 73-year-old female ex-smoker who presented to our emergency department on her own initiative with progressive dyspnea 3 days in duration and suspected pneumonia. Based on this situation, our aim is to sensitize the reader's clinical view to this extremely rare disease to avoid possible pitfalls. The patients' relatives were asked for their consent and agreed to publication.
A 73-year-old, fully conscious woman presented to our emergency department (Martha-Maria-Hospital in Dölau/Halle, Germany) due to dyspnea for the past 3 days. Her general condition was stable, and she denied any pain. At the time of presentation, she was an ex-smoker (cum. 10 PY). No cardiopulmonary events were recorded up to that time, and she denied taking anticoagulant medication. Physical examination revealed decreased breath sounds on the left side and no abnormalities on the right side. Her hemoglobin level was 7.0 mmol/L (reference range 7.1–9.9 mmol/L). The initial chest x-ray (see Figure 1) showed a left pleural effusion, which was managed by placing a 14 French chest tube in the left pleural cavity using the Seldinger technique. A total of 2,000 ml of bloody effusion fluid was fractionally drained. The patient was admitted to the pulmonary unit for further diagnosis and therapy (Table 1).
A contrast-enhanced computed tomography (CT) scan of the chest revealed persistent left pleural effusion, which was classified as serous to slightly hemorrhagic by density. There was no evidence of tumor formation, infiltration or pathologically enlarged thoracic lymph nodes. Bronchoscopy was unremarkable. Endobronchial ultrasound (EBUS) biopsy of the thoracic lymph nodes revealed squamous metaplasia. Cytology of the pleural effusion fluid revealed no malignant cells. Two erythrocyte concentrates were administered, resulting in a sufficient increase in hemoglobin. In the absence of malignant findings, the cause of the hemothorax was considered to be traumatic. Iron deficiency anemia was diagnosed, and oral iron supplementation was initiated.
The patient was transferred to our thoracic surgery unit because of the persistence of a bloody pleural effusion. We performed video-assisted thoracoscopic surgery (VATS). Lesions of the parietal pleura were subsequently diagnosed (Figure 2). The pleura was intact, thickened and irregular. There was no active bleeding. The surgeon initially thought it was mesothelioma because of the parietal pleural manifestation, although the macroscopic impression was different. Several pleural samples were taken (2 samples, maximum size 48 cm²). Due to evidence of persistent bloody pleural effusion, a double chest drain was used. The pathological findings showed no evidence of malignancy, only a hemothorax with siderin deposits and granulation tissue. However, mesothelioma was ruled out by immunohistochemical analysis. Histopathological examination revealed strong nuclear BAP expression in the proliferated MSLN cultures. After incubation with an antibody against Ber-EP4 (EpCAM), carcinosis was excluded. The proliferating cells expressed Pan-Cytokeratin and WT-1 as well as focally weak calretinin. The immunohistological findings were suggestive of reactive proliferation, and neoplastic proliferation was consequently excluded (Figure 3). The chest tube was removed on the 3rd postoperative day, and the patient was discharged to the pulmonary department on the 5th postoperative day.

Figure 2 Image of the 1st video-assisted thoracoscopic surgery (VATS) after pleural biopsy showing abnormalities in the parietal pleura.
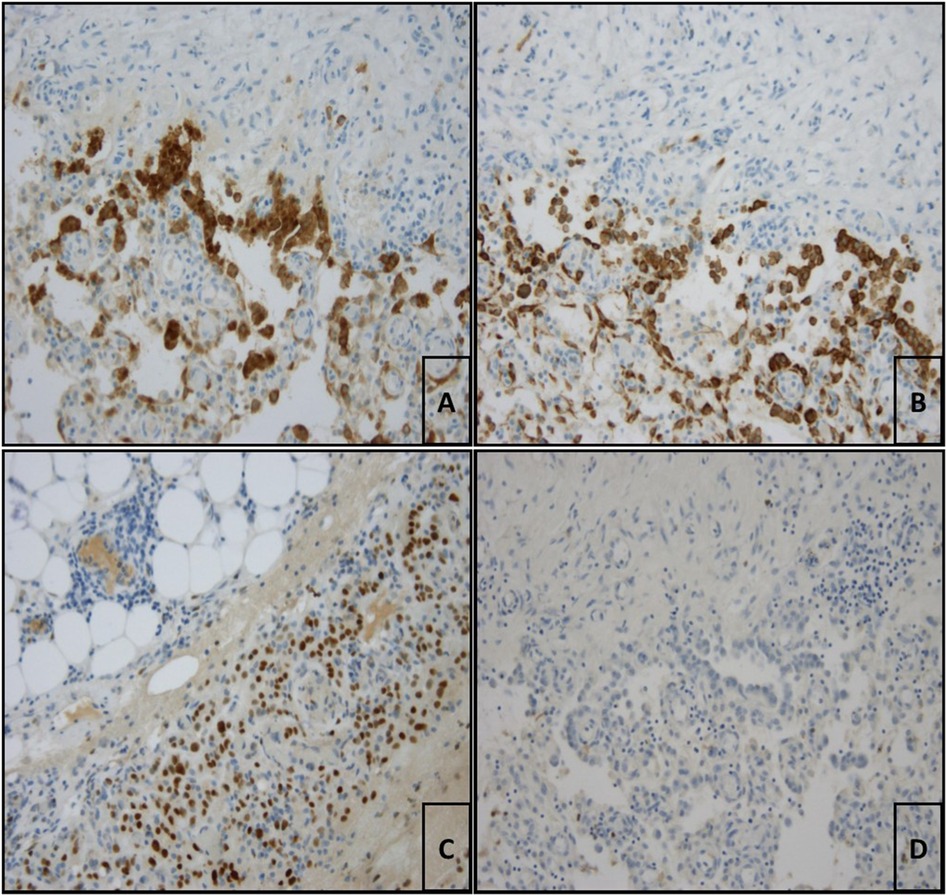
Figure 3 (A) Pleura visceralis with evidence of reactive proliferated mesothelia which are cytoplasmically labelled with antibodies against calretinin (calretinin (ZM85), 1:150 dilution, ZETA corporation, monospecific mouse-AK, IgG1/k; 200× magnification). (B) Pleura viszeralis showing proliferating mesothelia stained positive for panzytokeratin (Anti-Humanic Cytokeratin (pan), 1:75 dilution, Zytomed, AE1/AE3, mouse IgG1/k mouse IgG1/k; 200× magnification). (C) According to the immunstaining proliferating cells express WT-1 (WT49, 1:10 dilution, Leica; 200× magnification). (D) No immunohistological marking of the tumor cells with Ber-EP4 (EpCAM, Ber-EP4 (4), 1:100 dilution, DAKO; 200× magnification) which ruled out mesothelioma.
Surprisingly, the patient developed a right pleural effusion, which was also managed with a chest tube. Due to the persistence of the pleural effusion and the need for erythrocyte transfusion, VATS, which was performed by the same surgeon, was also indicated for the right hemothorax. The findings on the right side were almost identical to those on the left side. Multiple biopsies (3 samples, maximum size 12 cm²) were taken, the hemothorax was treated with a double chest tube, and the patient was transferred to our ICU. Unfortunately, the histopathological examination was inconclusive due to incorrect selection of antigens. However, due to increasing hemorrhagic effusion through the chest tube, surgical revision was mandatory. The bleeding was managed by right-sided thoracotomy, which allowed tamponade and extensive biopsy. The recurrent bleeding eventually led to the creation of a thoracostomy to facilitate repeated tamponade changes.
An additional pathological report with further immunohistochemical staining for cluster of differentiation (CD) revealed the following results: massively proliferating epithelioid cells strongly positive for Erythroblast-transformation-specific Related Gene (ERG) and CD31. CD34 staining was negative. The neoplastic cells coexpressed D2-40 (podoplanin) and Ki67, with a proliferation rate of 80%. The final diagnosis was pleural manifestation of epithelioid angiosarcoma (Figure 4).
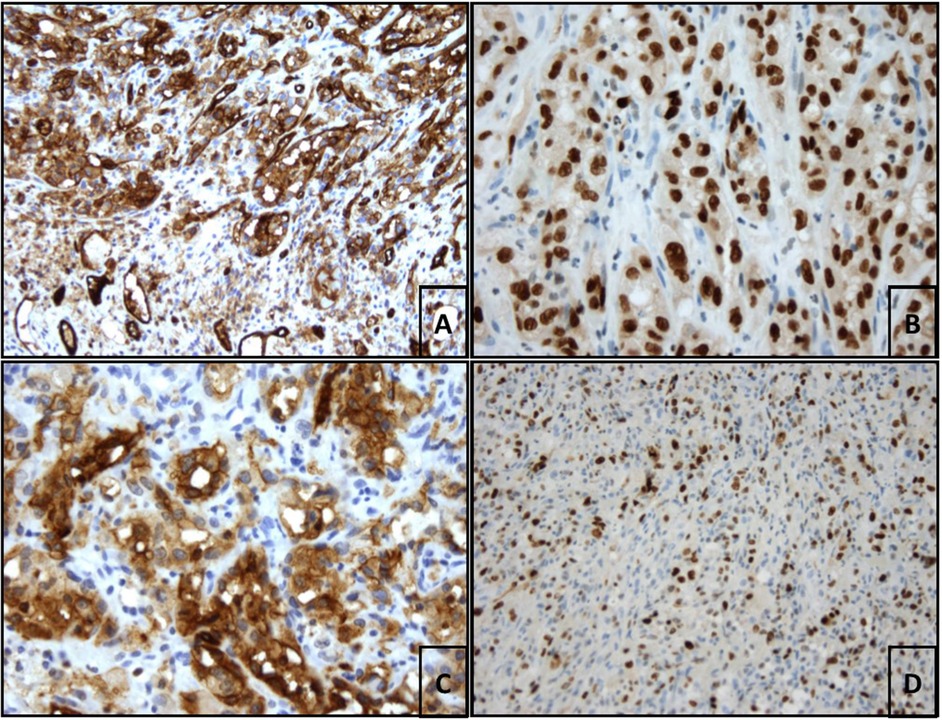
Figure 4 (A) Benign CD31-positive endothelia in the lower third of the image. In the upper 2 thirds of the image strongly CD31-positive epitheloid endothelial cells of the angiosarcoma (CD31 (JC70A), ready-to-use, Leica; 200× magnification. (B) Strong nuclear ERG staining of epitheloid tumor cells (EP111, ready-to-use, DAKO, 400× magnification). (C) Strong diffuse cytoplasmic staining of the neoplastic cells with antibodies against D2-40 (podoplanin, D2-40 (7), 1:50 delution, DAKO; 400× magnification). (D) Focal Ki67-proliferation rate of 80% (SP6, 1:100 delution, rabit IgG, Zytomed; 200× magnification).
Due to the poor prognosis and general condition with a Karnosky score of 30%, the patient was finally transferred to our palliative care unit. A CT scan of the head revealed multiple cerebral metastases. The patient was discharged at the request of her family.
According to Morgan et al., “spontaneous hemothorax is defined as a pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit and the absence of natural or iatrogenic trauma to the lung or pleural space” (10). However, the most common etiologies of hemathorax are traumatic, iatrogenic and coagulopathy (11). Cancer rarely causes true spontaneous hemathorax. However, bloody effusion is often associated with malignancy.
Rare cancers, such as hemangioendothelioma and hemangiosarcoma of the pleura or lung (12); schwannoma (13); primitive neuroectodermal, hepatocellular, vascular and germ cell tumors; primary lung cancer; mesothelioma; and malignancies leading to extramedullary hematopoiesis, are associated with spontaneous hematomas (10). Nonmalignant causes include endometriosis (14); neurofibromatosis type 1, also known as Recklinghausen's disease (15); and vascular events such as ruptured aortic aneurysm, pulmonary infarction from pulmonary embolism, necrotizing lung infection and hemopneumothorax (10). In addition, when spontaneous hemothorax occurs bilaterally, other differential diagnoses are more likely than when it occurs unilaterally.
Pleural epithelioid hemangiosarcoma has no specific clinical or imaging manifestations, so the diagnosis is based on histological findings, including immunohistochemical staining. It should be differentiated from adenocarcinoma, hemangioendothelioma, mesothelioma and pulmonary epithelioid hemangiosarcoma (12). Other authors have noted the difficulties in differentiating malignant mesothelioma from epithelioid hemangiosarcoma (7, 9) or even benign diagnoses (8).
As J. W. Goethe said, “You only see what you already know”. Therefore, physicians treating spontaneous hemothorax need to have broad knowledge of the possible, sometimes rare, etiologies. Preoperative investigations revealed no evidence of malignancy. Due to the persistence of the pleural effusion, the patient underwent surgery by an experienced thoracic surgeon who questioned the commonly suspected diagnosis of malignant mesothelioma because of lesions of the parietal pleura. This case highlights the need for appropriate communication between surgeons and pathologists. Even when biopsies are suitable for diagnosis, misdiagnosis can occur due to misinterpretation of the clinical course and misunderstanding of tumor localization and cell morphology, leading to inappropriate immunohistochemical staining programs. The cell morphology of angiosarcoma is diverse. Tumor cells may mimic fibroblasts or epithelial cells, making differential diagnosis difficult (7). According to the literature, pleural epithelioid angiosarcoma tumor cells are often positive for CD31 (5–7, 16, 17), vimentin (5–7, 16) and WT1 (17, 7). Analysis of CD34 may be positive (5, 6) or negative (14). The results published by Roh et al. showed weak positivity for factor VIII (5). Other authors published immunohistochemical analysis with negative factor VIII staining (16). Our staining was strongly positive for CD31, negative for CD34 and positive for D2-40 (podoplanin). The analysis published by Durani et al. was also positive for D2-40 (17). According to Sullivan et al., ERG staining is highly suitable for the cytological diagnosis of angiosarcoma (18).
Pleural angiosarcoma should be considered in the differential diagnosis of recurrent pleural effusion of unknown etiology. An excisional biopsy is required to rule out the diagnosis. However, if the clinical course and intraoperative findings are not consistent with the histopathological findings, the diagnosis must be questioned, and further immunohistochemical staining is mandatory.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
N-DJ: Conceptualization, Visualization, Writing – original draft, Writing – review & editing, Data curation. CM: Writing – review & editing, Supervision. JK: Visualization, Writing – review & editing, Supervision. SS: Supervision, Writing – review & editing. MK: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to acknowledge the legal representatives of the patient for allowing the case to be published.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. (2010) 11(10):983–91. doi: 10.1016/S1470-2045(10)70023-1
2. Alexiou C, Clelland CA, Robinson D, Morgan WE. Primary angiosarcomas of the chest wall and pleura. Eur J Cardiothorac Surg. (1998) 14(5):523–6. doi: 10.1016/s1010-7940(98)00211-5
3. Wang X, Lu Z, Luo Y, Cai J, Wei J, Liu A, et al. Characteristics and outcomes of primary pleural angiosarcoma: a retrospective study of 43 published cases. Medicine (Baltimore). (2022) 101(6):e28785. doi: 10.1097/MD.0000000000028785
4. Sturm EC, Marasco IS, Katz SC. Multidisciplinary management of angiosarcoma—a review. J Surg Res. (2021) 257:213–20. doi: 10.1016/j.jss.2020.07.026
5. Roh MS, Seo JY, Hong SH. Epithelioid angiosarcoma of the pleura: a case report. J Korean Med Sci. (2001) 16(6):792–5. doi: 10.3346/jkms.2001.16.6.792
6. Zhang S, Zheng Y, Liu W, Yu X. Primary epithelioid angiosarcoma of the pleura: a case report and review of literature. Int J Clin Exp Pathol. (2015) 8(2):2153–8.25973118
7. Fan C, Liu Y, Lin X, Han Y, He A, Wang E. Epithelioid angiosarcoma at chest wall which needs to be carefully distinguished from malignant mesothelioma: report of a rare case. Int J Clin Exp Pathol. (2014) 7(12):9056–60.25674287
8. Kurtz JE, Serra S, Duclos B, Brolly F, Dufour P, Bergerat JP. Diffuse primary angiosarcoma of the pleura: a case report and review of the literature. Sarcoma. (2004) 8(4):103–6. doi: 10.1080/1357-7140400003596
9. Kao YC, Chow JM, Wang KM, Fang CL, Chu JS, Chen CL. Primary pleural angiosarcoma as a mimicker of mesothelioma: a case report **VS**. Diagn Pathol. (2011) 6:130. doi: 10.1186/1746-1596-6-130
10. Morgan CK, Bashoura L, Balachandran D, Faiz SA. Spontaneous hemothorax. Ann Am Thorac Soc. (2015) 12(10):1578–82. doi: 10.1513/AnnalsATS.201505-305CC
11. Broderick SR. Hemothorax: etiology, diagnosis, and management. Thorac Surg Clin. (2013) 23(1):89–96, vi–vii. doi: 10.1016/j.thorsurg.2012.10.003
12. Sanxi A, Yalan B, Qinfeng Z, Xinchao L, Jing Z, Wei Z, et al. Pleural epithelioid hemangioendothelioma: a case report and review of the literature. Zhonghua Jie He He Hu Xi Za Zhi. (2015) 38(3):174–8.26269304
13. Xiang Y, Yan L, Lin X, Zhang X, Zhang F, Wu Z. Posterior mediastinal epithelioid angiosarcoma arising in schwannoma: a case report and review of the literature. Front Surg. (2021) 8:666389. doi: 10.3389/fsurg.2021.666389
14. Augoulea A, Lambrinoudaki I, Christodoulakos G. Thoracic endometriosis syndrome. Respiration. (2008) 75(1):113–9. doi: 10.1159/000105102
15. Degbelo FDA, Cito G, Guendil B, Christodoulou M, Abbassi Z. Spontaneous hemothorax in a patient with von Recklinghausen’s disease: a case report and review of the literature. Am J Case Rep. (2019) 20:674–8. doi: 10.12659/AJCR.915810
16. Maglaras GC, Katsenos S, Kakadelis J, Katsanos C, Metafratzi Z, Stefanou DG, et al. Primary angiosarcoma of the lung and pleura. Monaldi Arch Chest Dis. (2004) 61(4):234–6. doi: 10.4081/monaldi.2004.687
17. Durani U, Gallo de Moraes A, Beachey J, Nelson D, Robinson S, Anavekar NS. Epithelioid angiosarcoma: a rare cause of pericarditis and pleural effusion. Respir Med Case Rep. (2018) 24:77–80. doi: 10.1016/j.rmcr.2018.04.008
Keywords: sarcoma, angiosarcoma, thoracic surgery, rare disease (RD), pleura
Citation: Dörr-Jerat NM, May C, Knolle J, Schmidt S and Krüger M (2024) Case Report: Epithelioid angiosarcoma of the pleura. Front. Surg. 11:1393159. doi: 10.3389/fsurg.2024.1393159
Received: 28 February 2024; Accepted: 7 June 2024;
Published: 27 June 2024.
Edited by:
Marcelo Jimenez, University of Salamanca, SpainReviewed by:
Nicoletta Pia Ardò, Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, Italy© 2024 Dörr-Jerat, May, Knolle, Schmidt and Krüger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niels Michael Dörr-Jerat, bmllbHNkb2VyckBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.