
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Surg. , 05 April 2024
Sec. Surgical Oncology
Volume 11 - 2024 | https://doi.org/10.3389/fsurg.2024.1386708
Background: Managing postoperative pancreatic fistula (POPF) presents a formidable challenge after pancreatoduodenectomy. Some centers consider pancreatic duct occlusion (PDO) in reoperations following pancreatoduodenectomy as a pancreas-preserving procedure, aiming to control a severe POPF. The aim of the current study was to evaluate the short- and long-term outcomes of employing PDO for the management of the pancreatic stump during relaparotomy for POPF subsequent to pancreatoduodenectomy.
Methods: Retrospective review of consecutive patients at Oslo University Hospital undergoing pancreatoduodenectomy and PDO during relaparotomy. Pancreatic stump management during relaparotomy consisted of occlusion of the main pancreatic duct with polychloroprene Faxan-Latex, after resecting the dehiscent jejunal loop previously constituting the pancreaticojejunostomy.
Results: Between July 2005 and September 2015, 826 pancreatoduodenectomies were performed. Overall reoperation rate was 13.2% (n = 109). POPF grade B/C developed in 113 (13.7%) patients. PDO during relaparotomy was performed in 17 (2.1%) patients, whereas completion pancreatectomy was performed in 22 (2.7%) patients. Thirteen (76%) of the 17 patients had a persistent POPF after PDO, and the time from PDO until removal of the last abdominal drain was median 35 days. Of the PDO patients, 13 (76%) patients required further drainage procedures (n = 12) or an additional reoperation (n = 1). In-hospital mortality occurred in one patient (5.9%). Five (29%) patients developed new-onset diabetes mellitus, and 16 (94%) patients acquired exocrine pancreatic insufficiency.
Conclusions: PDO is a safe and feasible approach for managing severe POPF during reoperation following pancreatoduodenectomy. A significant proportion of patients experience persistent POPF post-procedure, necessitating supplementary drainage interventions. The findings suggest that it is advisable to explore alternative pancreas-preserving methods before opting for PDO in the management of POPF subsequent to pancreatoduodenectomy.
Postoperative pancreatic fistula (POPF) is a well-known and challenging complication following pancreatoduodenectomy. POPF is characterized by a high rate of morbidity and mortality. Percutaneous drainage is the most common primary intervention for severe POPF (1). In selected cases, a reoperation is deemed necessary to mitigate POPF. During relaparotomy, several strategies are feasible: completion pancreatectomy, disconnection of anastomosis with preservation of the pancreatic remnant, establishing internal or external wirsungostomy, salvage pancreaticogastrostomy, or optimal surgical drains along the pancreas (2–4). Current evidence suggests that opting for a pancreas-preserving procedure is a more favorable choice than completion pancreatectomy in patients requiring a relaparotomy (5). Prophylactic pancreatic duct occlusion (PDO) has been explored as a substitute to pancreaticojejunostomy in pancreatoduodenectomy, aiming to reduce the risk of POPF (6–8). Several techniques of pancreatic stump occlusion have been described (6). Some centers also consider PDO in post-pancreatoduodenectomy reoperations as a pancreas-preserving procedure, aiming to control severe POPF (7, 9). The aim of the current study was to evaluate the short- and long-term outcomes of PDO in managing the pancreatic remnant during a relaparotomy for POPF following pancreatoduodenectomy.
This was a retrospective review of consecutive patients undergoing pancreatoduodenectomy at Oslo University Hospital between July 2005 and September 2015. Patients who underwent PDO at reoperation to treat a POPF were identified from the institutional pancreatic database. Hospital records and pathology reports were reviewed. Postoperative complications were assessed according to the Clavien-Dindo classification. The Clavien-Dindo grading was used to calculate the Comprehensive Complication Index (CCI), by means of the online tool. POPF, including persistent POPF after PDO, was defined and graded according to criteria set by the International study Group on Pancreatic Fistula (10). The hospital review board approved the study (19/04710) according to the general guidelines provided by the Regional Ethics Committee. The manuscript was completed in accordance with the STROBE statement. Duration of hospital stay was calculated from the day of pancreatoduodenectomy until discharge. Ninety-day mortality and in-hospital mortality were defined as death within 90 days after surgery or during hospital stay, respectively. The surgical procedure consisted of pancreatoduodenectomy with standard lymphadenectomy. Pylorus-preserving pancreatoduodenectomy has been the standard approach since 2012, whereas a classic Whipple procedure was preferred between 2005 and 2011 (11). The pancreaticojejunostomy was performed in an end-to-side, two-layer, duct-to-mucosa technique (12). PDO was performed at the discretion of the treating surgeon. Pancreatic stump management during relaparotomy consisted of occlusion of the main pancreatic duct by polychloroprene Faxan-Latex (Oslo University Hospital Pharmacy, Oslo, Norway), after resecting the dehiscent jejunal loop previously anastomosed to the pancreas. In addition, the pancreatic duct was occluded at the resection margin with a non-absorbable U-suture. Faxan-Latex was no longer available in our institution from September 2015.
Descriptive statistics were collected and reported as a whole number (percentage) and median (range or interquartile range). Statistical analysis was carried out using IBM SPSS Statistics, Version 29.0.
Between July 2005 and September 2015, 826 pancreatoduodenectomies were performed, and 113 (13.7%) patients developed POPF grade B/C. Completion pancreatectomy was performed in 22 (2.7%) patients. PDO with polychloroprene Faxan-Latex during relaparotomy was performed in 17 (2.1%) patients. Baseline surgical and histopathological characteristics of these 17 patients are presented in Table 1. There were 8 male and 9 female with a median age of 71 years (range 63–79). Ten patients had periampullary adenocarcinoma (distal bile duct, ampullary, duodenal), two patients had pancreatic ductal adenocarcinoma, three patients had premalignant tumors, one patient had a neuroendocrine tumor, and one patient had chronic pancreatitis. Main outcomes are presented in Table 2. Hospital length of stay (LOS) was median 41 days. Thirteen (76%) patients had a persistent POPF after PDO, and time from PDO to removal of the last abdominal drain was median 35 days. These 13 patients required additional drainage procedures (n = 12) or a re-operation (n = 1). CCI was median 54, 90-day mortality was zero, whereas in-hospital mortality occurred in one patient (5.9%) after 135 days. No patients underwent a subsequent completion pancreatectomy. Postoperative new-onset exocrine or endocrine insufficiency occurred in 16 (94%) and 5 (29%) patients, respectively. In addition, two surgical complications were identified. One patient developed an intraabdominal abscess close to the pancreatic tail 17 months after the time of PDO, and one patient experienced perforation of the glue and a baby-feeding tube left behind in the pancreatic duct during PDO, to the jejunal loop, re-establishing a connection between the pancreatic duct and the jejunum.
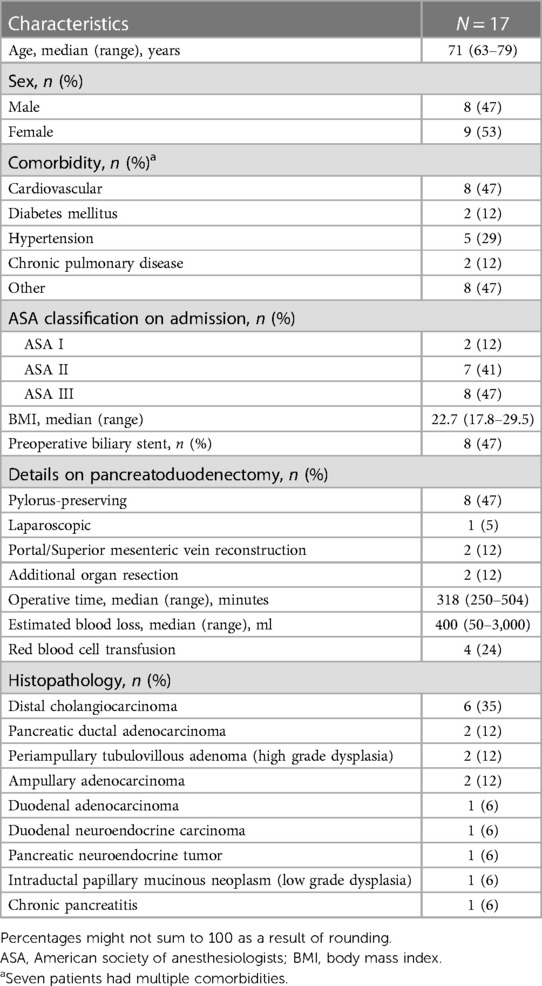
Table 1. Baseline, surgical and histopathological characteristics in patients treated with pancreatic duct occlusion.
Overall the reoperation rate after pancreatoduodenectomy during the study period was 13.2% (n = 109). PDO was performed in 17 (15.6%) of the reoperations, whereas completion pancreatectomy was performed in 22 (20.2%). For numerical comparison, CCI was median 84.3 and 90-day mortality 22.7% in the 22 patients undergoing completion pancreatectomy. Main procedures performed in the remaining 70 reoperations were: control of intraabdominal bleeding (n = 32), redo or suture of hepaticojejunostomy (n = 10), drainage or suture of bile leak (n = 5), suture of wound dehiscence (n = 4), delayed reconstruction of the three anastomoses (n = 4), revascularization of portal vein or hepatic artery due to thrombosis (n = 4), small bowel resection or adhesiolysis due to small bowel obstruction (n = 3), redo gastrojejunostomy due to duodeneojejunostomy leak (n = 2), redo gastrojejunostomy due to obstruction (n = 2), suture of stomach perforation (n = 1), splenectomy due to spleen necrosis (n = 1), suture of pancreaticojejunostomy (n = 1), and colectomy due to bowel necrosis (n = 1). Twenty-one (30%) of these 70 patients had a POPF needing optimal drainage during the reoperation.
For historical comparison, between October 2015 and December 2023, 896 patients underwent pancreatoduodenectomy. Reoperation rate was 11.5% (n = 103), and of these POPF was the main cause in 30 patients. Completion pancreatectomy was performed in nine (1%) patients, and no patients were treated with PDO.
The current study demonstrates that PDO is a safe and feasible approach for managing severe POPF after pancreatoduodenectomy. However, 76% of patients still had a persistent POPF after undergoing PDO. Moreover, 29% of patients developed new-onset diabetes mellitus, and two patients developed operative complications.
Only a few studies have examined the use of PDO for the treatment of POPF after pancreatoduodenectomy (9). Balzano's findings did not confirm the potential benefits of PDO, as they found that PDO and simple drainage through relaparotomy led to similar outcomes (9). The high rate of persistent POPF (76%) after PDO in this study is of concern, as it necessitated prolonged drainage for a median of 35 days. During PDO, the dehiscent jejunal loop is resected to convert an “activated” POPF into a “pure” POPF, thus preventing the mixing of pancreatic enzymes with bilioenteric secretions. The debate surrounding whether to perform a completion pancreatectomy or a pancreas-preserving procedure during relaparotomy for POPF after pancreatoduodenectomy is ongoing, and the rates of completion pancreatectomy vary among different centers (5, 13). The rate of completion pancreatectomy in the current study of 2.7%, is similar to the rate reported in a large study conducted in Heidelberg of 3% (120 out of 3,953), but higher than the rate reported in a multicenter study conducted in the Netherlands of 0.74% (36 out of 4,877) (5, 13). Starting from October 2015 and until December 2023, completion pancreatectomy was performed in 1% of all pancreatoduodenectomies, PDO was discontinued, and there was a shift towards a more conservative strategies, in line with several other reports (1, 9).
The Dutch Pancreatic Cancer Group conducted a study in 2017 showing that percutaneous drainage as the initial intervention for severe POPF after pancreatoduodenectomy was associated with better clinical outcomes, including lower mortality, in comparison to primary relaparotomy (1). The authors attribute the success of percutaneous drainage to adherence to two main surgical principles: ensuring adequate source control and avoiding further harm. The in-hospital mortality rate was 14.1% (nine out of 64) in the Dutch study with a mini-invasive pancreas-preserving procedure, compared to 5.9% (one out of 17) in the current study with relaparotomy and PDO. The higher mortality rate may be attributed to a higher disease severity and a higher incidence of single or multi-organ failure in the Dutch study of 34.4% compared to 23.5% (four out of 17) in our study. It is important to acknowledge that comparison of such studies is challenging because the choice of treatment for severe POPF after pancreatoduodenectomy is both center and surgeon dependent (14). Still, it is noteworthy that the time interval from pancreatoduodenectomy until last abdominal drain could be safely removed was median 29 days in the Dutch study that utilized primary percutaneous drainage. In the current study, this timeframe was extended to 41 days (with a median of 35 days after PDO) which indicates that PDO does not completely heal the POPF.
Importantly, 29% of the patients developed postoperative new-onset diabetes mellitus after PDO. Mazzaferro showed that 36.7% of the patients developed new-onset diabetes after neoprene-based PDO during the primary operation in a selected group of pancreatoduodenectomy patients at high risk of POPF (7). Another study comparing total pancreatectomy with pancreatoduodenectomy in patients with high risk pancreatic anastomosis observed new-onset diabetes in 13% of the patients undergoing pancreatoduodenectomy (15). Thus, the rate of new-onset diabetes mellitus is seemingly higher in patients undergoing elective or emergent PDO compared to conventional anastomosis.
Some limitations of this study should be acknowledged. Firstly, this is a small, retrospective, single-center study with a high risk of selection bias. The indication for relaparotomy may differ depending on the surgeons' evaluation of the patients' condition, and the criteria for performing PDO or completion pancreatectomy may have differed among surgeons and from case to case. Secondly, no comparison was made with alternative approaches, as it was beyond the scope of this study to compare PDO with completion pancreatectomy or conservative strategies. There is a lack of consensus among surgeons on the indications for reoperations and the appropriate intraoperative approach during reoperations in cases of severe POPF following pancreatoduodenectomy (14). Further studies are needed to clarify the dilemma of whether or not to reoperate for a severe POPF following pancreatoduodenectomy, and if a relaparotomy is deemed necessary, which approach should be attempted. The use of PDO in the treatment of severe POPF after pancreatoduodenectomy has been discontinued in our institution, primarily due to pharmaceutical regulations making the glue unavailable for routine clinical purposes. A recent review found that the potential advantages of PDO remain questionable when compared to debridement and drainage (4). The findings of this study, which include persistent POPF and prolonged drainage, long-term exocrine and endocrine pancreatic insufficiency, and local complications after PDO, suggest that it is advisable to explore alternative pancreas-preserving techniques before considering PDO.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Oslo University Hospital Review Board approved the study (Reference#: 19/04710). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this is a quality study of treatment performed at the hospital thus written informed consent was waived.
SY: Conceptualization, Data curation, Methodology, Project administration, Writing – original draft, Writing – review & editing. BR: Writing – original draft, Writing – review & editing. IG: Writing – original draft, Writing – review & editing. KL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Smits FJ, van Santvoort HC, Besselink MG, Batenburg MCT, Slooff RAE, Boerma D, et al. Management of severe pancreatic fistula after pancreatoduodenectomy. JAMA Surg. (2017) 152(6):540–8. doi: 10.1001/jamasurg.2016.5708
2. Bouras AF, Marin H, Bouzid C, Pruvot FR, Zerbib P, Truant S. Pancreas-preserving management in reinterventions for severe pancreatic fistula after pancreatoduodenectomy: a systematic review. Langenbecks Arch Surg. (2016) 401(2):141–9. doi: 10.1007/s00423-015-1357-0
3. Malgras B, Dokmak S, Aussilhou B, Pocard M, Sauvanet A. Management of postoperative pancreatic fistula after pancreaticoduodenectomy. J Visc Surg. (2023) 160(1):39–51. doi: 10.1016/j.jviscsurg.2023.01.002
4. Torres OJM, Moraes-Junior JMA, Fernandes ESM, Hackert T. Surgical management of postoperative grade C pancreatic fistula following pancreatoduodenectomy. Visc Med. (2022) 38(4):233–42. doi: 10.1159/000521727
5. Groen JV, Smits FJ, Koole D, Besselink MG, Busch OR, den Dulk M, et al. Completion pancreatectomy or a pancreas-preserving procedure during relaparotomy for pancreatic fistula after pancreatoduodenectomy: a multicentre cohort study and meta-analysis. Br J Surg. (2021) 108(11):1371–9. doi: 10.1093/bjs/znab273
6. Giglio MC, Cassese G, Tomassini F, Rashidian N, Montalti R, Troisi RI. Post-operative morbidity following pancreatic duct occlusion without anastomosis after pancreaticoduodenectomy: a systematic review and meta-analysis. HPB (Oxford). (2020) 22(8):1092–101. doi: 10.1016/j.hpb.2020.04.014
7. Mazzaferro V, Virdis M, Sposito C, Cotsoglou C, Droz Dit Busset M, Bongini M, et al. Permanent pancreatic duct occlusion with neoprene-based glue injection after pancreatoduodenectomy at high risk of pancreatic fistula: a prospective clinical study. Ann Surg. (2019) 270(5):791–8. doi: 10.1097/SLA.0000000000003514
8. Chierici A, Frontali A, Granieri S, Facciorusso A, De’ Angelis N, Cotsoglou C. Postoperative morbidity and mortality after pancreatoduodenectomy with pancreatic duct occlusion compared to pancreatic anastomosis: a systematic review and meta-analysis. HPB (Oxford). (2022) 24(9):1395–404. doi: 10.1016/j.hpb.2022.03.015
9. Balzano G, Pecorelli N, Piemonti L, Ariotti R, Carvello M, Nano R, et al. Relaparotomy for a pancreatic fistula after a pancreaticoduodenectomy: a comparison of different surgical strategies. HPB (Oxford). (2014) 16(1):40–5. doi: 10.1111/hpb.12062
10. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. (2017) 161(3):584–91. doi: 10.1016/j.surg.2016.11.014
11. Kleive D, Sahakyan MA, Berstad AE, Verbeke CS, Gladhaug IP, Edwin B, et al. Trends in indications, complications and outcomes for venous resection during pancreatoduodenectomy. Br J Surg. (2017) 104(11):1558–67. doi: 10.1002/bjs.10603
12. Lindholm E, Bergmann GB, Haugaa H, Labori KJ, Yaqub S, Bjornbeth BA, et al. Early detection of anastomotic leakage after pancreatoduodenectomy with microdialysis catheters: an observational study. HPB (Oxford). (2022) 24(6):901–9. doi: 10.1016/j.hpb.2021.10.020
13. Loos M, Konig AK, von Winkler N, Mehrabi A, Berchtold C, Muller-Stich BP, et al. Completion pancreatectomy after pancreatoduodenectomy: who needs it? Ann Surg. (2023) 278(1):e87–93. doi: 10.1097/SLA.0000000000005494
14. Casciani F, Bassi C, Vollmer CM Jr. Decision points in pancreatoduodenectomy: insights from the contemporary experts on prevention, mitigation, and management of postoperative pancreatic fistula. Surgery. (2021) 170(3):889–909. doi: 10.1016/j.surg.2021.02.064
Keywords: duct occlusion, postoperative pancreatic fistula, pancreatoduodenectomy, pancreatic surgery, reoperation
Citation: Yaqub S, Røsok B, Gladhaug IP and Labori KJ (2024) Pancreatic duct occlusion with polychloroprene-based glue for the management of postoperative pancreatic fistula after pancreatoduodenectomy—an outdated approach?. Front. Surg. 11:1386708. doi: 10.3389/fsurg.2024.1386708
Received: 15 February 2024; Accepted: 26 March 2024;
Published: 5 April 2024.
Edited by:
Luca Nespoli, University of Milano-Bicocca, ItalyReviewed by:
Salvatore Paiella, University of Verona, Italy© 2024 Yaqub, Røsok, Gladhug and Labori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheraz Yaqub c2hlcmF6LnlhcXViQG1lZGlzaW4udWlvLm5v
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.