- Department of Retroperitoneal Tumor Surgery, Peking University International Hospital, Beijing, China
Background: Castleman disease (CD) is a rare lymphoproliferative disorder that can occur anywhere along the lymphatic pathway. Retroperitoneal unicentric Castleman disease (UCD) is an extremely rare manifestation. This study aims to explore the clinical features and surgical treatment of retroperitoneal UCD.
Methods: We retrospectively reviewed patients who underwent retroperitoneal tumor surgery and were diagnosed with CD based on postoperative pathology before December 31, 2022. Data from these patients were collected and analyzed.
Results: A total of 15 patients were included in the final analysis. All patients underwent radical resection under general anesthesia. Two out of 15 patients (13.3%) experienced serious complications but recovered well. There were no perioperative deaths. The median follow-up time was 78.5 months (range: 18–107.5 months), and no deaths or recurrences occurred during this period.
Conclusions: Surgical treatment for retroperitoneal UCD is safe. Patients with retroperitoneal UCD can achieve long-time survival through complete resection.
1 Introduction
Castleman disease (CD) comprises a group of heterogeneous disorders involving lymphoid tissue and is considered very rare. Based on the number of lymph node stations involved, CD can be classified into unicentric CD (UCD) and multicentric CD (MCD) (1, 2). Histologically, CD can be further divided into hyaline-vascular, plasma cell, and mixed types. However, our understanding of the epidemiology and etiology of CD remains limited (3, 4).
The treatment and prognosis of MCD are complex and significantly differ. Although consensus exists that surgical resection should be considered for UCD patients whenever feasible, managing UCD occurring in the retroperitoneum remains challenging (5–7). The deep location, complex surrounding organs, and blood vessels make surgical treatment of retroperitoneal UCD high-risk. Additionally, distinguishing this disease from primary retroperitoneal sarcomas based on imaging examinations (such as lymphoma, leiomyoma, and paraganglioma) poses difficulties (8–12).
As a high-volume center specializing in retroperitoneal sarcoma treatment, we observed that some patients initially diagnosed with retroperitoneal tumors were pathologically confirmed to have CD after surgery. Given the rarity of this disease, we conducted a retrospective analysis of retroperitoneal CD patients treated in our center to gain insights into the disease's characteristics, treatment strategies, and prognosis.
2 Materials and methods
2.1 Patient selection
We retrospectively reviewed patients treated at our center from January 1, 2014 to December 31, 2022. Among them, 20 patients had a definitive pathological diagnosis of Castleman disease (CD), confirmed either by needle biopsy or surgical resection. Exclusion criteria included patients with a history of other malignancies, those who underwent needle biopsy only and declined surgery, and one patient diagnosed with multicentric Castleman disease (MCD) after thorough examination. Ultimately, 15 patients with retroperitoneal unicentric Castleman disease (UCD) were included in the final analysis.
2.2 Imaging and diagnostic criteria
All patients underwent contrast-enhanced computed tomography (CT) scans of the neck, chest, abdomen, and pelvis, or ultrasound examinations of the involved regions/organs and superficial lymph nodes. Additional systemic positron emission tomography (PET) scans were performed as needed. UCD was defined as a solitary site of mass without other suspicious lesions.
2.3 Data collection and statistical analysis
We established a comprehensive database from medical records, including patient demographics (gender, age), body mass index (BMI), presenting symptoms, blood test results, radiological lesion size, and pathology subtype. Surgical details, such as the surgical approach, operative time, estimated blood loss, length of postoperative stay, and postoperative complications, were also recorded. Patients were followed up via telephone conversations, with the last follow-up date set at November 1, 2023. The primary endpoint was disease-related death or disease recurrence. Survival analysis was conducted based on the occurrence of endpoint events during follow-up.
Categorical variables were expressed as frequencies and percentages, while continuous variables were presented as means with standard deviation (SD) or medians with ranges or interquartile ranges (IQRs), depending on the distribution normality. Data analyses were performed using SPSS v22 statistical software (Chicago, IL, USA).
2.4 Ethics approval and informed consent
This study adhered to the ethical standards outlined by the responsible committee on human experimentation (both institutional and national) and followed the principles of the Declaration of Helsinki (1964 and subsequent revisions). The Institutional Review Board of Peking University International Hospital approved the study, and informed consent (or an appropriate substitute) was obtained from all patients before their inclusion.
3 Results
3.1 Clinical features
The clinical characteristics of 15 retroperitoneal UCD patients are summarized in Table 1. The ratio of male to female patients was 2.75:1.00. The median age was 31 years (range, 24–58 years), with 80% patients younger than 40 years. The histology subtype was hyaline-vascular for 10 patients (66.7%), mixed type for 5 patients (33.3%), and 0 plasma cell type. B symptoms (fever, night sweats, and weight loss) were present in 2 patients (13.3%). Pleural effusion was found in 1 patient (6.7%). Splenomegaly was found in 1 patient (6.7%). Most lesion sizes were smaller than 10 cm (6.9 ± 2.3 cm) (showed in Table 1). The laboratory tests were generally normal for all patients, including blood routine examination, serum biochemical indicators, C-reactive protein and renal function (showed in Table 1). Only one patient had suspected TAFRO syndrome, with splenomegaly and pleural effusion.
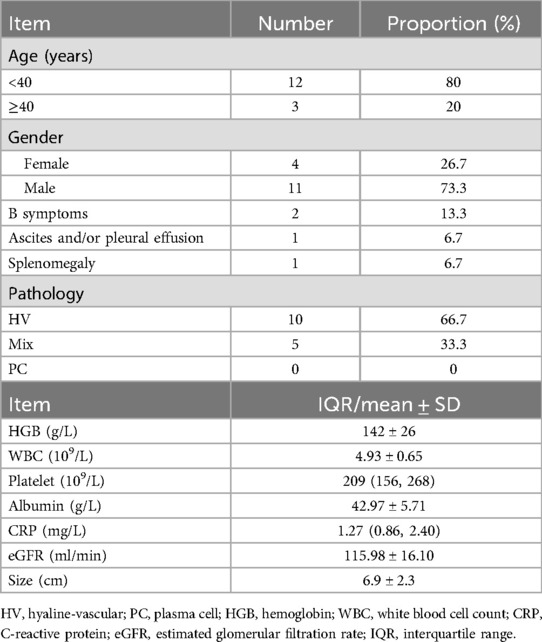
Table 1. Clinicopathological characteristics of 15 patients with retroperitoneal unicentric Castleman disease.
3.2 Surgical details
Patients were admitted to hospital for retroperitoneal lesions suspected of malignant sarcomas. The surgical strategy aimed for radical resection with adjacent tissue dissection, ensuring negative margins.
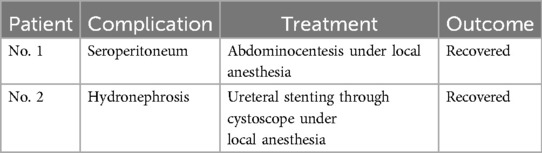
Thirteen patients underwent traditional open surgery, while two patients received laparoscopic surgery. The average operation time was 186 min (186 ± 57 min). Estimated intraoperation blood loss ranged from 50 ml to 1,500 ml, and median volume was 400 ml (showed in Table 2). Two patients experienced severe postoperative complications and recovered well after treatment (details in Table 3). All patients were discharged with satisfactory recovery. There were no perioperative deaths or readmissions within 30 days. The average length of stay after surgery was 11.2 days (11.2 ± 3.6 days).

Table 2. Details of surgical treatment for 15 retroperitoneal unicentric Castleman disease patients.
3.3 Follow-up results
The median follow-up time for all retroperitoneal UCD patients was 78.5 months (range, 18–107.5 months). Regular follow-up visits were conducted until the last recorded visit. Encouragingly, all patients remained alive during the follow-up period, and no evidence of disease recurrence was observed. Given the absence of endpoint events, survival analysis was omitted.
4 Discussion
Retroperitoneal Castleman disease cases are exceedingly rare worldwide. Existing literature primarily consists of case reports, often involving fewer than two cases (8–11). As a specialized center focused on the surgical management of retroperitoneal sarcomas, we present a comprehensive analysis of unicentric Castleman disease occurring in the retroperitoneum based on a cohort of patients.
4.1 Clinical characteristics and diagnostic challenges
Unlike multicentric Castleman disease (MCD), which frequently manifests with symptoms such as polyneuritis, organomegaly, endocrinopathy, and skin changes, most patients with unicentric Castleman disease (UCD) remain asymptomatic except for the localized mass. In our study, only one patient had suspected TAFRO syndrome, which was considered as a special subtype of multicentric Castleman disease (13). This patient presented with splenomegaly and pleural effusion but did not have fever or abnormal hematological markers. The remaining patients showed no significant symptoms, and objective laboratory tests and examinations revealed no abnormalities. This subtle presentation underscores the challenge of early diagnosis (14). In the case of retroperitoneal UCD, patients often lack symptoms until abdominal ultrasound or computed tomography is performed during routine physical examinations (5, 12).
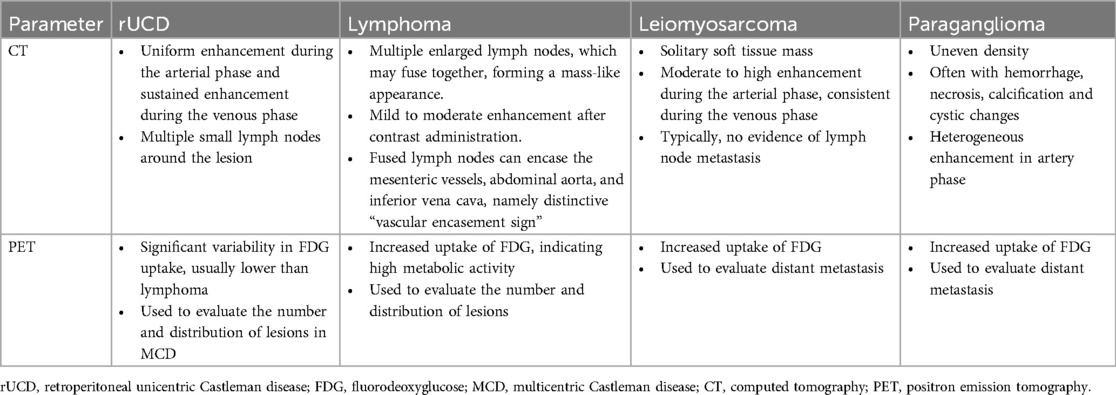
Histologically, Castleman disease encompasses three main subtypes: hyaline-vascular (accounting for 90%–91% of cases), plasma cell, and mixed type. The hyaline-vascular subtype is associated with UCD, while the plasma-cell subtype is linked to MCD (6). Definitive diagnosis of retroperitoneal UCD hinges on histological analysis of the mass. However, differential diagnosis remains challenging due to the absence of characteristic symptoms. Preoperative fine-needle aspiration is not recommended due to its low specificity and risk of tumoral seeding (15, 16, 22). Furthermore, fine-needle aspiration has limited utility in CD diagnosis, as it relies on cell architecture rather than cell morphology (15). When encountering patients with isolated retroperitoneal masses exhibiting contrast kinetics along the midline adjacent to the inferior vena cava and abdominal aorta, UCD should be considered, and differential diagnoses should include other highly hypervascular retroperitoneal tumors (e.g., lymphoma, leiomyosarcoma, and paraganglioma, et al.) (see Figures 1–3) (17–19). The imageological distinctions of retroperitoneal UCD and other retroperitoneal tumors are listed in Table 4.
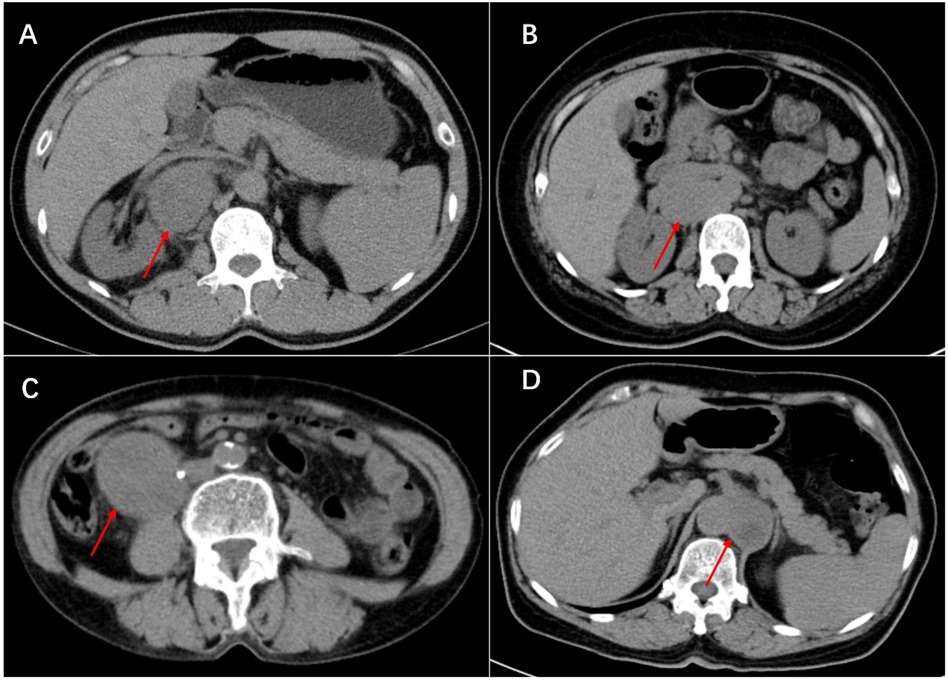
Figure 1. Plain scan of Castleman disease (A), lymphoma (B), leiomyosarcoma (C) and paraganglioma (D) in enhanced CT scan. Multiple enlarged small lymph nodes can be seen around lesion of Castleman disease and lymphoma. In the image, the leiomyosarcoma can be seen invading the right ureter, resulting in secondary hydronephrosis (not shown in the image), which was managed with a ureteral stent placement before surgery. In particular, splenomegaly can be seen in Castleman disease. They are all cases that have been definitely diagnosed by postoperative pathology.
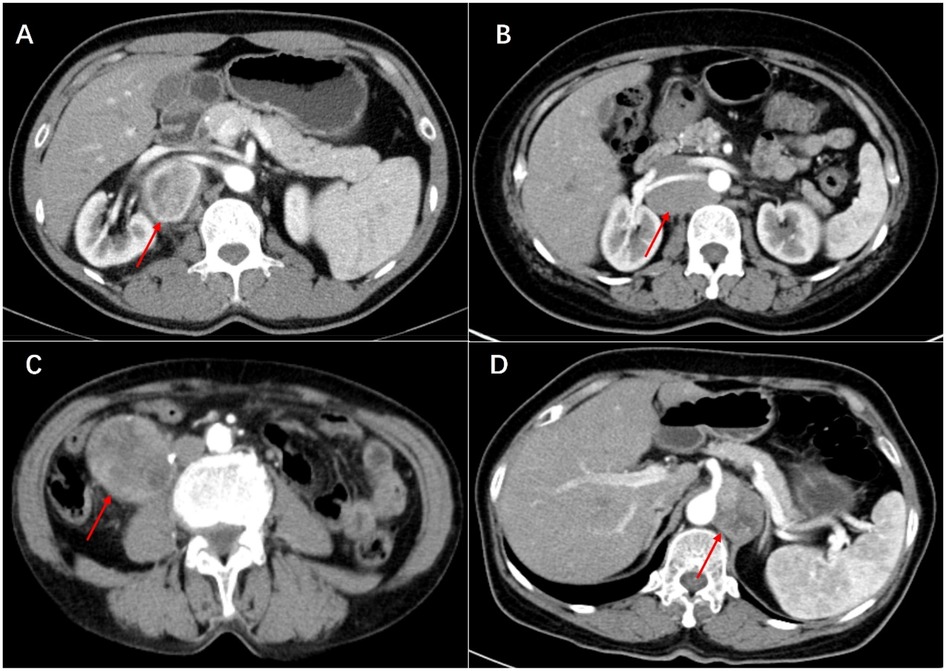
Figure 2. Artery phase of castleman disease (A), lymphoma (B), leiomyosarcoma (C) and paraganglioma (D) in enhanced CT scan. The tumors show heterogeneous enhancement in the arterial phase. All tumors are closely related to the blood vessels in the retroperitoneum, even encircling the renal artery or celiac trunk.
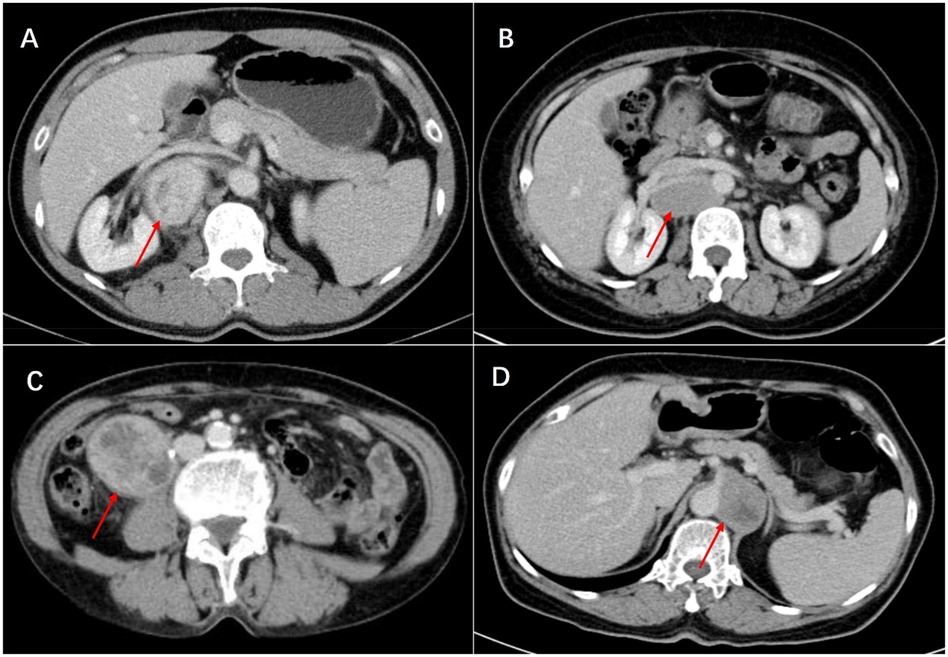
Figure 3. Venous phase of castleman disease (A), lymphoma (B), leiomyosarcoma (C) and paraganglioma (D) in enhanced CT scan. This lymphoma is highly similar to Castleman disease and surrounds the renal vessels, leading to a high risk of needle biopsy. Ultimately, surgical resection was performed, and postoperative pathology revealed invasive B-cell lymphoma.
4.2 Surgical approach and prognosis
Complete surgical resection remains the gold standard for treating UCD, including retroperitoneal UCD. The prognosis for retroperitoneal UCD is generally favorable. Most patients achieve long-term survival following R0 resection. Systematic reviews indicate that complete resection alone, without additional treatment, yields excellent outcomes, with 5-year disease-free survival (DFS) rates exceeding 80% and overall survival (OS) rates surpassing 90% (5, 6).
In our study, all 15 patients with retroperitoneal UCD underwent complete resection of the primary lesion as the initial treatment, without additional therapies. Remarkably, all patients remained alive during the follow-up period, and no evidence of disease recurrence was observed. Although our patient cohort was small, our surgical strategy—favoring extended resection margins to ensure radical cure—may have contributed to these positive outcomes. This finding underscores the importance of radical resection with negative margins in patients suspected of having UCD but lacking definitive diagnosis. Longer follow-up and larger patient cohorts are needed to validate the impact of extended resection on retroperitoneal UCD. Currently, no standardized follow-up protocol exists for resected UCD. Based on existing literature, we recommend CT scans every 6 months during the first 3 years postoperatively, followed by annual scans thereafter.
4.3 Surgical challenges and strategies
Our experience highlights the significant challenge posed by intraoperative bleeding during resection of retroperitoneal UCD. The adjacent and surrounding blood vessels, primarily branches of the abdominal aorta and inferior vena cava, contribute to this complexity. To mitigate operative risks and ensure safety, comprehensive radiographic examinations play a crucial role. These examinations should include:
• Color Ultrasonography: Provides real-time visualization of blood flow patterns and helps assess vascular relationships.
• Contrast-Enhanced Computed Tomography (CT): Offers detailed anatomical information, aiding in precise evaluation of lesion-to-vessel proximity.
• Angiography (if necessary): Allows direct visualization of vascular structures and assists in surgical planning.
By meticulously assessing the relationship between lesions and adjacent vessels, surgeons can navigate the retroperitoneal space safely. Notably, laparoscopic surgery emerged as a viable option for selected patients. In our cohort, two patients underwent laparoscopic procedures without perioperative complications, and long-term follow-up revealed no recurrences.
4.4 Study limitations
First, as a retrospective study, inherent biases in patient selection and data collection may exist. Second, Patients were often screened by other hospitals and departments before seeking our specialized team's expertise, potentially introducing additional selection bias. Besides, the rarity of retroperitoneal UCD limited the number of patients available for final analysis. Due to the small sample size, we could not directly compare different treatment strategies (e.g., incomplete resection vs. radiotherapy). As patients were initially managed as malignant sarcomas, certain Castleman disease-related details (e.g., human herpes virus 8 status, serum immunoglobulin G, interleukin-6 levels) were lacking (20, 21).
5 Conclusion
Our findings underscore that complete resection remains the gold standard for treating retroperitoneal UCD. Achieving excellent survival outcomes with minimal surgery-related morbidity validates this approach. Furthermore, experienced surgeons can safely explore laparoscopic surgery in carefully selected patients. Future studies should validate our results and deepen our understanding of this rare disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Institutional Review Board of Peking University International Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HG: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. WL: Data curation, Software, Writing – review & editing. BZ: Data curation, Writing – review & editing. SL: Data curation, Writing – review & editing. CM: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Peking University International Hospital Research Fund (No. YN2021ZD04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. (2020) 135(16):1353–64. doi: 10.1182/blood.2019000931
2. Munshi N, Mehra M, van de Velde H, Desai A, Potluri R, Vermeulen J. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma. (2015) 56(5):1252–60. doi: 10.3109/10428194.2014.953145
3. Cronin DMP, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. (2009) 16(4):236–46. doi: 10.1097/PAP.0b013e3181a9d4d3
4. Oksenhendler E, Boutboul D, Fajgenbaum D, Mirouse A, Fieschi C, Malphettes M, et al. The full spectrum of Castleman disease: 273 patients studied over 20 years. Br J Haematol. (2018) 180(2):206–16. doi: 10.1111/bjh.15019
5. van Rhee F, Oksenhendler E, Srkalovic G, Voorhees P, Lim M, Dispenzieri A, et al. International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Adv. (2020) 4(23):6039–50. doi: 10.1182/bloodadvances.2020003334
6. Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. (2012) 255(4):677–84. doi: 10.1097/SLA.0b013e318249dcdc
7. Zhang MY, Jia MN, Chen J, Feng J, Cao XX, Zhou DB, et al. UCD with MCD-like inflammatory state: surgical excision is highly effective. Blood Adv. (2021) 5(1):122–8. doi: 10.1182/bloodadvances.2020003607
8. Cheng JL, Cui J, Wang Y, Xu ZZ, Liu F, Liang SB, et al. Unicentric Castleman disease presenting as a retroperitoneal peripancreatic mass: a report of two cases and review of literature. World J Gastroenterol. (2018) 24(34):3958. doi: 10.3748/wjg.v24.i34.3958
9. Carrion DM, Alvarez-Maestro M, Gómez Rivas J, Brygadyr Y, García-Fernandez E, Martínez-Piñeiro L. Challenging diagnosis of a solitary retroperitoneal mass: a case report of Castleman’s disease and review of the literature. Urol Int. (2019) 103(2):245–8. doi: 10.1159/000493511
10. Shimokihara K, Kawahara T, Kasahara R, Kasuga J, Sugiura S, Tajiri R, et al. Retroperitoneal Castleman’s disease. Case Rep Oncol. (2020) 12(3):885–9. doi: 10.1159/000504700
11. Singh R, Prasher R, Mohan S, Thakur B. Retroperitoneal unicentric Castleman’s disease—a case report and review of literature. Int J Surg Case Rep. (2021) 86:106325. doi: 10.1016/j.ijscr.2021.106325
12. Aguilar-Rodriguez R, Milea SL, Demirci I, Herold S, Flasshove M, Klosterhalfen B, et al. Localized retroperitoneal Castleman’s disease: a case report and review of the literature. J Med Case Rep. (2014) 8(1):1–5. doi: 10.1186/1752-1947-8-93
13. Masaki Y, Arita K, Sakai T, Takai K, Aoki S, Kawabata H. Castleman disease and TAFRO syndrome. Ann Hematol. (2022) 101(3):485–90. doi: 10.1007/s00277-022-04762-6
14. van Rhee F, Voorhees P, Dispenzieri A, Fosså A, Srkalovic G, Ide M, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. (2018) 132(20):2115–24. doi: 10.1182/blood-2018-07-862334
15. Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol. (2005) 129(1):3–17. doi: 10.1111/j.1365-2141.2004.05311.x
16. Xu J, Zhou BO, Cao HL, Wang BO, Yan S, Zheng SS. Surgical management of isolated retroperitoneal Castleman’s disease: a case report. Oncol Lett. (2016) 11(3):2123–6. doi: 10.3892/ol.2016.4177
17. Gao YJ, Yang Z, Yu JY, Li N, Wang XJ, Zhou NN. Potential application value of PET/computed tomography in retroperitoneal leiomyosarcoma and a literature review. Nucl Med Commun. (2021) 42(7):800–10. doi: 10.1097/MNM.0000000000001388
18. Benz MR, Crompton JG, Harder D. PET/CT variants and pitfalls in bone and soft tissue sarcoma. Semin Nucl Med. (2021) 51(6):584–92. doi: 10.1053/j.semnuclmed.2021.06.009
19. Sharon CE, Straker RJ 3rd, Karakousis GC. The role of imaging in soft tissue sarcoma diagnosis and management. Surg Clin North Am. (2022) 102(4):539–50. doi: 10.1016/j.suc.2022.04.003
20. Fajgenbaum DC, Uldrick TS, Bagg A, Frank D, Wu D, Srkalovic G, et al. International, evidence-based consensus diagnostic criteria for HHV-8–negative/idiopathic multicentric Castleman disease. Blood. (2017) 129(12):1646–57. doi: 10.1182/blood-2016-10-746933
21. Zhang L, Li Z, Cao X, Feng J, Zhong D, Wang S, et al. Clinical spectrum and survival analysis of 145 cases of HIV-negative Castleman’s disease: renal function is an important prognostic factor. Sci Rep. (2016) 6(1):23831. doi: 10.1038/srep23831
Keywords: unicentric Castleman disease, retroperitoneal, surgery, complications, prognosis
Citation: Gao H, Li W, Zou B, Liu S and Miao C (2024) Clinical features and outcomes of retroperitoneal unicentric Castleman disease resected as sarcomas: insights from a high-volume sarcoma center. Front. Surg. 11:1371968. doi: 10.3389/fsurg.2024.1371968
Received: 17 January 2024; Accepted: 27 August 2024;
Published: 5 September 2024.
Edited by:
Hanxing Tong, Fudan University, ChinaReviewed by:
Makoto Ide, Takamatsu Red Cross Hospital, JapanZou Liaonan, Guangzhou University of Chinese Medicine, China
Copyright: © 2024 Gao, Li, Zou, Liu and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haicheng Gao, Z2FvaGFpY2hlbmdAcGt1aWguZWR1LmNu
 Haicheng Gao
Haicheng Gao Wenjie Li
Wenjie Li Boyuan Zou
Boyuan Zou