- 1Skull Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Brain and Spinal Cord Injury Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran
- 3Student Research Committee, School of Medicine, Islamic Azad University, Qeshm International Branch, Qeshm, Iran
Hemangiopericytoma (HPC) constitutes less than 1% of all primary central nervous system tumors. It is a vascular neoplasm with potential malignancy that, in rare instances, manifests as a primary lesion within the brain. Typically, it originates from the meninges. Here, we describe an exceptionally uncommon sellar region solitary fibrous tumor/hemangiopericytoma (SFT/HPC) that mimicked a nonfunctional pituitary adenoma.
Introduction and importance:
Case presentation: A 54-year-old male was referred to our hospital due to progressive blurred vision in the left eye over the past year. A homogeneous iso-dense extra-axial intrasellar round mass with extension into the suprasellar region, mainly on the left side, along with bony erosion and osteolysis around the sellar region, was observed on a brain computed tomography (CT) scan. Brain magnetic resonance imaging (MRI) revealed a well-defined 251,713 mm mass with iso-signal on T1-weighted images and hypersignal on T2-weighted images, originating from the pituitary gland within the sella turcica. The mass avidly enhanced following Gadolinium injection and adhered to both carotid arteries without vascular compression or invasion. It extended to the suprasellar cistern and compressed the optic chiasm. The diagnosis was nonfunctional pituitary macroadenoma, leading to the decision for Endoscopic Trans-Sphenoidal Surgery (ETSS). A non-sustainable, soft, grayish mass was grossly and totally resected during the operation. Subsequently, there was a significant improvement in visual acuity during the early postoperative period. Histopathologic examination confirmed hemangiopericytoma (WHO grade II).
Conclusion: Due to its malignant nature, hemangiopericytoma should be included in the differential diagnosis of a sellar mass, both from a clinical and morphological perspective.
Highlights
• A vascular neoplasm known as hemangiopericytoma has the potential for malignancy and occasionally appears as a primary lesion within the brain.
• In terms of both ophthalmological manifestations and endocrine dysfunction, intracranial hemangiopericytoma can simulate the characteristics of a pituitary tumor.
• The recommended approach for treating hemangiopericytoma involves surgical removal followed by additional radiation therapy.
Introduction
The rare tumor known as solitary fibrous tumor (SFT)/hemangiopericytoma (HPC) was initially documented by Begg and Garret in 1954. It originates from the capillary walls and has historically been believed to have the potential to develop anywhere in the body, although occurrences in the sellar region are infrequent (1, 2). In 1942, Stout and Murray coined the term “hemangiopericytoma” to describe a vascular tumor arising from Zimmermann's pericytes, which are altered smooth muscle cells located within the capillary walls, forming endothelial tubes and sprouts (3). Since then, HPCs have undergone several changes in nomenclature. Ultimately, extracranial HPCs have been reclassified under the category of “solitary fibrous tumors,” while neuropathologists continue to use the term “hemangiopericytoma” (4). SFTs or HPCs make up less than 1% of all primary central nervous system (CNS) tumors. Predominantly situated in the dura, these tumors are believed to originate from meningeal capillary pericytes, placing them in locations similar to meningiomas. However, SFTs or HPCs in the sellar and suprasellar regions are even more uncommon (4, 5). We present an exceedingly rare case of a sellar region SFT/HPC that resembles a nonfunctional pituitary adenoma.
Case presentation
A 54-year-old male was referred to our hospital due to progressive blurred vision in the left eye over the past year. He reported a gradual loss of visual acuity in the left eye and experienced daily throbbing biparietal headaches, relieved by analgesics. Additionally, he mentioned polyuria, polydipsia, and a loss of libido dating back approximately one year. His medical history included rheumatoid arthritis, hypertension, and diabetes mellitus induced by glucocorticoids. He was recently taking losartan (50 mg daily) and prednisolone (5 mg daily). There was no notable family history.
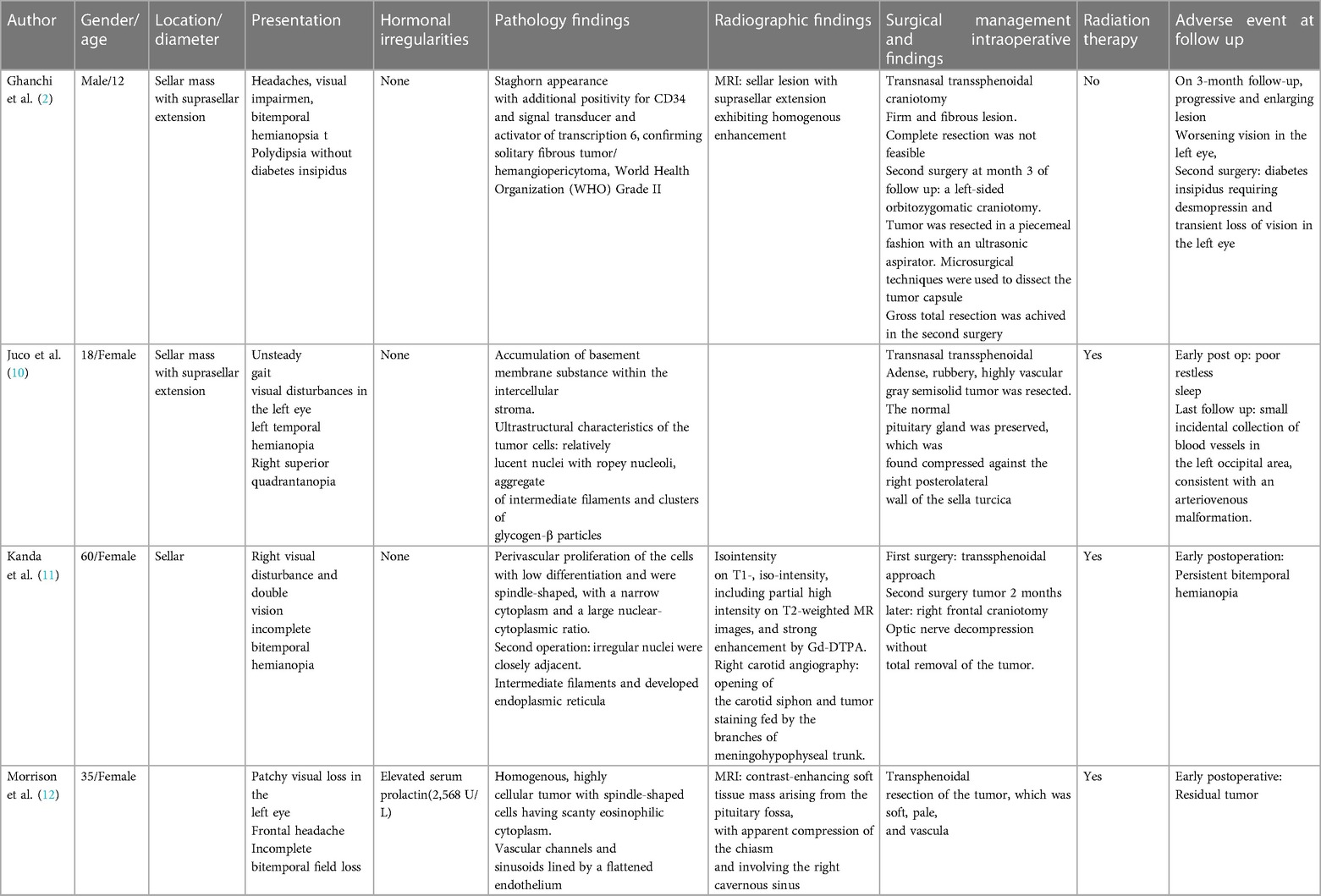
During the physical examination, bilateral visual acuity loss was observed (right eye: 5 meters counting fingers, left eye: 0.5 meters counting fingers). Ocular movements and other cranial nerves were normal, with no abnormalities detected in the peripheral nervous system and other neurological examinations. A brain computed tomography (CT) scan revealed a homogeneous iso-dense extra-axial intrasellar round mass extending to the suprasellar region, predominantly on the left side, with bony erosion and osteolysis around the sellar region (Figure 1). Brain magnetic resonance imaging (MRI) showed a well-defined mass with iso-signal on T1-weighted images and hypersignal on T2-weighted images, originating from the pituitary gland within the sella turcica. The mass avidly enhanced following Gadolinium injection and was adherent to both carotid arteries without vascular compression or invasion, extending to the suprasellar cistern and compressing the optic chiasm (Figures 2, 3). Hypothalamic-pituitary-adrenal axis evaluation revealed no hormonal abnormalities.
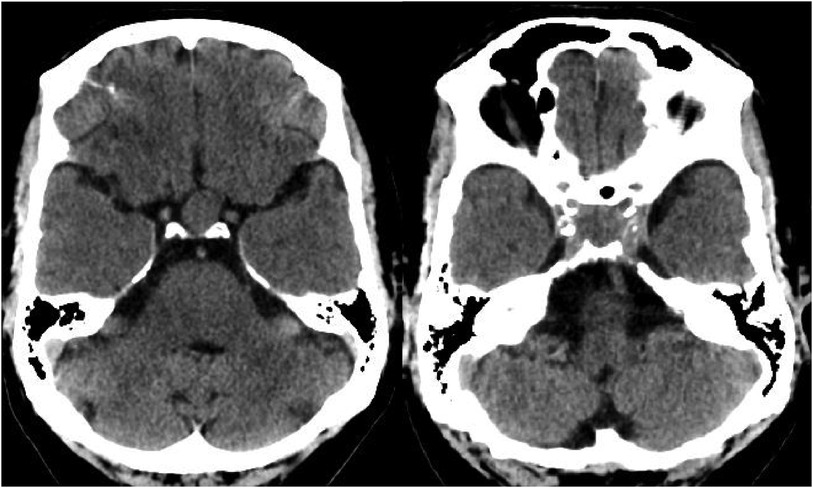
Figure 1. Brain CT scan without contrast, axial view. Intrasellar mass with extension to suprasellar cistern and adjacent bone erosion.
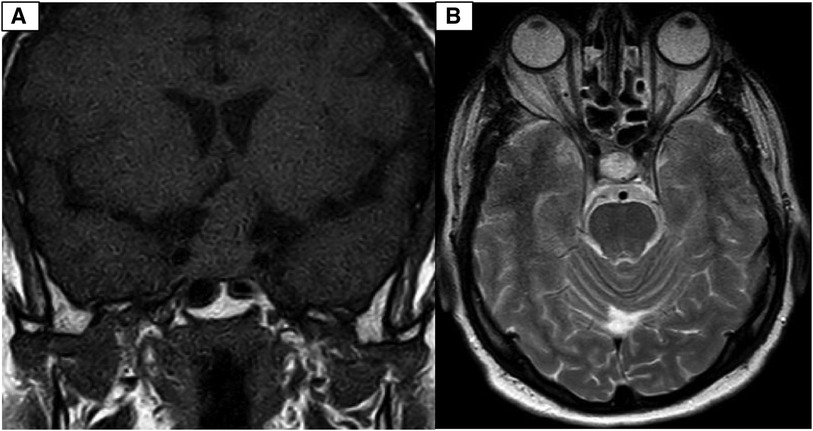
Figure 2. (A) Coronal view of T1-weighed brain MRI without Gadolinium injection. Well defined sellar mass with extension to suprasellar cistern. (B) Axial view of T2-weighed brain MRI without Gadolinium injection. Well defined sellar mass with extension to suprasellar cistern and adherent to both carotid arteries.
Figure 3. (A) Coronal T1-weighted brain MRI with Gadolinium contrast injection depicting a distinctive avidly enhanced sellar mass extending to the suprasellar cistern and adhering to both carotid arteries. (B) Sagittal T1-weighted brain MRI with Gadolinium contrast injection reveals a well-defined, prominently enhanced sellar mass extending into the suprasellar cistern and adherence to both carotid arteries.
The diagnosis was a non-functional pituitary macroadenoma, leading to the decision for endoscopic trans-sphenoidal surgery (ETSS). Intraoperatively, a soft grayish non-sustainable mass was grossly resected. There was a remarkable improvement in visual acuity during the early postoperative period (right eye: 5 meters counting fingers, left eye: 5 meters counting fingers), and a postoperative brain CT scan showed no residual mass (Figure 4). During the operation, we encountered a soft grayish sellar mass with suprasellar extension and peripheral bone invasion and erosion.
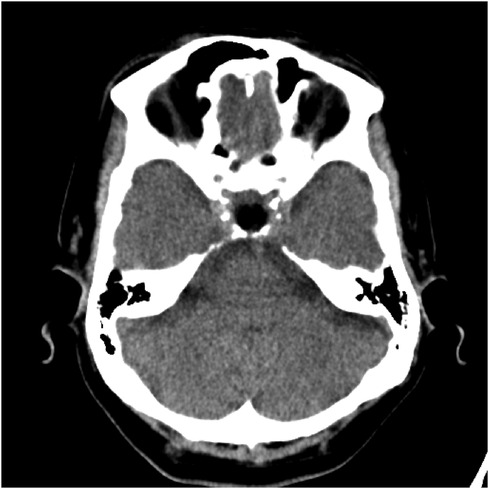
Figure 4. A post-operative brain CT scan, conducted without contrast injection, depicted the resection of the sellar and suprasellar mass, along with the implantation of abdominal subcutaneous fat tissue.
The tumor had extended to the floor of the 3rd ventricle. Following surgery, the patient experienced meningitis and cerebrospinal fluid (CSF) leakage. Treatment included administration of intravenous antibiotics and acetazolamide. After completing a 21-day regimen, there was no CSF leak detected. Despite the tumor's lateral extension beyond the carotid arteries, into the middle fossa, and temporal region, which poses challenges for the endoscopic trans-nasal approach, the patient opted for ETSS due to the presence of approximately midline suprasellar extension without lateral spread.
Histopathologic examination revealed a hypervascular neoplasm of branching vessels surrounded by bland-looking pericytes with oval to spindle nuclei without evidence of anaplasia, necrosis, or hemorrhage. Immunohistochemistry staining was positive for STAT 6 in tumoral cells, while Ki67 was low, and CD-34 highlighted vascular proliferation—all consistent with HPC [World Health Organization (WHO) grade II] (Figure 5).
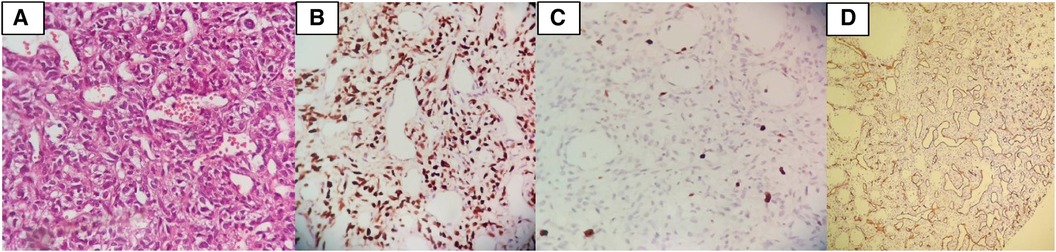
Figure 5. (A) Histopathologic examination revealed a hypervascular neoplasm of branching vessels surrounded by bland-looking pericytes with oval to spindle nuclei without evidence of anaplasia, necrosis, or hemorrhage. (B) IHC stain positive for STAT6 in tumoral cells. (C) IHC stain demonstrates that Ki67 is low. (D) IHC stain for CD34 highlights vascular proliferation.
The patient had a history of ETSS, with observed postoperative changes in the sella. No abnormal enhancement affecting the brain parenchyma or meninges was detected. Both supra and infratentorial structures appeared grossly normal (Figures 6, 7).
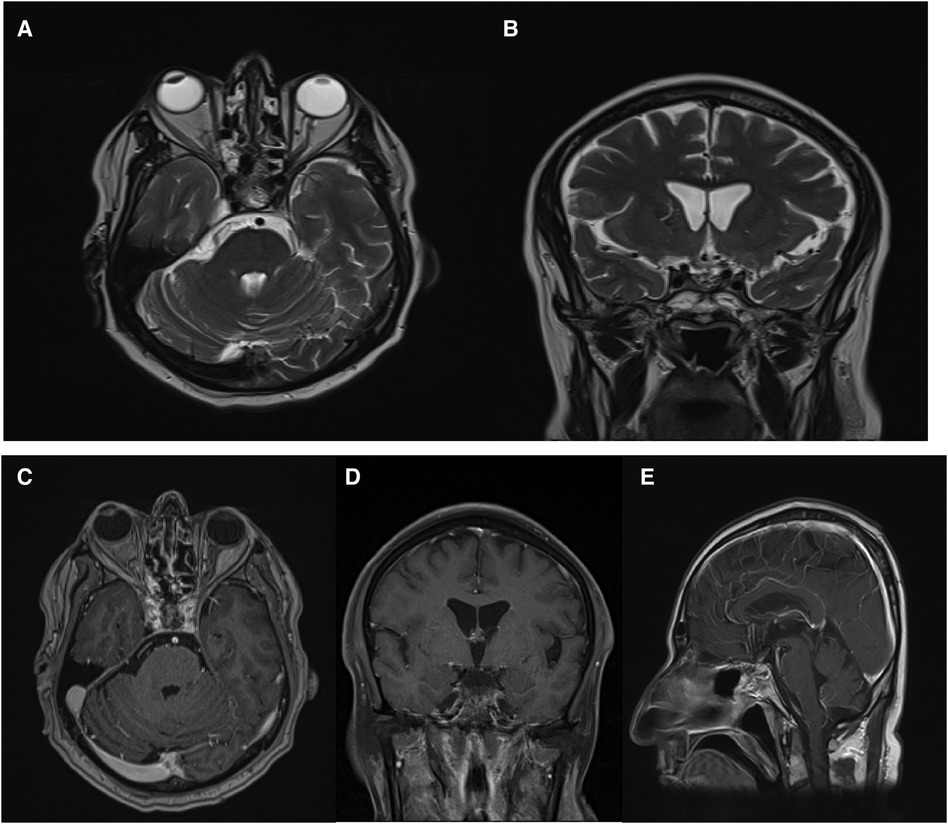
Figure 6. Post-operative brain MRI revealed no evidence of residual mass. Axial (A), coronal (B), axial with Gadolinium injection (C), sagittal with Gadolinium injection (E).
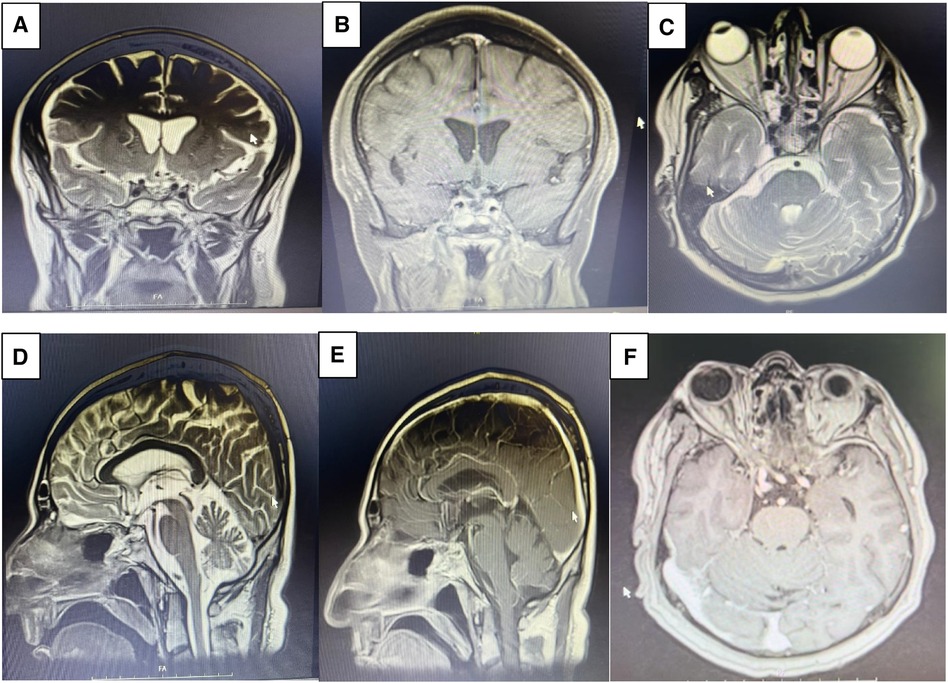
Figure 7. Post op MRI on month 2 demonstrates gross total resection (GTR) of the sellar mass with suprasellar extension. Coronal view of T2 weighed (A), coronal view of T1 weighted following Gadolinium injection (B), axial view of T2 weighed (C), sagittal view of T2 weighted (D), sagittal view of T1 weighted Gadolinium injection (E), axial view of T1 weighted following Gadolinium injection (F).
Discussion
HPCs and SFTs represent uncommon primary intracranial tumors. HPC constitutes only 1% of all intracranial tumors (6, 7). Approximately 15% of SFTs exhibit malignant biological behavior, while the majority display benign and mesenchymal characteristics (8). Recent investigations have identified a novel fusion gene involving Nab2 and STAT6 in samples of SFT and HPC. This discovery has led to the reclassification of these tumors into a new category known as SFT/HPC in the WHO classification of CNS tumors in 2016 (4). Despite several published reports detailing the pathological and clinical features, as well as survival outcomes of SFT/HPC, more comprehensive clinical data for this newly defined entity is still needed (9). Table 1 outlines the prominent characteristics of the previously reported sellar HPC mimicking pituitary adenoma.
Extracranial SFT/HPCs have undergone reclassification within the spectrum of SFTs, while neuropathologists still commonly employ the term HPC (Table 2). Both entities exhibit the 12q3 inversion and fusion of the NGFI-A-binding protein 2 (NAB2) and STAT6 genes, with observable nuclear expression of STAT6 on immunohistochemistry (13). In the 2016 WHO classification of CNS tumors, these two tumors were collectively designated as a single entity, acknowledging their shared inversions at 12q13, resulting in the fusion of NAB2–STAT6 genes and subsequent nuclear expression of STAT6 (14).
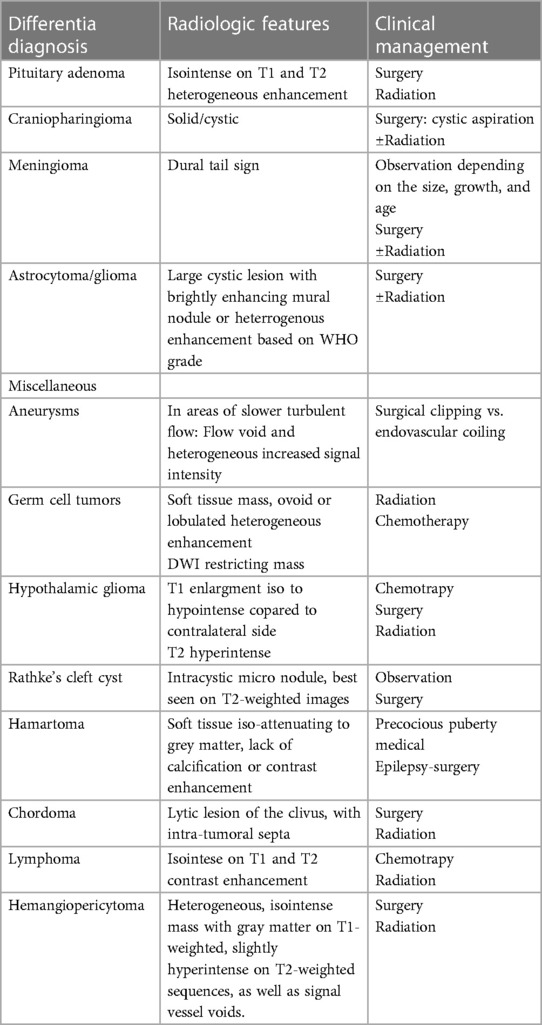
Table 2. The most common differential diagnosis of sellar masses and therapeutic approach (18, 36, 37).
STAT6, or Signal Transducer and Activator of Transcription 6, plays a pivotal role in a myriad of cellular processes, encompassing growth, survival, differentiation, and immune responses. Recent investigations have unveiled a distinctive molecular profile linked to hemangiopericytoma, characterized by recurring NAB2-STAT6 gene fusions. This fusion event leads to the perpetual activation of the STAT6 signaling pathway, speculated to augment the oncogenic potential of HPC. Several studies indicated that robust nuclear STAT6 expression, as identified through immunohistochemistry, serves as a highly sensitive and specific marker for HPC. Furthermore, the presence of the NAB2-STAT6 fusion gene has been correlated with a more favorable prognosis in HPC patients (15).
While the precise origin of SFT or HPC remains uncertain, there is consensus on its mesenchymal nature characterized by HPC-like features, such as a monotonous cell population, varying cellularity, and the presence of branching blood vessels (16). Ultrastructurally, both SFT and HPC exhibit diverse degrees of pericytic, fibroblastic, or myofibroblastic differentiation. Gengler and Guillou clarified that, excluding myopericytoma, infantile myopericytosis, and HPC of the sinonasal tract, all HPCs lacking pericytic differentiation are considered variants of SFT, resembling a cellular form of SFT within a morphological continuum (16). Tumors characterized by lower cellularity and high collagen content are designated as grade I (formerly SFT), whereas those with increased cellularity, reduced collagen, and characteristic staghorn vasculature are categorized as grade II (formerly HPC). Grade III encompasses HPC or SFT with five or more mitoses per 10 high-power fields, inclusive of both anaplastic HPC and malignant SFT types (4).
Sellar SFTs or HPCs are frequently misidentified as pituitary adenomas and meningiomas (17). Visual disturbance followed by headaches is the predominant presenting symptom of sellar SFT or HPC. Like other suprasellar tumors, these growths often compress the pituitary gland and stalk, resulting in common symptoms of pituitary dysfunction (10, 17).
Imaging characteristics of HPC encompass several aspects: a broad-based attachment to the dura, absence of calcifications and hyperostosis, multilobulated tumor morphology, heterogeneously hyperdense patterns with focal areas of hypodensity on unenhanced brain CT, varied enhancement patterns on enhanced brain CT (homogeneous or heterogeneous), and an isointense signal compared to cortical gray matter on T1- and T2-weighted brain MRI. Additionally, they exhibit heterogeneous enhancement on Gadolinium-enhanced T1-weighted brain MRI (18). Intracranial SFT's CT features closely resemble those of extracranial SFT, displaying isoattenuation and marked enhancement following intravenous iodinated contrast injection. Brain MRI highlights SFT's extra-axial, multilobulated nature with heterogeneous signal intensity on T2-weighted images, hypointense T2 areas exhibiting robust enhancement after gadolinium administration, while adjacent meninges remain unenhanced (19). Table 2 outlines the most common differentiation diagnosis of sellar masses and therapeutic approach.
For intraspinal SFT/HPC, a comprehensive en-bloc surgical resection is advisable to mitigate postoperative recurrence or regrowth (17). However, due to the fibrous, hypervascular, and invasive nature of these tumors, partial resection is the more commonly employed surgical management. While total removal has been achieved in certain instances, some cases have reported new-onset visual impairment and pituitary dysfunction postoperatively (20, 21).
Divergent opinions exist among researchers regarding the optimal strategy for HPC management. Some advocate for complete surgical resection followed by postoperative radiotherapy to the tumor bed as the most effective approach (22, 23). A perspective suggests that external irradiation of the tumor bed after surgery may delay recurrence (24). Postoperative radiotherapy has been traditionally considered a primary treatment for HPC, although a previous study indicated that it did not significantly enhance local control and overall survival. Notably, the local control rates were comparable between intensity-modulated radiotherapy (IMRT) and stereotactic radiosurgery (SRS), despite IMRT having a substantially higher biological dose (25).
In our management, since HPC was not initially suspected as the etiology, and both intraoperative findings and postoperative MRI results confirmed gross total resection (GTR), we followed the protocol for pituitary adenoma, considering it the most likely diagnosis. However, upon receiving the pathology report confirming the diagnosis of HPC, the patient was referred to a senior oncologist for further advice regarding the potential need for radiotherapy.
Several studies indicate that postoperative radiotherapy following subtotal resection (STR) can enhance overall survival (OS) and recurrence-free survival (RFS) compared to STR alone (26–28). Additionally, postoperative radiotherapy following GTR has been associated with prolonged OS (6, 23, 29, 30) or improved local control (31, 32). In contrast, some authors report that postoperative radiotherapy after GTR has no significant impact on survival (26, 33) or should only be considered for recurrent cases (34, 35). Gou et al. (9) found that different postoperative radiotherapy (PORT) strategies significantly influenced disease-free survival (DFS). Patients receiving postoperative radiotherapy via IMRT had a median DFS of 8.33 years, a statistically significant difference compared to those undergoing postoperative radiotherapy via SRS. Multivariate analysis revealed that postoperative radiotherapy had no predictive effect on survival compared to surgery alone, but when compared to other treatments, both postoperative radiotherapy and surgery were beneficial for DFS.
In a series report involving 29 patients with intracranial adult HPC, the 5-, 10-, and 15-year overall survival rates were recorded at 85%, 68%, and 43%, respectively, for individuals undergoing this particular treatment regimen (27). Another series, encompassing 43 patients, reported 1-, 5-, and 10-year overall survival rates of 100%, 94.4%, and 72.2%, respectively (23). The outcomes are intricately linked to the presence of metastatic disease and the effectiveness of local control, with GTR being advocated due to its association with a 5-year local disease control rate of 84%, significantly higher than the 38% observed with STR (33).
Conclusions
Although intracranial HPC is a rare entity, its clinical presentation can mimic a pituitary tumor both ophthalmologically and in terms of endocrine dysfunction. However, the prognosis is considerably more severe. The treatment approach is markedly distinct, necessitating extended follow-up. Histological confirmation remains crucial for optimal management, even when clinical features appear indicative of the diagnosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Skull base research center, Loghman Hakim Hospital. This study was conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants because the procedures were based on the conventional procedures. Written informed consent was obtained from the individual(s), for the publication of any potentially identifiable images or data included in this article.
Author contributions
KE: Writing – original draft, Writing – review & editing, Conceptualization, Methodology, Project administration, Supervision. MMM: Writing – original draft, Writing – review & editing, Data curation, Investigation. MA: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Validation. AAD: Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgment
The authors acknowledge the assistance of ChatGPT 3.5 in refining our initial drafts and enhancing the quality of the English language. We meticulously ensured the accuracy of our content and implemented rigorous measures to prevent plagiarism.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yip CM, Hsu SS, Liao WC, Liu SH, Lin YS, Hsu YH, et al. Intracranial solitary fibrous tumor/hemangiopericytoma - a case series. Surg Neurol Int. (2020) 11:414. doi: 10.25259/SNI_490_2020
2. Ghanchi H, Patchana T, Christian E, Li C, Calayag M. Pediatric sellar solitary fibrous tumor/ hemangiopericytoma: a rare case report and review of the literature. Surg Neurol Int. (2020) 11:238. doi: 10.25259/SNI_234_2020
3. Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericytes. Ann Surg. (1942) 116(1):26. doi: 10.1097/00000658-194207000-00004
4. Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
5. Wu W, Shi J-X, Cheng H-L, Wang H-D, Hang C-H, Shi Q-L, et al. Hemangiopericytomas in the central nervous system. J Clin Neurosci. (2009) 16(4):519–23. doi: 10.1016/j.jocn.2008.06.011
6. Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma: long-term outcome revisited. J Neurosurg. (2011) 114(3):747–55. doi: 10.3171/2010.6.JNS091660
7. Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. (1989) 25(4):514–22. doi: 10.1227/00006123-198910000-00003
8. Gubian A, Ganau M, Cebula H, Todeschi J, Scibilia A, Noel G, et al. Intracranial solitary fibrous tumors: a heterogeneous entity with an uncertain clinical behavior. World Neurosurg. (2019) 126:e48–56. doi: 10.1016/j.wneu.2019.01.142
9. Gou Q, Xie Y, Ai P. Intracranial solitary fibrous tumor/hemangiopericytoma: role and choice of postoperative radiotherapy techniques. Front Oncol. (2022) 12:994335. doi: 10.3389/fonc.2022.994335
10. Juco J, Horvath E, Smyth H, Rotondo F, Kovacs K. Hemangiopericytoma of the sella mimicking pituitary adenoma: case report and review of the literature. Clin Neuropathol. (2007) 26(6):288–93. doi: 10.5414/NPP26288
11. Kanda Y. Sellar hemangiopericytoma mimicking pituitary adenoma. Surg Neurol. (2001) 55:113–5. doi: 10.1016/S0090-3019(01)00330-5
12. Morrison DA, Bibby K. Sellar and suprasellar hemangiopericytoma mimicking pituitary adenoma. Arch Ophthalmol. (1997) 115(9):1201–3. doi: 10.1001/archopht.1997.01100160371022
13. Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. (2013) 125:651–8. doi: 10.1007/s00401-013-1117-6
14. Antonescu C, Paulus W, Perry A, Rushing E, Hainfellner J, Bouvier C, et al. WHO classification of tumours of the central nervous system. IARC, Lyon (2016).
15. Liu J, Wu S, Zhao K, Wang J, Shu K, Lei T. Clinical features, management, and prognostic factors of intracranial solitary fibrous tumor. Front Oncol. (2022) 12:915273. doi: 10.3389/fonc.2022.915273
16. Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. (2006) 48(1):63–74. doi: 10.1111/j.1365-2559.2005.02290.x
17. Thapa S, Fujio S, Kitazono I, Yonenaga M, Masuda K, Kuroki S, et al. Solitary fibrous tumor or hemangiopericytoma of the sella in an older patient treated with partial removal followed by fractionated gamma knife radiosurgery. NMC Case Rep J. (2021) 8(1):697–703. doi: 10.2176/nmccrj.cr.2021-0103
18. Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytomas: MR and CT features. Am J Neuroradiol. (1996) 17(7):1365–71. PMCID: PMC83385398871726
19. Clarençon F, Bonneville F, Rousseau A, Galanaud D, Kujas M, Naggara O, et al. Intracranial solitary fibrous tumor: imaging findings. Eur J Radiol. (2011) 80(2):387–94. doi: 10.1016/j.ejrad.2010.02.016
20. Furlanetto TW, Pinheiro CFP, Oppitz PP, de Alencastro LC, Asa SL. Solitary fibrous tumor of the sella mimicking pituitary adenoma: an uncommon tumor in a rare location—a case report. Endocr Pathol. (2009) 20:56–61. doi: 10.1007/s12022-009-9063-5
21. Yang X, Jiang Q, Yu B. Solitary fibrous tumor located in the sella turcica: a report of two cases and review of the literature. Oncol Lett. (2015) 10(1):354–8. doi: 10.3892/ol.2015.3162
22. Kano H, Niranjan A, Kondziolka D, Flickinger JC, Lunsford LD. Adjuvant stereotactic radiosurgery after resection of intracranial hemangiopericytomas. Int J Radiat Oncol Biol Phys. (2008) 72(5):1333–9. doi: 10.1016/j.ijrobp.2008.03.024
23. Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer. (2004) 100(7):1491–7. doi: 10.1002/cncr.20109
24. Dufour H, Métellus P, Fuentes S, Murracciole X, Régis J, Figarella-Branger D, et al. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery. (2001) 48(4):756–63. doi: 10.1097/00006123-200104000-00011
25. Xiao J, Xu L, Ding Y, Wang W, Chen F, Zhou Y, et al. Does post-operative radiotherapy improve the treatment outcomes of intracranial hemangiopericytoma? A retrospective study. BMC Cancer. (2021) 21(1):1–10. doi: 10.1186/s12885-020-07763-8
26. Ghia AJ, Allen PK, Mahajan A, Penas-Prado M, McCutcheon IE, Brown PD. Intracranial hemangiopericytoma and the role of radiation therapy: a population based analysis. Neurosurgery. (2013) 72(2):203–9. doi: 10.1227/NEU.0b013e31827b9e68
27. Melone AG, D’Elia A, Santoro F, Salvati M, Delfini R, Cantore G, et al. Intracranial hemangiopericytoma—our experience in 30 years: a series of 43 cases and review of the literature. World Neurosurg. (2014) 81(3-4):556–62. doi: 10.1016/j.wneu.2013.11.009
28. Staples J, Robinson R, Wen B-C, Hussey D. Hemangiopericytoma—the role of radiotherapy. Int J Radiat Oncol Biol Phys. (1990) 19(2):445–51. doi: 10.1016/0360-3016(90)90556-Y
29. Sonabend AM, Zacharia BE, Goldstein H, Bruce SS, Hershman D, Neugut AI, et al. The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: a surveillance, epidemiology, and end results analysis. J Neurosurg. (2014) 120(2):300–8. doi: 10.3171/2013.10.JNS13113
30. Stessin AM, Sison C, Nieto J, Raifu M, Li B. The role of postoperative radiation therapy in the treatment of meningeal hemangiopericytoma—experience from the SEER database. Int J Radiat Oncol Biol Phys. (2013) 85(3):784–90. doi: 10.1016/j.ijrobp.2012.05.042
31. Kim JH, Jung H-W, Kim Y-S, Kim CJ, Hwang S-K, Paek SH, et al. Meningeal hemangiopericytomas: long-term outcome and biological behavior. Surg Neurol. (2003) 59(1):47–53. doi: 10.1016/S0090-3019(02)00917-5
32. Ghia AJ, Chang EL, Allen PK, Mahajan A, Penas-Prado M, McCutcheon IE, et al. Intracranial hemangiopericytoma: patterns of failure and the role of radiation therapy. Neurosurgery. (2013) 73(4):624–31. doi: 10.1227/NEU.0000000000000064
33. Rutkowski MJ, Sughrue ME, Kane AJ, Aranda D, Mills SA, Barani IJ, et al. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. (2010) 113(2):333–9. doi: 10.3171/2010.3.JNS091882
34. Olson C, Yen C-P, Schlesinger D, Sheehan J. Radiosurgery for intracranial hemangiopericytomas: outcomes after initial and repeat gamma knife surgery. J Neurosurg. (2010) 112(1):133–9. doi: 10.3171/2009.3.JNS0923
35. Rutkowski MJ, Bloch O, Jian BJ, Chen C, Sughrue ME, Tihan T, et al. Management of recurrent intracranial hemangiopericytoma. J Clin Neurosci. (2011) 18(11):1500–4. doi: 10.1016/j.jocn.2011.04.009
36. Al-Bader D, Hasan A, Behbehani R. Sellar masses: diagnosis and treatment. Front Ophthalmol. (2022) 2:970580. doi: 10.3389/fopht.2022.970580
Keywords: hemangiopericytoma, sellar hemangiopericytoma, pituitary adenoma, sellar tumor, endoscopic trans-sphenoidal surgery
Citation: Ebrahimzadeh K, Mirahmadi Eraghi M, Ansari M and Dmytriw AA (2024) Sellar hemangiopericytoma masquerading as pituitary adenoma: an overlooked intriguing case study unveiling a rare surgical conundrum. Front. Surg. 11:1359787. doi: 10.3389/fsurg.2024.1359787
Received: 22 December 2023; Accepted: 18 April 2024;
Published: 22 May 2024.
Edited by:
Nicola Montano, Fondazione Policlinico Universitario Agostino Gemelli IRCCS—Università Cattolica del Sacro Cuore, ItalyReviewed by:
Xulei Huo, Beijing Neurosurgical Institute, ChinaSalvatore Chibbaro, Strasbourg University Hospital, France
© 2024 Ebrahimzadeh, Mirahmadi Eraghi, Ansari and Dmytriw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Ansari, bWhkYW5zMzZAeWFob28uY29t
 Kaveh Ebrahimzadeh1
Kaveh Ebrahimzadeh1 Mohammad Mirahmadi Eraghi
Mohammad Mirahmadi Eraghi Mohammad Ansari
Mohammad Ansari Adam A. Dmytriw
Adam A. Dmytriw