
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 19 January 2024
Sec. Thoracic Surgery
Volume 11 - 2024 | https://doi.org/10.3389/fsurg.2024.1335144
A correction has been applied to this article in:
Corrigendum: Safety assessment of poly-ε-caprolactone in the treatment of primary spontaneous pneumothorax
 Cheng-Hung How1,2,†
Cheng-Hung How1,2,† Pei-Hsing Chen1,3,4,†
Pei-Hsing Chen1,3,4,† Yu-Ching Chen3
Yu-Ching Chen3 Yong-Chong Lin1
Yong-Chong Lin1 Ke-Cheng Chen4*
Ke-Cheng Chen4* Jin-Shing Chen4
Jin-Shing Chen4 Tai-Horng Young1*
Tai-Horng Young1*
Background/purpose: Biomaterial-based implants are gaining traction as an option for pleurodesis treatment, yet the search for the best biomaterial or the most suitable shape to handle spontaneous pneumothorax continues. This forward-looking research assessed the use of a poly-ε-caprolactone membrane for its safety when applied as a sclerosant in pleurodesis procedures in human patients.
Methods: From July 2017 to February 2018, we conducted a Phase I trial in which 10 patients with primary spontaneous pneumothorax were treated using video-assisted thoracoscopic surgery with a poly-ε-caprolactone membrane. These procedures encompassed bleb resection and mechanical pleurodesis through parietal pleura scrubbing. After resection, a 150 × 150 mm poly-ε-caprolactone membrane was applied to the apex. The primary outcome measures were the adverse events and laboratory outcomes.
Results: After surgery, we observed no cardiopulmonary-related adverse events or indications of systemic inflammation. Furthermore, no episodes of hypothermia or hyperthermia occurred. Chest radiographs showed no evident pneumonitis or effusion associated with tissue reactions. The average follow-up duration was 31.7 ± 17.7 months, during which two patients exhibited recurrence.
Conclusion: This study is the first to show the biocompatibility of poly-ε-caprolactone in humans, suggesting its potential as a treatment option for patients with primary spontaneous pneumothorax. Despite the relatively small number of patients, we maintain confidence in the reliability and safety profile of the PCL membrane, bolstered by its previously established efficacy in applications involving other organs. Phase II and phase III clinical studies are needed to support these observations.
The management of spontaneous pneumothorax includes the cessation of air leak, elimination of pleural air, and prevention of recurrence (1). Surgical management is generally suggested for recurrent or complicated spontaneous pneumothorax; recent advancements in thoracic surgery, particularly in video-assisted thoracoscopic surgery (VATS) (2, 3), have established it as a forefront method for primary spontaneous pneumothorax treatment due to its minimally invasive nature (4). Yet, pneumothorax recurrence post-stapled bullectomy via VATS remains a concern, with rates spanning between 2% and 16% (5–7). This rate is influenced by the pleurodesis technique employed and is notably higher than the recurrence rate associated with open thoracotomy, which is reported to be less than 1% (8–10). The elevated postoperative recurrence observed with VATS can often result from overlooked leaking blebs proximate to the pleural staple line, coupled with a milder pleural inflammatory response elicited by VATS compared to thoracotomy. Further compounding this issue are instances of partial bleb resection or those performed adjacent to, or traversing, the lungs' emphysematous regions, leading to air leaks near the staple line (11, 12). Given this heightened recurrence risk, the effectiveness of simple bullectomy video-assisted thoracic surgery in PSP management has come under scrutiny.
Pleurodesis is considered an adjunctive therapy to surgery. Although many chemical sclerosants, including talc powder, minocycline, bleomycin, autologous blood patch, iodopovidone, picibanil and 50% dextrose, and silver nitrate, have been used to induce pleurodesis, all of which have drawbacks, especially related to pain sensation or unsatisfactory recurrence rates (13). Biomaterial implants are emerging as a treatment choice for pleurodesis; however, the ideal biomaterial or optimal form for the management of spontaneous pneumothorax is still under investigation (14–17). Several biomaterials have been reported for the treatment of repetitive catamenial pneumothorax (18, 19).
Previously, we demonstrated significant pleurodesis in New Zealand white rabbit animal models using poly-ε-caprolactone (PCL) membranes and found satisfactory results. However, the safety and efficacy of PCL membranes in humans remains unclear. Hence, this study aimed to determine the safety and/or adverse events of PCL membranes in a clinical phase I study.
This Phase I trial was conducted from July 2017 to February 2018 at National Taiwan University Hospital. The eligibility criteria were patients aged 15–50 years, with an indication for thoracoscopic bullectomy and pleurodesis, such as prolonged air leaks >3 days, ipsilateral pneumothorax recurrence, and contralateral pneumothorax. Written informed consent was obtained from all patients. Patients were included when they met the minimal requirement of hematological data (hemoglobin level > 10 g/dl, absolute neutrophil count > 1.5 × 103/μl, and platelet count > 100 × 103/μl). Patients were excluded if they had underlying chronic obstructive pulmonary disease, hemothorax, pneumothorax, catamenial pneumothorax, or other serious concomitant illnesses or malignancy or ongoing pregnancy.
This Phase I clinical trial was registered in the National Clinical Trial (clinicaltrials.gov number, NCT03227978) database and received ethics committee approval (201610065DINA).
PCL (SigmaeAldrich, St. Louis, MO, USA) is bioresorbable polyester approved by the U.S. Food and Drug Administration and the European Union Food and Drug Administration. The sterile PCL membranes were fabricated using a solvent-casting method as described previously (20). The final membrane size was 150 × 150 mm (Figures 1A,B).
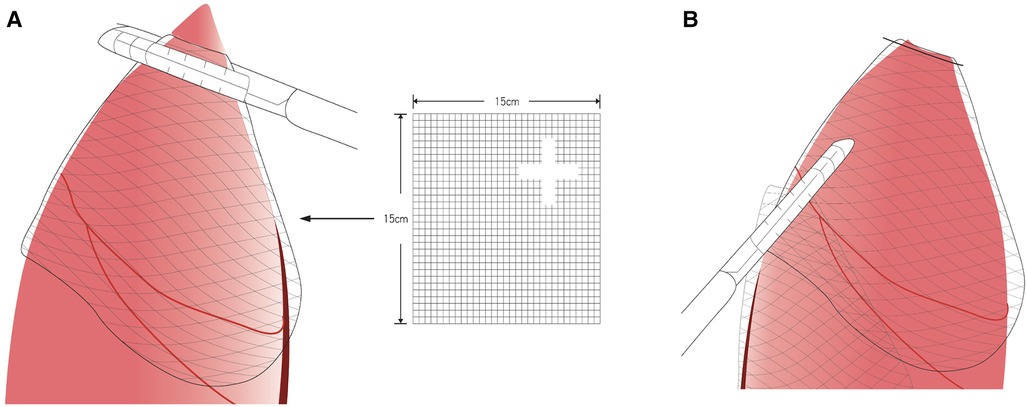
Figure 1. Poly-ε-caprolactone membrane fixed with the latest staple following the method by ikeda et al. (19) (A) Fix the membrane with the stapler (Illustration). (B) After stapler fixation (Illustration).
The surgical and anesthesia techniques were performed as previously described (4, 16, 21). Briefly, all patients were intubated with a double-lumen endotracheal tube and placed in the lateral decubitus position. The ipsilateral lung was deflated during the procedure. The remaining steps of the thoracoscopy procedure was described in our previous study (14). A single skin incision (approximately 2.5–3 cm in length) was made at the fifth intercostal space at the middle axillary line (Figures 2A,2B). A vagus nerve block was performed to reduce the cough reflex, and an intercostal nerve block was used for pain control.
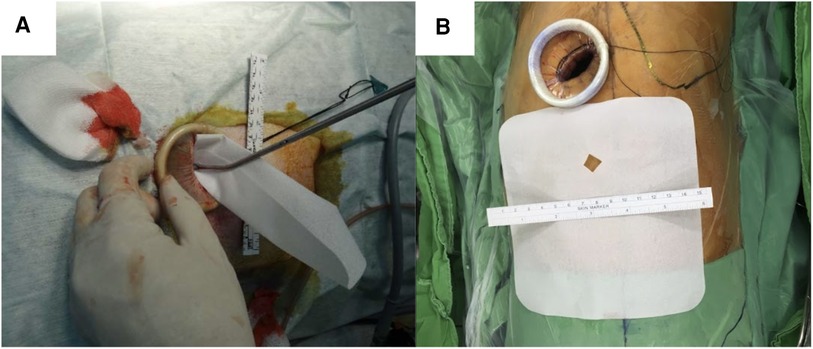
Figure 2. Poly-ε-caprolactone membrane (A) membrane structure. (B) Insert membrane in the chest cavity through the thoracoscopic wound.
The lung was inspected for any possible bleb, and the adhesion was identified according to Vanderschueren's classification (22, 23). When a bleb was observed, an endoscopic stapler was introduced for resection. In cases of no or ruptured blebs, the resection was performed at the most suspicious area. Participants would undergo thoracoscopic bullectomy and pleural abrasion, then the PCL membrane (same material and size as used in the animal model experiments) was fixed with the latest staple following the method by Ikeda et al. (19) Thus, the membrane was sutured on the remaining lung (Figures 1A,B). The remaining procedure was identical to that previously described (24). After all the procedures were performed, a chest tube or pigtail was placed in the apex through the wound. The wound was closed after lung re-expansion. The bleb or apex lung was sent for pathological examination.
The primary outcome of the clinical trial was abnormal arterial blood pressure or body temperature results. Laboratory data related to possible allergic responses, such as C-reactive protein levels, eosinophil levels, and white blood cell counts, were recorded. Data related to postoperative hospital stays were also analyzed.
Opioids were administered to patients every 4–6 h when the pain was not relieved by oral analgesics, or if the Visual Analogue Scale pain level score was >7. Chest radiography was routinely performed after surgery. The chest tube was removed after full lung expansion with no air leak in a 24-h period.
Post-operative complications were meticulously documented. Specifically, prolonged air leaks were characterized as air leaks persisting for a duration exceeding 5 days. Pleural detachment referred to the development of a pneumothorax immediately subsequent to the extraction of the chest tube.
Following hospital discharge, patients were scheduled for outpatient clinic visits at intervals of 1 week, 1 month, 3 months, and 6 months. During each of these visits, chest radiography was conducted. All participants were suggested to be monitored for a minimum of 12 months. Furthermore, patients were advised to revisit the clinic or seek emergency care if they experienced symptoms such as chest pain, dyspnea, or any indications suggestive of a pneumothorax recurrence (Figure 3).
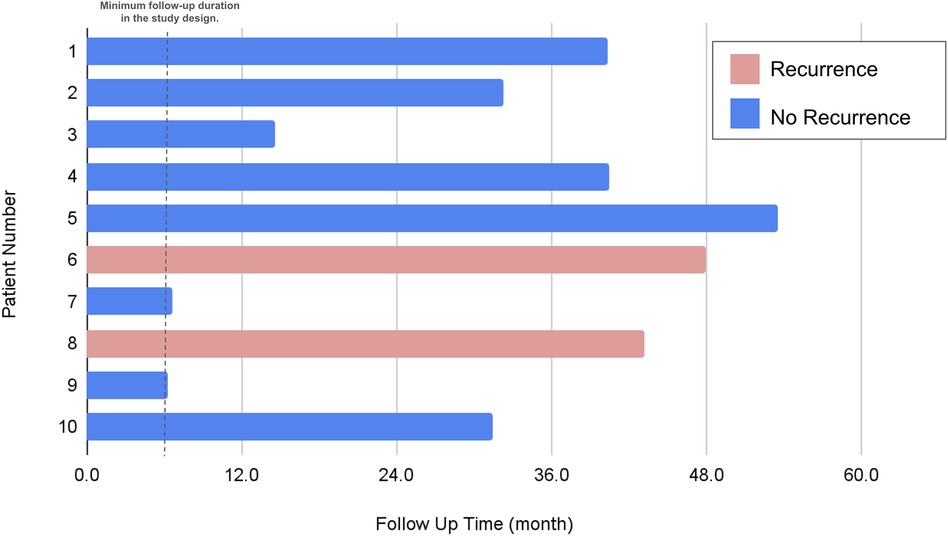
Figure 3. Evaluation of recurrence status. Of the two patients who experienced recurrence, both were taller than 170 cm and had a BMI below 20. The recurrence site for both was on the left side.
The demographic data and related surgical results, including operative findings, complications, and body weights, were recorded and analyzed statistically. Continuous variables, such as body weight and height, are expressed as mean ± standard deviation. Categorical variables, such as sex, are represented as frequency (%). Fisher's exact test was performed as a statistical significance test of contingency tables. Statistical analyses were performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA), R (version 3.4.3; R Foundation for Statistical Computing, Vienna, Austria), and R Studio (version 1.1.414; RStudio, PBC, Boston, MA, USA).
The extended investigation in the Phase I trial included a cohort of 10 patients, all diagnosed with primary spontaneous pneumothorax, who were administered treatments utilizing PCL membrane. Detailed patient demographics and clinical features are systematically cataloged in Table 1. The average age of the cohort was 27.6 years, with a standard deviation of 10.2 years, spanning from 20 to 46 years, and the majority of these patients (80%) were male. Stature and mass metrics averaged at 169.7 cm in height and 56.3 kg in weight, respectively. A significant outcome of the study was the absence of fatalities, coupled with a complete lack of necessity to escalate to multi-port VATS, as shown in Table 2. Blood loss was minimal across the board, with all patients losing less than 20 cc during surgery. The surgical intervention duration averaged at slightly over 54 min, with a variability of 18.5 min. Notably, none of the patients had a history of smoking.
Following the surgery, the patients' recovery was notable for the lack of fever presentations. Furthermore, within the initial postoperative triad of days, there were no documented cases of temperature dysregulation, blood pressure abnormalities, or severe adverse reactions. Allergy assessments, indicated by vital signs and laboratory analysis, showed no significant hypersensitive responses. Inflammatory markers, such as C-reactive protein levels, and counts for white blood cells and eosinophils, were reported to be stable and within normal parameters (Table 3). Radiographic imaging post-procedure did not reveal any evidence of pneumonitis or effusions that could indicate a tissue reaction to the implant.
The median hospitalization period post-procedure was 4 days, but this varied from as brief as 2 days to as extended as 18 days. Chest tubes were utilized in the vast majority (90%) of patients, typically remaining in place for a median of 3 days. One case stood out where a patient experienced prolonged air leakage for up to 25 days. This individual was discharged with a chest tube outfitted with a one-way valve system, which was later removed during a subsequent outpatient visit. The follow-up period for the study participants averaged at almost 32 months, with a spectrum ranging from half a year to just over three years, as depicted in Figure 3. Within this follow-up timeframe, two instances of pneumothorax recurrence were observed, as elaborated in Table 2. Notably, no perioperative complications were recorded. A commonality among patients who experienced a recurrence was a height exceeding 170 cm and a body mass index below 20, with recurrences exclusively occurring on the left side. At the start, these patients were all categorized as grade III under the Vanderschueren classification, which denotes the presence of blebs or bullae smaller than 2 cm.
This research showed that the PCL membrane, when combined with bullectomy via VATS, is safe and effective for clinical pleurodesis procedures. No significant cardiopulmonary changes or adverse effects were observed in the clinical study, and there was no increase in inflammation indicators. The follow-up period remained uneventful.
Since pleurodesis is a possible treatment for primary pneumothorax, several studies have evaluated its effect in clinical situations (21, 24, 25). The most intensive pleurodesis agent may not always be the best choice due to the possibility of a severe inflammation response, pain sensation, or bleeding. The effect of a biomaterial with the conventional pleurodesis method, such as mechanical pleurodesis and bullectomy, remains uncertain (12, 22, 26).
Previous research demonstrated the PCL membrane's ability to create adhesive effects. It induced and enhanced tissue adhesion by changing the membrane from dense to porous tissue (27, 28). Our previous work also proved the adhesion ability, fibronectin level change, and fibrosis effects of PCL membrane in a rabbit model, and the PCL membrane showed potential in short-term results (30 days) (14, 20).
While its biocompatibility has been clinically evaluated for safety in human participants, the recurrence rate in this trial appears to be higher than in previous studies (21, 25, 29), even when compared to simple pleural abrasion (18). While the recurrence rate of pneumothorax after thoracoscopic surgery typically ranges from 2% to 14% according to previous studies (30), our study observed two patients experiencing recurrence. However, it is essential to note that the primary objective of this trial was to evaluate safety. In our routine clinical practice, as described in our recently published work (4), we employ a specific method: we use a woven absorbable mesh (Vicryl mesh, 30 cm × 30 cm). In our previously published technique, the mesh is introduced into the pleural cavity through an access port, and we meticulously position it on the apical visceral pleura to ensure coverage of the staple line, adhering to the method of Ikeda et al. (19). Furthermore, some cases were performed using a non-intubated method, representing a surgical innovation (31, 32).
However, in this study, we employed only a quarter of the typical mesh size, primarily as a safety precaution, which differs from the methodologies in previous studies (4, 19). This decision to use a reduced mesh size might be the reason for the observed recurrence in patients taller than 170 cm. We expect the recurrence rate to be lower in future phase II or phase III trials when using the mesh at its intended size. In our study, we observed a patient with a prolonged air leak that persisted for 25 days. Utilizing a larger size of the PCL membrane for coverage could potentially have ameliorated this situation. Furthermore, no postoperative adverse events were observed in the human participants. This suggests that the PCL membrane is both safe and reliable as a biomaterial for treating patients with primary spontaneous pneumothorax.
Limitations of this study include the small number of patients in the clinical trial. Further, the clinical trial demonstrated only the PCL membrane's safety, but not its effectiveness; two patients experienced recurrence during the follow-up period. Further studies (Phase II and Phase III) are required to establish the effectiveness of the PCL membrane for patients with primary pneumothorax. Despite the relatively small number of patients, we maintain confidence in the reliability and safety profile of the PCL membrane, bolstered by its previously established efficacy in applications involving other organs.
In conclusion, this study is the first to report the safe use of PCL in humans when utilized in combination with VATS bullectomy. Our results demonstrate the PCL membrane's potential for treating patients with primary spontaneous pneumothorax.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The phase I clinical trial was registered in the National Clinical Trial (clinicaltrials.gov number, NCT03227978) database and received ethics committee approval (201610065DINA). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
C-HH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. P-HC: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. Y-CC: Formal Analysis, Investigation, Writing – original draft. Y-CL: Data curation, Formal Analysis, Writing – original draft. K-CC: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing. J-SC: Project administration, Writing – review & editing. T-HY: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
The author acknowledges the support from the National Science and Technology Council project, Grant No. 112-2314-B-002-226-MY3 and 110-2314-B-002-037-MY3.
This research was jointly supported by the Far Eastern Memorial Hospital and the National Taiwan University Hospital Joint Research Program.
We thank Far Eastern Memorial Hospital, National Taiwan University Hospital Joint Research Program and National Science and Technology Council for their support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. How CH, Hsu HH, Chen JS. Chemical pleurodesis for spontaneous pneumothorax. J Formos Med Assoc. (2013) 112:749–55. doi: 10.1016/j.jfma.2013.10.016
2. Chen PH, Hung WT, Chen JS. Nonintubated video-assisted thoracic surgery for the management of primary and secondary spontaneous pneumothorax. Thorac Surg Clin. (2020) 30:15–24. doi: 10.1016/j.thorsurg.2019.08.001
3. Chen PH, Hsu HH, Yang SM, Tsai TM, Tsou KC, Liao HC, et al. Preoperative dye localization for thoracoscopic lung surgery: hybrid versus computed tomography room. Ann Thorac Surg. (2018) 106:1661–7. doi: 10.1016/j.athoracsur.2018.07.030
4. Hsu HH, Liu YH, Chen HY, Chen PH, Chen KC, Hsieh MJ, et al. VICRYL Mesh coverage reduced recurrence after bullectomy for primary spontaneous pneumothorax. Ann Thorac Surg. (2021) 112:1609–15. doi: 10.1016/j.athoracsur.2020.11.012
5. Nakanishi K. An apical symphysial technique using a wide absorbable mesh placed on the apex for primary spontaneous pneumothorax. Surg Endosc. (2009) 23:2515–21. doi: 10.1007/s00464-009-0436-0
6. Sakamoto K, Takei H, Nishii T, Maehara T, Omori T, Tajiri M, et al. Staple line coverage with absorbable mesh after thoracoscopic bullectomy for spontaneous pneumothorax. Surg Endosc. (2004) 18:478–81. doi: 10.1007/s00464-003-8918-y
7. Tsou KC, Huang PM, Hsu HH, Chen KC, Kuo SW, Lee JM, et al. Role of computed tomographic scanning prior to thoracoscopic surgery for primary spontaneous pneumothorax. J Formos Med Assoc. (2014) 113:606–11. doi: 10.1016/j.jfma.2014.02.011
8. Horio H, Nomori H, Fuyuno G, Kobayashi R, Suemasu K. Limited axillary thoracotomy vs video-assisted thoracoscopic surgery for spontaneous pneumothorax. Surg Endosc. (1998) 12:1155–8. doi: 10.1007/s004649900805
9. Gebhard FT, Becker HP, Gerngross H, Brückner UB. Reduced inflammatory response in minimal invasive surgery of pneumothorax. Arch Surg. (1996) 131:1079–82. doi: 10.1001/archsurg.1996.01430220073016
10. Hung WT, Chen HM, Wu CH, Hsu WM, Lin JW, Chen JS. Recurrence rate and risk factors for recurrence after thoracoscopic surgery for primary spontaneous pneumothorax: a nationwide population-based study. J Formos Med Assoc. (2021) 120:1890–6. doi: 10.1016/j.jfma.2020.12.011
11. Lee S, Park SY, Bae MK, Lee JG, Kim DJ, Chung KY, et al. Efficacy of polyglycolic acid sheet after thoracoscopic bullectomy for spontaneous pneumothorax. Ann Thorac Surg. (2013) 95:1919–23. doi: 10.1016/j.athoracsur.2013.03.011
12. Hatz RA, Kaps MF, Meimarakis G, Loehe F, Müller C, Fürst H. Long-term results after video-assisted thoracoscopic surgery for first-time and recurrent spontaneous pneumothorax. Ann Thorac Surg. (2000) 70:253–7. doi: 10.1016/s0003-4975(00)01411-9
13. How CH, Tsai TM, Kuo SW, Huang PM, Hsu HH, Lee JM, et al. Chemical pleurodesis for prolonged postoperative air leak in primary spontaneous pneumothorax. J Formos Med Assoc. (2014) 113:284–90. doi: 10.1016/j.jfma.2012.12.016
14. How CH, Lin CW, Lin YC, Young TH, Chen KC, Chen JS. Evaluation of pleurodesis by poly-ε-caprolactone (PCL) gel in an animal model using New Zealand white rabbits. Asian J Surg. (2019) 42:495–500. doi: 10.1016/j.asjsur.2018.07.001
15. MacDuff A, Arnold A, Harvey J, BTS Pleural disease guideline group. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax (2010) 65(Suppl 2):ii18–31. doi: 10.1136/thx.2010.136986
16. Chen JS, Hsu HH, Chen RJ, Kuo SW, Huang PM, Tsai PR, et al. Additional minocycline pleurodesis after thoracoscopic surgery for primary spontaneous pneumothorax. Am J Respir Crit Care Med. (2006) 173:548–54. doi: 10.1164/rccm.200509-1414OC
17. Lee YC, Yasay JR, Johnson JE, Parker RE, Thompson PJ, Lane KB, et al. Comparing transforming growth factor-beta2, talc and bleomycin as pleurodesing agents in sheep. Respirology. (2002) 7:209–16. doi: 10.1046/j.1440-1843.2002.00397.x
18. Ling ZG, Wu YB, Ming MY, Cai SQ, Chen YQ. The effect of pleural abrasion on the treatment of primary spontaneous pneumothorax: a systematic review of randomized controlled trials. PLoS One. (2015) 10:e0127857. doi: 10.1371/journal.pone.0127857
19. Ikeda T, Sasaki M, Sakon K, Koshiji T. An effective method of pleurodesis involving absorbable mesh for repetitive catamenial pneumothorax. Eur J Cardiothorac Surg. (2012) 42:370–2. doi: 10.1093/ejcts/ezs101
20. Chen KC, Hsieh MS, Li YR, Lin YC, Lai HS, Shieh MJ, et al. Study of poly-ɛ-caprolactone membranes for pleurodesis. J Formos Med Assoc. (2017) 116:880–7. doi: 10.1016/j.jfma.2016.12.009
21. Chen JS, Hsu HH, Huang PM, Kuo SW, Lin MW, Chang CC, et al. Thoracoscopic pleurodesis for primary spontaneous pneumothorax with high recurrence risk: a prospective randomized trial. Ann Surg. (2012) 255:440–5. doi: 10.1097/SLA.0b013e31824723f4
22. Cardillo G, Facciolo F, Giunti R, Gasparri R, Lopergolo M, Orsetti R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: a 6-year experience. Ann Thorac Surg (2000) 69:357–61; discussion 361. doi: 10.1016/s0003-4975(99)01299-0
23. Vanderschueren RG. The role of thoracoscopy in the evaluation and management of pneumothorax. Lung. (1990) 168(Suppl):1122–5. doi: 10.1007/BF02718252
24. Chen JS, Cheng WC, Lien HC, Wu SW, Hsu HH, Lee SC, et al. Patterns, effects, and thoracic volume changes of thoracoscopic pleurodesis in rabbits. J Surg Res. (2008) 147:34–40. doi: 10.1016/j.jss.2007.04.033
25. Chen JS, Chan WK, Tsai KT, Hsu HH, Lin CY, Yuan A, et al. Simple aspiration and drainage and intrapleural minocycline pleurodesis versus simple aspiration and drainage for the initial treatment of primary spontaneous pneumothorax: an open-label, parallel-group, prospective, randomised, controlled trial. Lancet. (2013) 381:1277–82. doi: 10.1016/S0140-6736(12)62170-9
26. Chan P, Clarke P, Daniel FJ, Knight SR, Seevanayagam S. Efficacy study of video-assisted thoracoscopic surgery pleurodesis for spontaneous pneumothorax. Ann Thorac Surg. (2001) 71:452–4. doi: 10.1016/s0003-4975(00)02446-2
27. Park SN, Jang HJ, Choi YS, Cha JM, Son SY, Han SH, et al. Preparation and characterization of biodegradable anti-adhesive membrane for peritoneal wound healing. J Mater Sci Mater Med. (2007) 18:475–82. doi: 10.1007/s10856-007-2007-z
28. Hamaji M, Kojima F, Komatsu T, Tsuruyama T, Date H, Nakamura T. A synthetic bioabsorbable sheet may prevent postoperative intrapleural adhesions following thoracotomy: a canine model. Interact Cardiovasc Thorac Surg. (2014) 19:914–20. doi: 10.1093/icvts/ivu299
29. Chen JS, Hsu HH, Kuo SW, Huang PM, Lee JM, Lee YC. Management of recurrent primary spontaneous pneumothorax after thoracoscopic surgery: should observation, drainage, redo thoracoscopy, or thoracotomy be used? Surg Endosc. (2009) 23:2438–44. doi: 10.1007/s00464-009-0404-8
30. Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med (2000) 342(12):868–74. doi: 10.1056/NEJM200003233421207
31. Chen KC, Cheng YJ, Hung MH, Tseng YD, Chen JS. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis. (2012) 4(4):347–51. doi: 10.3978/j.issn.2072-1439.2012.08.07
Keywords: pleurodesis, pneumothorax, poly-ε-caprolactone, thoracoscopy, Phase I trial
Citation: How C-H, Chen P-H, Chen Y-C, Lin Y-C, Chen K-C, Chen J-S and Young T-H (2024) Safety assessment of poly-ε-caprolactone in the treatment of primary spontaneous pneumothorax. Front. Surg. 11:1335144. doi: 10.3389/fsurg.2024.1335144
Received: 8 November 2023; Accepted: 8 January 2024;
Published: 19 January 2024.
Edited by:
Marco Anile, Sapienza University of Rome, ItalyReviewed by:
Yu-Wei Liu, Kaohsiung Medical University Hospital, Taiwan© 2024 How, Chen, Chen, Lin, Chen, Chen and Young. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke-Cheng Chen Y3NrY2hlbkBnbWFpbC5jb20= Tai-Horng Young dGh5b3VuZ0BudHUuZWR1LnR3
†These authors have contributed equally to this work and share first authorship
Abbreviations PCL, poly-ε-caprolactone; VATS, video-assisted thoracoscopic surgery.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.