
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 18 March 2024
Sec. Surgical Oncology
Volume 11 - 2024 | https://doi.org/10.3389/fsurg.2024.1252131
This article is part of the Research Topic Advance and Innovation of Oncoplastic Breast Surgery View all 6 articles
Introduction: Accessory breast cancer (ABC) is an extremely rare condition, particularly the presence of triple-negative ABC with ipsilateral invasive in situ breast cancer. Binary breast tumors are controversial in terms of surgical methods and comprehensive treatment.
Case presentation: We share the case of a 64-year-old postmenopausal woman who presented with an underarm mass for 3 months. Ultrasonography and computed tomography suggested possible breast cancer with axillary lymph node metastasis. The patient underwent a left modified radical mastectomy combined with axillary lymph node dissection. The postoperative pathology confirmed a binary tumor, prompting us to initiate comprehensive treatment.
Conclusion: We present the treatment approach for a rare case of triple-negative para-breast cancer complicated with carcinoma in situ of the breast, hoping to contribute new therapeutic ideas for the treatment of this disease.
The development of mammary tissue typically occurs along the embryonic mammary ridge, which extends from the axilla to the groin area and emerges during the sixth week of gestation (1, 2). While the embryonic mammary ridge usually regresses, except in the breast region where normal mammary tissue forms, there is a possibility for incomplete degeneration of this ridge, leading to the development of accessory mammary glands. During the embryonic period, approximately 90% of accessory mammary glands develop along the mammary ridge, predominantly in the axillary region. However, reports have also documented their occurrence in other areas such as the chest wall, abdominal femoral groove, face, ear, neck, arms, lateral aspect of the leg, and vulva (1, 3). The prevalence of accessory mammary glands in the axilla is estimated to be between 2% and 6% among women (4), with a female-to-male ratio of 5:1 (3).
Accessory mammary development is as hormone-dependent as the normal breast; in other words, any factor that induces breast cancer can also trigger the development of accessory mammary glands. However, accessory breast cancer (ABC) is an extremely rare condition, accounting for approximately 0.2%–0.6% of all breast cancer cases (5). ABC carries a poor prognosis primarily due to early lymph node invasion, high misdiagnosis rates, and the absence of standardized treatment guidelines. The coexistence of ABC and primary invasive breast cancer is exceptionally uncommon. In this report, we present a case study highlighting the diagnosis and management of this unique clinical scenario.
The coexistence of ABC and primary invasive breast cancer is exceedingly rare. In this report, we present a case study highlighting the diagnosis and management of this unique clinical scenario. The patient had a history of good health with no underlying medical conditions such as hypertension, diabetes, or heart disease. She was not taking any medications and had no known drug allergies. Menarche occurred at the age of 17 years, and menopause occurred at 54 years old. She experienced regular menstrual cycles and breastfed her son for 1 year. There was no family history of diseases or tumors.
The patient's left axilla exhibited a palpable mass with a diameter of 2 cm. It displayed a firm texture, indistinct boundaries, adherence to surrounding tissues, and limited mobility. Multiple enlarged lymph nodes were palpable in the left axilla. In addition, several nodules measuring approximately 0.5 cm in diameter were observed in the left breast, while no evident masses were detected in the right breast or right axilla.
The chest computed tomography (CT) image revealed scattered chronic inflammation and fibrous lesions in both lungs, while two low-density soft tissue nodules were observed in the left axilla (Figure 1).
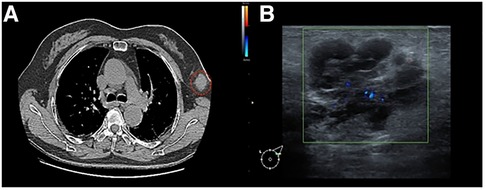
Figure 1. (A) Chest CT image: the red circle indicates a mass of the left accessory milk, which is not clearly connected to the breast tissue. (B) Mammary ultrasound: A glandular echo with a thickness of approximately 0.8 cm can be seen in the left axilla, and a hypoechoic nodule measuring approximately 3.0 cm × 2.1 cm can be seen on the inside. The boundary is clear and irregular in shape, with early lobulation, and the internal echo is uneven and separated.
Breast color ultrasound (US) revealed low-echo nodules in both mammary glands. The larger one was approximately 0.4 cm × 0.3 cm (left breast at 3 o’clock) and displayed an oval shape, smooth edges, a horizontal position, and uniform internal echo. A hypoechoic nodule measuring approximately 3.0 cm × 2.1 cm could be observed internally. The boundary was clear and irregular in shape, with early lobulation, and the internal echo was uneven and separated. Color Doppler flow imaging (CDFI) revealed slightly rich blood flow signals within the nodules. Pulsed wave Doppler indicated that the arterial spectrum could be measured, with a resistance index (RI) of 0.7. In addition, lymph nodes measuring 2.1 cm × 0.8 cm were found in the left armpit, showing smooth edges, low echo, and an unclear skin medulla structure. CDFI exhibited slightly rich blood flow signals. In addition, a lymph node echo with a size of 2.9 cm × 0.7 cm was visible, exhibiting a clear boundary and an even internal echo with no visible echo area inside. CDFI exhibited slightly rich blood flow signals. The left axillary accessory mammary gland appeared as hypoechoic nodules, graded BI-RADS 4b, while the left axillary lymph node structure was abnormal (Figure 1).
Based on the medical history and auxiliary examinations, we diagnosed the tumor as an ABC. The TNM classification indicated it as cT2N1M0, corresponding to clinical stage IIb.
After multidisciplinary discussion and considering the patient's tumor clinical stage of cT2N1M0 (clinical stage IIb), combined with the patient's preferences, we performed a modified radical mastectomy for left breast cancer and axillary lymph node dissection. Preoperative tumor location markers and postoperative specimens are shown in Figure 2. In addition to the left ABC, postoperative pathology revealed an invasive ductal carcinoma in the upper inner quadrant of the left breast.
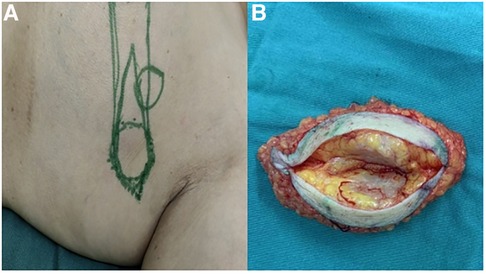
Figure 2. (A) Preoperative tumor location markers. (B) Postoperative specimen: No definite continuous breast tissue was found in the adipose tissue inside the mass.
Postoperative pathology revealed that the left accessory breast mass was a non-specific type of invasive breast cancer with medullary features, measuring approximately 2.8 cm × 2.5 cm, and the histologic grade was characterized as grade III (Figure 3). Immunohistochemical staining revealed the following results: ER and PR expression were negative, p53 was wild-type, and Ki-67 was positive in 90% of cases. Molecular detection results revealed a negative HER2 gene status (no amplification), as identified by fluorescence in situ hybridization (FISH), and diploid chromosome 17. Macro-metastasis was found in 5 out of 7 sentinel lymph nodes on the left side, while no cancer metastasis was found in 26 axillary lymph nodes on the left side. The cutting margins, including the basal cutting margin, skin cutting margin, and nipple cutting margin, were all negative. Postoperative pathology showed that the tumor in the medial superior quadrant of the left breast was multifocal invasive ductal carcinoma with a diameter of approximately 0.5–1.5 cm; in addition, carcinoma in situ was identified. There was no tumor stromal lymphocyte infiltration, but there was more neurovascular involvement with no intravascular tumor thrombus. The histologic grade was identified as grade II (Figure 3). Immunohistochemical staining results revealed positive expression of ER (3+, 90%), PR (3+, 80%), p53 (wild-type), and Ki-67 (+, 5%). Molecular testing revealed a negative HER2 gene status (no amplification), as identified by FISH, and diploid chromosome 17. The cutting margins, including the basal cutting margin, skin cutting margin, and nipple cutting margin, were all negative. The pathological stage was identified as pT2N1bN0.

Figure 3. (A) Pathology of the left internal upper quadrant mass (invasive ductal carcinoma): non-specific invasive carcinoma in situ (approximately 0.5 cm × 1.5 cm, grade II). (B) Pathology of the left axillary mass: non-specific invasive breast cancer with medullary features (approximately 2.8 cm × 2.5 cm, grade III).
According to the results of postoperative pathological analysis and immunohistochemical staining, the patient received postoperative chemotherapy with four cycles of epirubicin and cyclophosphamide, followed by four cycles of docetaxel. The patient has completed all chemotherapy, and there has been no progression of the tumor since surgery.
The accessory mammary gland, also known as an ectopic mammary gland, is the most common breast malformation, which is formed by incomplete degeneration of the mammary gland primordium during the embryonic period (3). The accessory mammary gland shares a tissue structure similar to that of the normal breast; therefore, diseases that can occur in the normal breast can also affect the accessory mammary gland. Any risk factors that may induce breast cancer, such as age, menstruation, fertility, genetics, living environment, and mental factors, cause endocrine system disorders and result in excessive estrogen levels, which can cause ABC. The clinical manifestations of ABC are often the first symptom of an axillary mass or axillary discomfort. Due to the extremely rare nature of this disease in clinical practice, clinicians often treat patients with axillary lymphadenitis or lymphadentuberculosis using routine treatments, which poses difficulty in achieving an early and accurate diagnosis and is prone to clinical misdiagnosis (6).
The treatment principle for ABC is basically equivalent to that of breast cancer, and comprehensive treatment based on surgical treatment is adopted. There is no consensus on the optimal surgical approach for paranasal breast cancer. Madej et al. (7) proposed that unless ABC is very close to or connected with the breast, or cancer cells have been confirmed to invade the side of the breast, there is no need to remove the ipsilateral breast. Evans (8) performed local enlarged axillary tumor resection in addition to axillary lymph node dissection. Compared with radical or modified radical resection, there was no statistically significant difference in survival between the two groups. Drawing on the successful experience of breast-conserving surgery for breast cancer, other experts believe that the scope of surgery for ABC can also be reduced; unless the tumor is very close to the breast or closely connected with the breast, removing the ipsilateral breast is not better than conserving the breast (9). In this case, due to unclear preoperative diagnosis, axillary lymph node metastasis of breast cancer was initially considered, so mastectomy combined with axillary lymph node dissection was performed on the patient. However, the intraoperative frozen section and postoperative pathological analysis revealed the presence of ABC and breast cancer in situ. This indicates that clinicians’ understanding of ABC is still insufficient. Therefore, the relationship between the tumor and the surrounding tissue structure can be observed by breast CT, MRI, or even PET-CT before surgery to judge whether the breast has been involved and assess the condition of axillary lymph nodes, which can help in accurately choosing the surgical method to avoid the blindness associated with mastectomy. Although mastectomy is no better than breast-conserving surgery in many cases, the experience gained from this case suggests that if a mastectomy is not performed, particularly careful follow-up is required to rule out any manifestation of a latent primary breast tumor. For some patients with axillary masses appearing fused and fixed, making complete resection during surgery challenging, it is suggested that a coarse needle aspiration biopsy be performed before surgery. If ABC is confirmed, neoadjuvant chemotherapy can be performed first until the tumor shrinks significantly and then surgery can be performed. The clinical characteristics and surgical methods of ABC cases on PubMed over the last decade are shown in Table 1.
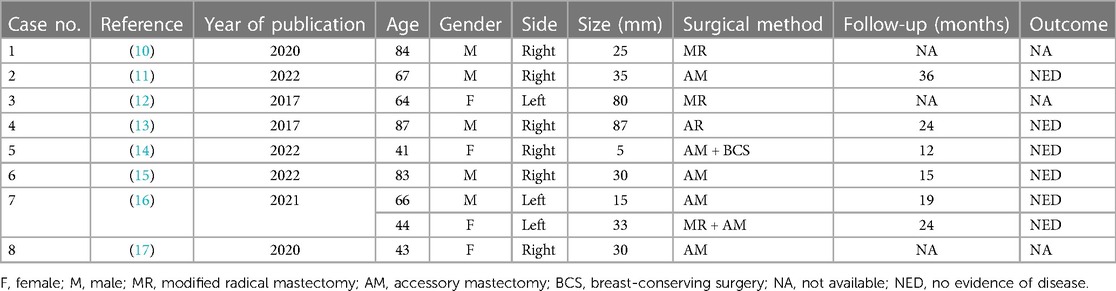
Table 1. Clinical characteristics and surgical methods of ABC cases on PubMed in the recent 10 years.
The principles of postoperative treatment for ABC are basically the same as those for breast cancer. However, because it mostly occurs in the armpit where blood vessels and lymphatic vessels are abundant, early infiltration and metastasis are more likely; thus, the scope of surgical resection will be limited to a certain extent. Therefore, compared with conventional breast cancer, the indications for radiotherapy and chemotherapy can be moderately relaxed in ABC cases (18). Specific chemotherapy regimens can be selected according to the clinical stage and postoperative pathological type of the cancer. Considering that the patient in this case presented with binary cancer, since the breast cancer is in situ, our center has established a comprehensive treatment plan mainly for ABC. In this case, ABC is of a triple-negative subtype, so chemotherapy is the main treatment. The patient is currently receiving chemotherapy with four cycles of epirubicin and cyclophosphamide, followed by four cycles of docetaxel. Triple-negative breast cancer (TNBC) patients have been the focus of research due to their poor prognosis, high mortality rates, and invasiveness. Given that triple-negative ABC is extremely rare and has a worse prognosis, our center will proceed with further treatment after standard chemotherapy. The SYSUCC-001 study (18) demonstrated that intensive adjuvant therapy with ciapecitabine following standard treatment outperformed chemotherapy in TNBC patients, bringing significant clinical benefits. Currently, the combination regimen of platinum-containing drugs has also emerged as a major area of research focus in TNBC treatment. Sharma (19) found that a regimen combining docetaxel with carboplatin achieved a higher pathological complete response rate in neoadjuvant chemotherapy for TNBC. These findings provide a new idea for our follow-up treatment, which warrants further exploration. With the further development of precision medicine, a new generation of antibody–drug conjugates, such as T-DXd, has been introduced. Modi et al. (20) suggested that patients with low expression of HER2 could derive significant survival benefits from T-DXd treatment. Immunotherapy has also gained popularity in the treatment of TNBC in recent years due to its significant efficacy. Currently, studies have proved that the anti-PD-1 antibody pabolizumab has a definite effect on the treatment of TNBC (21). The emergence of these studies provides more treatment possibilities for TNBC patients. However, due to the abundance of blood vessels, nerves, and lymphatic vessels in the armpit, different from radiotherapy in the chest wall and supraclavicular region of traditional breast cancer, radiotherapy for ABC has not been studied clearly and is still being explored.
Patients suspected of ABC should be carefully examined to strive for early diagnosis and treatment, and to improve the survival rate of patients. Surgical indications for accessory mammary gland treatment should be strictly mastered, and individualized surgery and comprehensive treatment programs should be formulated for patients to improve their quality of life.
Due to the rarity and particularity of accessory breast cancer, there is still no clear expert consensus on its standardized treatment. The selection of a surgical program for accessory breast tumors should consider the patient's condition comprehensively and exclude the existence of other tumors fully. We have shared a comprehensive treatment plan for this rare case. We will continue to follow up on the patient's prognosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZW, LC, and GW wrote the main manuscript. CG, SW, and HW collected data and prepared all figures, and all authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pathak S, Preston J. A rare case of multiple accessory breast tissue in the axillae, lower abdomen and vulval areas. J Obstet Gynaecol. (2007) 27(5):531–3. doi: 10.1080/01443610701467473
2. Visconti G, Eltahir Y, Van Ginkel RJ, Bart J, Werker PM. Approach and management of primary ectopic breast carcinoma in the axilla: where are we? A comprehensive historical literature review. J Plast Reconstr Aesthet Surg. (2011) 64(1):e1–11. doi: 10.1016/j.bjps.2010.08.015
3. Hao JY, Yang CC, Liu FF, Yang YL, Li S, Li WD, et al. Accessory breast cancer occurring concurrently with bilateral primary invasive breast carcinomas: a report of two cases and literature review. Cancer Biol Med. (2012) 9(3):197–201. doi: 10.7497/j.issn.2095-3941.2012.03.008
4. Bartsich SA, Ofodile FA. Accessory breast tissue in the axilla: classification and treatment. Plast Reconstr Surg. (2011) 128(1):35e–36e. doi: 10.1097/PRS.0b013e3182173f95
5. Francone E, Nathan MJ, Murelli F, Bruno MS, Traverso E, Friedman D. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. (2013) 37(4):746–9. doi: 10.1007/s00266-013-0125-1
6. Nihon-Yanagi Y, Ueda T, Kameda N, Okazumi S. A case of ectopic breast cancer with a literature review. Surg Oncol. (2011) 20(1):35–42. doi: 10.1016/j.suronc.2009.09.005
7. Madej B, Balak B, Winkler I, Burdan F. Cancer of the accessory breast–a case report. Adv Med Sci. (2009) 54(2):308–10. doi: 10.2478/v10039-009-0031-6
8. Evans DM, Guyton DP. Carcinoma of the axillary breast. J Surg Oncol. (1995) 59(3):190–5. doi: 10.1002/jso.2930590311
9. Yoshida Y, Sakakibara A, Watanabe T, Noto K, Sakita K, Sakai Y, et al. Extraordinarily large protruding accessory breast cancer in a man. J Am Acad Dermatol. (2012) 67(5):e230–1. doi: 10.1016/j.jaad.2012.04.034
10. Bi M, Li D, Su Y, Sun P, Gao Y. Male axillary accessory breast cancer: a case report. Medicine (Baltimore). (2020) 99(11):e19506. doi: 10.1097/MD.0000000000019506
11. Li C, Qian B. Male triple negative axillary accessory breast cancer—a case report. Transl Cancer Res. (2022) 11(8):2926–30. doi: 10.21037/tcr-22-33
12. Kuritzky A, Walheim L, Khakpour N. Cancer identified in accessory breast tissue within the mid axilla. Breast J. (2018) 24(3):414–5. doi: 10.1111/tbj.12912
13. Wang CX, Guo SL, Han LN. Successful treatment of accessory breast cancer with endocrine therapy. J Zhejiang Univ Sci B. (2017) 18(1):70–5. doi: 10.1631/jzus.B1600220
14. Kim H, Ko EY, Han BK, Kim JY, Chae BJ, Lee H. Multicentric breast cancer of the axillary and pectoral breasts: a case report and literature review. J Breast Cancer. (2022) 25(5):436–42. doi: 10.4048/jbc.2022.25.e33
15. Wu J, Chen H, Dong J, Cao Y, Li W, Zhang F, et al. Axillary masses as clinical manifestations of male sweat gland carcinoma associated with extramammary Paget's disease and accessory breast carcinoma: two cases report and literature review. World J Surg Oncol. (2022) 20(1):109. doi: 10.1186/s12957-022-02570-w
16. Van den Bruele AB, Gemignani ML. Management of ipsilateral supernumerary nipple at time of breast cancer diagnosis. Breast J. (2020) 26(10):2042–4. doi: 10.1111/tbj.13973
17. Mandal S, Bethala MG, Dadeboyina C, Khadka S, Kasireddy V. A rare presentation of an invasive ductal carcinoma of ectopic axillary breast tissue. Cureus. (2020) 12(8):e9928. doi: 10.7759/cureus.9928
18. Wang X, Wang SS, Huang H, Cai L, Zhao L, Peng RJ, et al., South China Breast Cancer Group (SCBCG). Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA. (2021) 325(1):50–58. doi: 10.1001/jama.2020.23370
19. Sharma P, López-Tarruella S, García-Saenz JA, Ward C, Connor CS, Gómez HL, et al. Efficacy of neoadjuvant carboplatin plus docetaxel in triple-negative breast cancer: combined analysis of two cohorts. Clin Cancer Res. (2017) 23(3):649–57. doi: 10.1158/1078-0432.CCR-16-0162
20. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al., DESTINY-Breast04 Trial Investigators. Trastuzumab deruxtecan in previously treated her2-low advanced breast cancer. N Engl J Med. (2022) 387(1):9–20. doi: 10.1056/NEJMoa2203690
21. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al., KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. (2020) 396(10265):1817–28. doi: 10.1016/S0140-6736(20)32531-9
Keywords: accessory breast cancer, binary breast cancer, case report, radical mastectomy, triple-negative breast cancer
Citation: Chen L, Wu Z, Guo C, Wan H, Wu S and Wang G (2024) Triple-negative accessory breast cancer occurring concurrently with primary invasive breast carcinoma: a case report. Front. Surg. 11:1252131. doi: 10.3389/fsurg.2024.1252131
Received: 3 July 2023; Accepted: 13 February 2024;
Published: 18 March 2024.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Tito Brambullo, University of Padua, Italy© 2024 Chen, Wu, Guo, Wan, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoping Wang ZG9jdG9yOTg3MUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Abbreviations ABC, accessory breast cancer; US, ultrasound; CT, computed tomography; CDFI, color Doppler flow imaging.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.