
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 03 January 2024
Sec. Colorectal and Proctological Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1340869
This article is part of the Research Topic Newest Challenges and Advances in the Treatment of Colorectal Disorders; From Predictive Biomarkers to Minimally Invasive Techniques View all 23 articles
 Zhizhong Zheng1,†
Zhizhong Zheng1,† Fenfen Kang2,†
Fenfen Kang2,† Yugang Yang1,†
Yugang Yang1,† Yicong Fang1
Yicong Fang1 Kaiyuan Yao1
Kaiyuan Yao1 Qunzhang Zeng1
Qunzhang Zeng1 Muhai Fu1
Muhai Fu1 Lixiong Luo1
Lixiong Luo1 Xiajuan Xue1
Xiajuan Xue1 Shuijie Lin1
Shuijie Lin1 Xingpeng Shi1
Xingpeng Shi1 Xun Fang1
Xun Fang1 Baohua Zhou1
Baohua Zhou1 Yincong Guo1*
Yincong Guo1*
Background: The cosmetic benefits of natural orifice specimen extraction (NOSE) are easily noticeable, but its principles of aseptic and tumor-free procedure have caused controversy.
Methods: We conducted a retrospective analysis of the clinical data of patients who underwent laparoscopic-assisted transanal NOSE or conventional laparoscopic surgery (CLS) for sigmoid and rectal cancer at our hospital between January 2018 and December 2018. The study aimed to compare the general characteristics, perioperative indicators, postoperative complications, and five-year follow-up results between the two groups.
Results: A total of 121 eligible patients were enrolled, with 52 underwent laparoscopic-assisted transanal NOSE and 69 underwent CLS. There were no significant differences observed between the two groups in terms of gender, age, body mass index (BMI), TNM stage, etc. (P > 0.05). However, the NOSE group exhibited significantly shorter total incision length and longer operation time compared to the CLS group (P < 0.05). There were no statistically significant differences observed between the two groups in terms of positive rate of bacterial culture, incidence rates of intraabdominal infections or anastomotic leakage (P > 0.05). Furthermore, during follow-up period there was no statistically significant difference observed between these two groups concerning overall survival rate and disease-free survival outcomes (P > 0.05).
Conclusions: The management of surgical complications in CLS is exemplary, with NOSE presenting a sole advantage in terms of incision length albeit at the cost of prolonged operative time. Therefore, NOSE may be deemed appropriate for patients who place high emphasis on postoperative cosmetic outcomes.
Despite the gradual acceptance of early screening for colorectal tumors, the global incidence and mortality rates of colorectal cancer remain alarmingly high, currently ranking third in terms of incidence and second in terms of mortality worldwide (1), this persistent trend poses significant challenges to colorectal surgeons. Surgical intervention represents the foremost and efficacious modality for managing colorectal neoplasms (2, 3). The transition from open surgery to laparoscopic surgery represents a groundbreaking milestone in the management of colorectal cancer. Modern surgeons strive not only for radical tumor cure but also for minimizing surgical trauma. Moreover, extensive evidence supports the safety and efficacy of laparoscopic colorectal surgery, which is associated with smaller incisions, faster postoperative recovery, and even improved tumor prognosis compared to open surgery (4–6). Consequently, it has gained widespread utilization in clinical practice. However, laparoscopic surgery inevitably necessitates a lengthy abdominal incision for specimen extraction and digestive tract reconstruction. This lengthy incision has led to numerous complications associated with wounds, including infection and hernia formation, which contradicts the fundamental principle of minimally invasive surgery. In the pursuit of achieving a more minimally invasive approach, the emergence of NOSE has revolutionized surgeons' perspectives. By utilizing the natural cavity passage for specimen retrieval, it eliminates the need for lengthy abdominal incisions, resulting in reduced trauma and enhanced aesthetic outcomes. Since its introduction by Franklin et al. (7) in 1993, who reported a series of patients undergoing laparoscopic sigmoid colon resection with transanal specimen removal, this technique has gained widespread recognition and adoption in China (8).
Despite the numerous advantages associated with NOSE, its principle of aseptic and tumor-free procedure remains a subject of controversy (9). The intraperitoneal opening of the intestinal cavity poses an increased risk for intraperitoneal infection and tumor dissemination, whereas extraction of specimens through the natural duct may potentially lead to rectal stump implantation and metastasis. Some studies pertaining to the NOSE have indeed substantiated its safety; however, there exists a dearth of outcomes derived from bacterial culture analysis of postoperative abdominal drainage fluid. Furthermore, the majority of these investigations suffer from limited availability of data. Meanwhile, the limited duration of NOSE surgery and the lack of long-term survival analysis preclude a comprehensive assessment of the efficacy of NOSE cancer treatment (10). This retrospective study aimed to investigate the short-term clinical outcomes and five-year follow-up of laparoscopic-assisted transanal NOSE compared to CLS for the treatment of sigmoid and rectal cancer.
This retrospective study (Registration No. 2020LWB035) was conducted at Zhangzhou Affiliated Hospital of Fujian Medical University, with approval from the ethics committee and informed consent obtained from all patients involved. The inclusion criteria encompassed: (1) patients who underwent laparoscopic-assisted transanal NOSE or CLS for sigmoid and rectal cancer between January 2018 and December 2018 at our institution; (2) patients with confirmed diagnoses of sigmoid or rectal cancer through preoperative colonoscopy and pathology assessments; (3) patients classified as T0–3N0–2M0 stage based on CT or MRI evaluations prior to surgery; (4) patients without evidence of distant metastasis or invasion into adjacent organs; and finally; (5) patients without any concurrent malignant tumors or significant systemic diseases such as cardiac, hepatic, renal conditions, among others. The exclusion criteria were as follows: (1) patients who had to undergo open surgery due to the discovery of distant metastasis or invasion of adjacent organs during the operation; (2) patients who were unable to provide complete follow-up data after surgery. Based on the different surgical methods, the included patients were divided into two groups: NOSE group and CLS group. The general characteristics, perioperative indicators, postoperative complications, and five-year follow-up results of these two groups were compared (Figure 1).

Figure 1. Patient recruitment process. NOSE, natural orifice specimen extraction; CLS, conventional laparoscopic surgery.
All patients were given a prescription for metronidazole tablets two days prior to their surgery and received oral laxatives the evening before the procedure to prepare their bowels. Before the surgery, a single infusion of cefmetazole was routinely administered intravenously 30 min beforehand. If the surgical procedure lasted longer than 3 h, the same dosage was repeated. General anesthesia was uniformly administered to all patients during anesthetic induction.
The patient underwent a modified lithotomy position and achieved pneumoperitoneum at 13 mmHg. The abdominal wall was punctured with five trocars: one 10-mm camera port above the navel, one 12-mm surgeon's operation port in the lower right quadrant, two 5-mm ports on the left side aligned with the spina iliaca anterior superior in both middle and lower abdomen, and one 5-mm port on the right side in the middle abdomen. Tumor and lymphoid tissue dissection were conducted following total mesorectal excision (TME). Sigmoidectomy was performed for tumors located in the sigmoid colon, anterior resection was performed for tumors located in the upper rectum, and low anterior resection was performed for tumors located in the lower rectum.
The CLS group underwent a conventional laparoscopic-assisted procedure for radical sigmoidectomy or proctectomy, involving the creation of a hypogastric incision measuring 4–6 cm in length. After separating the mesocolon, they proceeded to divide the proximal colon and remove tumor tissue. Subsequently, an anvil was introduced into the distal colon to facilitate bowel anastomosis.
The NOSE group received treatment using CRC-NOSES VI (11). A linear cutter stapler was utilized to divide the proximal 10 cm of the colon tumor and the lower edge of the tumor (up to 2–3 cm for rectal cancer and 10 cm for sigmoid colon cancer). Following that, a thorough disinfection of the rectal lumen was conducted using diluted povidone-iodine, followed by an incision in the rectum. Subsequently, a sterile protective sleeve was inserted into the abdominal cavity through which excised diseased tissue could be safely extracted along with the protective sleeve. Next, the circular stapling device's anvil was inserted into the abdominal cavity through the rectal stump. Sterile gauze was carefully placed around the proximal colon. A precise longitudinal incision of approximately 2 cm was made on the wall of the proximal colon to allow for insertion of the anvil in this area. Finally, using an endoscopic linear stapler, both the exposed proximal colon and rectal stump were expertly closed.
In both study groups, the circular stapling device was meticulously inserted into the rectum, followed by a laparoscopic-guided end-to-end anastomosis with the anvil junction positioned in the proximal colon. Subsequently, thorough irrigation of the abdomen and pelvis was performed using a substantial volume of normal saline solution, while concurrently placing a pelvic drainage tube. On postoperative day one, peritoneal drainage fluid samples were collected for bacterial culture analysis.
According to the guidelines provided by the NCCN, adjuvant chemotherapy was administered to all patients who had undergone surgery for T3/T4 or postoperative node-positive tumors. Follow-up appointments were scheduled every 3–6 months within the first three years, which included physical examinations and laboratory tests incorporating tumor biomarkers such as CEA and CA-199. Biannual CT scans of the chest, abdomen, and pelvis were performed, while a complete colonoscopy was planned on an annual basis. The patients were observed at intervals of 6–12 months after the surgery, either through outpatient visits or telephone communication, until the occurrence of CRC recurrence and metastasis or October 01, 2023. The main goals of this study were to assess the long-term outcomes of overall survival (OS) and disease-free survival (DFS) over a period of five years. This approach is in line with the stringent standards expected by Nature journal for scholarly writing.
The statistical data were processed using SPSS software version 27.0 for Windows (IBM Corp., Armonk, NY, United States). Quantitative variables were analyzed utilizing the Student's t-test and expressed as mean ± standard deviation (SD). Categorical variables were presented as a percentage (%) and compared employing Pearson's Chi-Square (χ2) test or Fisher's exact test when appropriate. The Kaplan–Meier method was employed to calculate survival outcomes of patients in both groups, and differences in survival curves (OS and DFS) were compared through the log-rank test. A significance level of P < 0.05 was considered statistically significant according to established conventions.
The CLS group comprised a total of 39 males and 30 females, with an average age of 60.7 ± 11.4 years. Similarly, the NOSE group consisted of 28 males and 24 females, with an average age of 62.2 ± 10.0 years. There were no statistically significant differences in clinical characteristics between the NOSE and CLS groups, including age, gender, BMI, history of abdominal operations, and metastasis (TNM) stages (P > 0.05; Table 1).
No conversions to open surgery were observed, and there were no incidences of incision infection. When comparing NOSE with CLS group, significant differences were noted in the effect on operation time (213.9 ± 20.0 min vs. 194.1 ± 20.6 min, t = 5.292, p < 0.01) and total incision length (7.0 ± 0.0 cm vs. 11.7 ± 0.8 cm, t = −12.435, p < 0.01). However, the differences between the groups regarding positive rate of bacterial culture (15.4% vs. 8.7%, χ2 = 1.297, p = 0.255) and intraabdominal infections (9.6% vs. 2.9%, χ2 = 2.455, P = 0.117) did not reach statistical significance. Eight patients in the NOSE group tested positive for bacterial culture; among them, five patients had escherichia coli cultured from drainage fluid. Six patients in the CLS group tested positive for bacterial culture and five patients had escherichia coli cultured from drainage fluid as shown in Table 2.
The median follow-up period was 64.0 months (range, 14–68). Throughout the entire follow-up duration, a total of 14 out of the initial cohort of 121 patients succumbed to mortality, while an additional 17 patients experienced either local recurrence or distant metastasis. Notably, there was no statistically significant disparity observed in terms of tumor recurrence between the NOSE group and the CLS group. Within the NOSE group specifically, one patient exhibited local recurrence and five patients encountered distant recurrence following a median follow-up period of 64 months (range, 23–68). Conversely, within the CLS group, one patient demonstrated local recurrence and ten patients manifested distant recurrence after being monitored for a median follow-up duration of 63 months (range, 14–68). Only one patient in the NOSE group experienced recurrence at the anastomotic site. The Kaplan curves revealed that the overall survival (p = 0.531) and disease-free survival (p = 0.460) of the NOSE group were comparable to those of the CLS group. In the NOSS group, the 5-year overall survival rate was 90.4% and disease-free survival rate was 88.5%, while in the CLS group, these rates were slightly lower at 87.0% and 84.1%, respectively (Figures 2, 3).
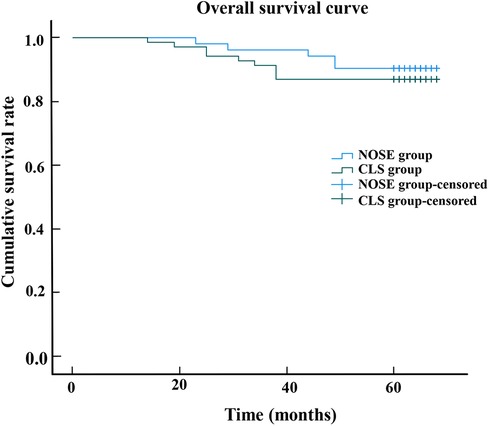
Figure 2. The overall survival curve shows that 5-year overall survival rate in the NOSE group and CLS group were 90.4% and 87.0%, respectively. There was no significant difference between the NOSE and CLS groups (p = 09.531).
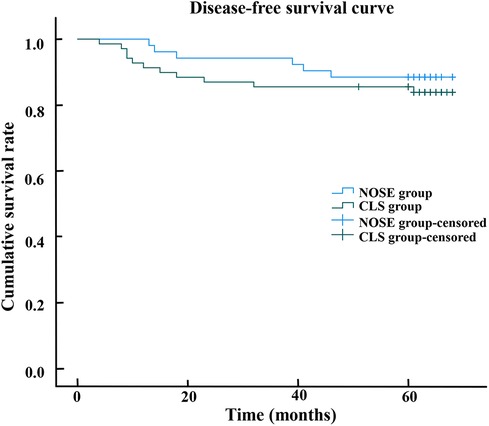
Figure 3. The disease-free survival curve shows that 5-year disease-free survival rate in the NOSE group and CLS group were 88.5% and 84.1%, respectively. There was no significant difference between the NOSE and CLS groups (p = 0.460).
In recent years, technological advancements and innovations in surgical instruments have facilitated the performance of surgeries with reduced incisions (12, 13). However, conventional laparoscopic colorectal cancer surgery inevitably entails a longer auxiliary incision for specimen extraction and reconstruction of the digestive tract. Compared to CLS, the key distinguishing feature of NOSE in colorectal surgery lies in its ability to extract specimens through natural orifices, perform complete intraperitoneal anastomosis, and avoid lengthy abdominal incisions (14–16). Patients undergoing NOSE experience enhanced pain management and reduced incidence of incision infections, among other notable benefits.
Numerous studies had conducted comparisons between NOSE and CLS, yielding invaluable insights for the clinical application in colorectal oncology. Studies had demonstrated that NOSE are predominantly conducted using laparoscopic techniques, which offer enhanced precision and obviate the need for lengthy surgical incisions. Consequently, this approach minimized surgical bleeding and did not prolong the duration of the operation (17). Our study demonstrated that the NOSE group exhibited significantly longer surgery times. Clearly, the laparoscopic procedure for NOSE entailed greater complexity, and certain patients undergoing this technique may encounter challenges in extracting specimens due to the narrow rectal cavity and pelvis, consequently leading to prolonged operative duration. Therefore, we propose conducting preoperative assessments for rhinoplasty patients to not only assess tumor dimensions but also evaluate pelvic measurements. As demonstrated by several studies, patients undergoing NOSE exhibit reduced reliance on postoperative analgesia and report lower pain scores (18–20). Additionally, NOSE has been shown to positively impact postoperative intestinal function recovery and lead to a decreased length of hospital stay (19, 21, 22). There may be multiple factors contributing to this outcome: (Ⅰ) The implementation of complete laparoscopic dissection and reconstruction of the digestive tract effectively minimizes excessive traction on the intestinal tract; (Ⅱ) The utilization of smaller incisions resulting in reduced postoperative pain enables patients to regain mobility earlier after surgery. Our study demonstrates comparable postoperative recovery outcomes among patients undergoing NOSE with CLS.
The postoperative abdominal or pelvic infection resulting from the dissemination of intestinal bacteria during bowel opening and anvil passage through the anorectum had garnered significant attention. Previous studies had substantiated this potential bacterial contamination following NOSE by assessing the prevalence of positive bacterial culture in intraoperative pelvic fluid (23, 24). Our research findings indicate that the predominant bacterial cultures in abdominal drainage were primarily escherichia coli, as a consequence of the dissemination of bacteria due to intestinal cavity opening. Numerous preventive measures had been implemented to impede the ingress of bacteria into the abdominal cavity, including ensuring meticulous bowel preparation, employing a linear cutter stapler for closure of both the proximal and lower edge of the tumor, irrigating with diluted 1% povidone-iodine prior to opening the rectal stump, and utilizing a sterile protective sleeve. Nevertheless, there remained an increased likelihood for bacterial dissemination through the aperture of the proximal bowel and rectal stump (25). However, it should be noted that not all instances of bacterial spread result in intraabdominal infections, and there were cases where patients with intraabdominal infections did not yield positive results in bacterial culture. Furthermore, our study revealed no significant disparity in celiac infections between the NOSE and CLS groups. Consequently, it is plausible to suggest that intracorporeal bowel opening did not augment the likelihood of abdominal or pelvic contamination. Additionally, patients did not encounter an extension in their hospital stay duration subsequent to receiving appropriate anti-infection treatment.
Another concern of the NOSE pertains to whether intraperitoneal dissection of the tumor bowel, opening of the rectal stump and proximal colon, and transrectal removal of the specimen result in exfoliation of cancer cells, potentially leading to recurrence in the abdominal and rectal stump. However, conclusive evidence regarding this matter is still lacking as only a limited number of studies have conducted comprehensive five-year survival analyses. The fundamental principle of tumor surgery is to achieve maximal resection, and the adoption of NOSE does not pose additional challenges in achieving complete tumor removal, particularly during lymph node dissection and mesangial separation. Notably, studies have demonstrated that both NOSE and CLS approaches yield comparable oncological outcomes over follow-up periods (20, 26, 27). Our study findings indicated that patients in the NOSE group demonstrated improved disease-free survival and overall survival outcomes compared to those in the CLS group; however, these differences did not reach statistical significance. We propose that this result may be attributed to limitations associated with CLS. Specifically, the vertical pull-out technique employed for excising diseased tissue through an abdominal incision, along with the compression of the incision protector, potentially increased the risk of tumor cells falling outside the protected area due to gravitational forces. Moreover, it is noteworthy that immediate removal of the incision protector following extraction of diseased tissue from the abdominal cavity was not performed. It was imperative to employ the incision protector for safeguarding the wound during the resection of the proximal colon and placement of the anvil of circular stapling device. However, this inadvertently facilitated tumor cell infiltration into the abdominal cavity, potentially leading to metastasis. In contrast, within the NOSE group, we utilized sterile protective sleeves to facilitate smooth specimen extraction and prevent tumor deposition at the open rectal stump. Subsequently, these sleeves were meticulously removed along with the specimen.
The study has certain limitations, as it did not employ a prospective design. Moreover, due to the restricted sample size, obtaining valid data on specific key findings was unattainable. For instance, no statistically significant difference was detected in bacterial culture results. Peritoneal drainage was only cultured for bacteria on the first day after surgery and was not continuously sampled to prevent potential false-negative results. Regrettably, further investigation into the correlation between bacterial culture in abdominal drainage fluid and intraperitoneal metastasis could not be conducted.
The management of surgical complications in CLS is exemplary, with NOSE presenting a sole advantage in terms of incision length albeit at the cost of prolonged operative time. Therefore, NOSE may be deemed appropriate for patients who place high emphasis on postoperative cosmetic outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Zhangzhou Affiliated Hospital of Fujian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZZ: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. FK: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. YY: Data curation, Investigation, Writing – review & editing. YF: Data curation, Investigation, Writing – original draft. KY: Investigation, Writing – original draft. QZ: Investigation, Writing – original draft. MF: Formal Analysis, Writing – original draft. LL: Formal Analysis, Writing – original draft. XX: Formal Analysis, Writing – original draft. SL: Writing – original draft. XS: Writing – original draft. XF: Writing – original draft. BZ: Writing – original draft. YG: Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank Zhiwei Huang of Minnan Normal University for his help in editing this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram L. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Hospital Authority of National Health Commission of the People’s Republic of China, Chinese Society of Oncology, Chinese Medical Association. National health commission of China colorectal cancer diagnosis and treatment standards (2023 edition). Chin J Pract Surg. (2023) 43(06):602–30. doi: 10.19538/j.cjps.issn1005-2208.2023.06.02
3. National Comprehensive Cancer Network. Colon cancer (version 2.2023). Available at: http://www.nccn.org/patients (Accessed April 25, 2023).
4. Yamaguchi S, Tashiro J, Araki R, Okuda J, Sugihara K. Laparoscopic versus open resection for transverse and descending colon cancer: short-term and long-term outcomes of a multicenter retrospective study of 1,830 patients. Asian J Endosc Surg. (2017) 10(3):268–75. doi: 10.1111/ases.12373
5. Kai C, Zhuqing Z, Yunfei Z, Shuangyi R. Comparison of the clinical outcomes of laparoscopic-assisted versus open surgery for colorectal cancer. Oncol Lett. (2014) 7(4):1213–8. doi: 10.3892/ol.2014.1859
6. Seshadri RA, Swaminathan R, Srinivasan A. Laparoscopic versus open surgery for rectal cancer after neoadjuvant chemoradiation: long-term outcomes of a propensity score matched study. J Surg Oncol. (2018) 3:506–13. doi: 10.1002/jso.24868
7. Franklin ME. Laparoscopic colonic procedures. World J Surg. (1993) 17(1):51–6. doi: 10.1007/BF01655705
8. Guan X, Wang G, Zhou Z, Zhou H, Chen Y, Tang Q, et al. Retrospective study of 718 colorectal neoplasms treated by natural orifice specimen extraction surgery in 79 hospitals. Chin J Colorectal Dis. (2017) 6(06):469–77. doi: 10.3877/CMA.J.ISSN.2095-3224.2017.06.006
9. Hu Q, Bai JG, Liu Z, Wang XS. Key points and clinical evidence of standardized “aseptic tumor free” specimen extraction through natural cavity passage. Colorectal Anal Surg. (2022) 28(5):432–7. doi: 10.19668/j.cnki,issn1674-0491.2022.05.002
10. Liu RJ, Zhang CD, Fan YC, Pei JP, Zhang C, Dai DQ, et al. Safety and oncological outcomes of laparoscopic NOSE surgery compared with conventional laparoscopic surgery for colorectal diseases: a meta-analysis. Front Oncol. (2019) 9:597. doi: 10.3389/fonc.2019.00597
11. Guan X, Liu Z, Parvaiz A, Longo A, Saklani AA, Cai JC, et al. International consensus on natural orifice specimen extraction surgery (NOSES) for gastric cancer (2019). Gastroenterol Rep. (2020) 8(1):5–10. doi: 10.1093/gastro/goz067
12. Athanasiou CD, Robinson J, Yiasemidou M, Lockwood S, Markides GA. Laparoscopic vs open approach for transverse colon cancer. A systematic review and meta-analysis of short and long term outcomes. Int J Surg. (2017) 41:78–85. doi: 10.1016/j.ijsu.2017.03.050
13. Reza MM, Blasco JA, Andradas E, Cantero R, Mayol J. Systematic review of laparoscopic versus open surgery for colorectal cancer. Br J Surg. (2006) 93(8):921–28. doi: 10.1002/bjs.5430
14. Franklin ME, Liang S, Russek K. Natural orifice specimen extraction in laparoscopic colorectal surgery: transanal and transvaginal approaches. Tech Coloproctol. (2013) 17(S1):S63–7. doi: 10.1007/s10151-012-0938-y
15. Akamatsu H, Omori T, Oyama T, Tori M, Ueshima S, Nakahara M, et al. Totally laparoscopic sigmoid colectomy: a simple and safe technique for intracorporeal anastomosis. Surg Endosc. (2009) 23(11):2605–9. doi: 10.1007/s00464-009-0406-6
16. Awad ZT, Qureshi I, Seibel B, Sharma S, Dobbertien MA. Laparoscopic right hemicolectomy with transvaginal colon extraction using a laparoscopic posterior colpotomy: a 2-year series from a single institution. Surg Laparosc Endosc Percutan Tech. (2011) 21(6):403–8. doi: 10.1097/SLE.0b013e31823945ac
17. Ng HI, Sun WQ, Zhao XM, Jin L, Shen XX, Zhang ZT, et al. Outcomes of trans-anal natural orifice specimen extraction combined with laparoscopic anterior resection for sigmoid and rectal carcinoma: an observational study. Medicine (Baltimore). (2018) 97(38):e12347. doi: 10.1097/MD.0000000000012347
18. Mingguang Z, Xiyue H, Xu G, Wei Z, Zheng L, Zheng J, et al. Surgical outcomes and sexual function after laparoscopic colon cancer surgery with transvaginal versus conventional specimen extraction: a retrospective propensity score matched cohort study. Int J Surg. (2022) 104:106787. doi: 10.1016/J.IJSU.2022.106787
19. Fuqiang Z, Wei Z, Tixian X, Zhijie W, Fei H, Wei X, et al. Evaluating short-term and survival outcomes of natural orifice specimen extraction surgery for colorectal cancer: a single-centre retrospective study. Front Surg. (2023) 10:1078316. doi: 10.3389/FSURG.2023.1078316
20. Zhou S, Wang X, Zhao C, Pei W, Zhou H, Liu Q, et al. Comparison of short-term and survival outcomes for transanal natural orifice specimen extraction with conventional mini-laparotomy after laparoscopic anterior resection for colorectal cancer. Cancer Manag Res. (2019) 11:5939–48. doi: 10.2147/CMAR.S209194
21. Awad ZT, Griffin R. Laparoscopic right hemicolectomy: a comparison of natural orifice vs. transabdominal specimen extraction. Surg Endosc. (2014) 28(10):2871–6. doi: 10.1007/s00464-014-3540-8
22. Weiwei W, Feipeng X, Feng G, Meixia L, Junjun L, Qidong L, et al. Short-term clinical effects and inflammatory response of natural orifice specimen extraction surgery versus conventional laparoscopic-assisted surgery for the treatment of sigmoid and rectal cancer. J Gastrointest Oncol. (2023) 14(2):815–23. doi: 10.21037/JGO-23-144
23. Costantino FA, Diana M, Wall J, Leroy J, Mutter D, Marescaux J. Prospective evaluation of peritoneal fluid contamination following transabdominal vs. transanal specimen extraction in laparoscopic left-sided colorectal resections. Surg Endosc. (2012) 26(6):1495–500. doi: 10.1007/s00464-011-2066-6
24. Ngu J, Wong ASY. Transanal natural orifice specimen extraction in colorectal surgery: bacteriological and oncological concerns. Aust N Z J Surg. (2016) 86(4):299–302. doi: 10.1111/ans.13383
25. Shan M, YiBo G, Xu G, Tang QC, Shao F, Guiyu W, et al. Laparoscopic natural orifice specimen extraction, a minimally invasive surgical technique for mid-rectal cancers: retrospective single-center analysis and single-surgeon experience of selected patients. J IntMed Res. (2022) 50(11):1–14. doi: 10.1177/03000605221134472
26. Wang S, Tang J, Sun W, Yao H, Li Z. The natural orifice specimen extraction surgery compared with conventional laparoscopy for colorectal cancer: a meta-analysis of efficacy and long-term oncological outcomes. Int J Surg. (2022) 97:106196. doi: 10.1016/j.ijsu.2021.106196
Keywords: laparoscopy, survival analysis, intraabdominal infections, colorectal surgery, sigmoid
Citation: Zheng Z, Kang F, Yang Y, Fang Y, Yao K, Zeng Q, Fu M, Luo L, Xue X, Lin S, Shi X, Fang X, Zhou B and Guo Y (2024) Short-term clinical outcomes and five-year survival analysis of laparoscopic-assisted transanal natural orifice specimen extraction versus conventional laparoscopic surgery for sigmoid and rectal cancer: a single-center retrospective study. Front. Surg. 10:1340869. doi: 10.3389/fsurg.2023.1340869
Received: 19 November 2023; Accepted: 7 December 2023;
Published: 3 January 2024.
Edited by:
Francesk Mulita, General University Hospital of Patras, GreeceReviewed by:
Angelis Peteinaris, University of Patras, Greece© 2024 Zheng, Kang, Yang, Fang, Yao, Zeng, Fu, Luo, Xue, Lin, Shi, Fang, Zhou and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yincong Guo Zmp6emd5Y0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.