- 1Department of Geriatrics, Taizhou First People’s Hospital, Taizhou, China
- 2Department of Anus & Intestine Surgery, Taizhou First People’s Hospital, Taizhou, China
- 3Department of General Surgery, Taizhou First People’s Hospital, Taizhou, China
Purpose: It was aimed at assessing the benefits of adjuvant chemotherapy (ACT) for patients with node-negative colorectal cancer (CRC) either with or without perineural invasion (PNI).
Methods: We systematically searched PubMed, Cochrane Library, Embase, and Web of Science from database inception through October 1, 2023. Survival outcomes were analyzed using hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). The methodological quality of included studies was assessed using the Newcastle-Ottawa Scale (NOS). Heterogeneity for the descriptive meta-analyses was quantified using the I2 statistic.
Results: Ten studies included in this review. ACT improved overall survival (OS) (HR 0.52, 95% CI 0.40–0.69) and disease-free survival (DFS) (HR 0.53, 95% CI 0.35–0.82) in PNI + patients but did not affect DFS (HR 1.13, 95% CI 0.72–1.77) in PNI- patients. A disease-specific survival (DSS) benefit with chemotherapy was observed in PNI + (HR 0.76, 95% CI 0.58–0.99) and PNI- patients (HR 0.76, 95% CI 0.57–1.00). And PNI decreased DFS (HR 1.94, 95% CI 1.52–2.47) and OS (HR 1.75, 95% CI 0.96–3.17) in node-negative CRC.
Conclusions: In conclusion, chemotherapy appears most beneficial for survival outcomes in node-negative patients with PNI, but may also confer some advantage in those without PNI.
Systematic Review Registration: Identifier INPLASY2021120103.
Introduction
Colorectal cancer is the third most common type of cancer in both men and women. Globally, almost 1.5 million new cases of CRC are diagnosed every year, of which more than a third are fatal (1). The most common cause of death is complications arising from metastasis (2). The primary treatment for stage I–II CRC is radical surgery (3). However, undetected micrometastases that persist after curative surgery may cause cancer recurrence (4). This micrometastasis is eradicated with ACT to enhance cure rates (5). It is unfortunate that few reliable prognostic and predictive markers exist to identify patients at a high risk for disease progression during the early stages of CRC (6). Stage II CRC recurrence rates range from 7.9%–22%, whereas only 2%–5% of patients benefit from ACT (7–10). Due to these reasons, the National Comprehensive Cancer Network (NCCN) does not recommend conventional ACT for stage II CRC unless certain risk factors exist. There were pT4 lesions, intestinal perforation, obstruction, 12-sample lymph nodes (LNs), lymph vascular invasion, PNI, poorly differentiated histology and margins that are positive, indeterminate, or close (11). Patients with these risk factors have a relatively poor prognosis (12). According to Lin et al., ACT was beneficial to patients with CRC and certain risk factors (13). In contrast, O'Connor et al. reported that ACT had no effect on any of these risk factors (14). Kumar et al. found that ACT was most effective for patients with pT4 in high-risk patients (15). Recent studies suggest, however, that ACT can benefit patients with PNI (16–18). PNI refers to tumor cells spreading through nerves. It was Bataskis who first described the prognostic value of PNI, which he defined as “tumor invasion around and through nerves (19).” PNI has been recognized as an unfavorable prognostic factor in CRC since it is associated with poor survival rates (20).
ACT, however, remains controversial because it is unclear whether these patients will benefit patients with PNI (21). This study was conducted to determine whether node-negative CRC patients with and without PNI receive different benefits from ACT.
Materials and methods
Search strategy
Our search focused on academic papers published in English between inception through October 1, 2023 in PubMed, Cochrane Library, Web of Science, and Embase databases. The following keywords were used: “perineural invasion”, “PNI”, “colon cancer”, “rectal neoplasms”, “Corectal cancer”, “colorectal neoplasms”, “adjuvant chemotherapy”, “cohort”, “randomized controlled trial”, and “randomized trial”. Additionally, we searched the references of relevant articles.
Selection criteria and exclusion criteria
Studies were included if they met the following criteria: (1) Enrolled patients with stage II CRC who underwent radical resection, confirmed by postoperative histopathology. (2) Assessed the association between PNI and survival among patients receiving ACT. (3) Published in English. (4) Reported sufficient data to calculate HRs and 95% CIs. Studies were excluded if they: (1) Were not published in English. (2) Included node-positive or mixed stage CRC patients. (3) Were case reports or case series with <50 patients. (4) Did not report outcomes of interest including OS, DFS.
Data extraction and quality assessment
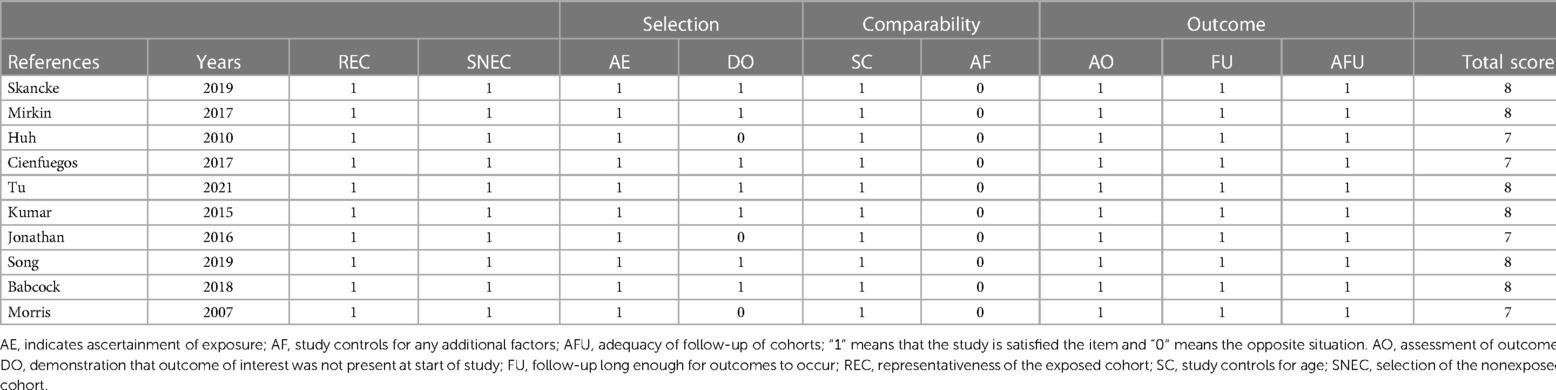
The researchers (W. Yu and H. Ying) independently assessed the eligibility of all the studies and extracted the following information: The first author's name, the country in which the study was conducted, the sample size, the year of the study, the ages of the participants, the stage of their cancer, the chemotherapy regimen, and the period of follow-up. As well as OS, DFS, DSS, recurrence-free survival (RFS), and NOS. We consulted with a third reviewer (W. Hong) to resolve any discrepancies between the reviewers. In order to rate the quality of the articles, we used the NOS score. Articles that have an NOS score >6 (on a scale of 0–9) were considered to be of high quality (22).
Risk of bias analysis
Using non-parametric correlation tests, we examined the association between quality of reporting and HR. Begg and Egger tests were also conducted to determine whether publication bias was present (23).
Statistical analysis
Our analysis used HRs and 95% CIs to compare PNI with survival. When HRs and 95% CIs were not included, data were derived from survival curves according to Parmar et al. and Tierney et al. (24, 25). Study heterogeneity was examined using I2 statistics. Whenever there was obvious heterogeneity, as indicated by a p-value < 0.10 or I2 exceeding 50%, a random effect model was used. In other cases, a fixed effect model was used. Our findings were further enhanced by performing meta-regressions and subgroup analyses in order to identify the sources of heterogeneity. We conducted sensitivity analysis to determine the stability of our combined results, and we assessed publication bias using the Begg and Egger test (26, 27). Statistical significance was set at p < 0.05 using STATA 16.0 (Stata Corporation, College Station, TX, USA).
Results
Search results and quality assessment
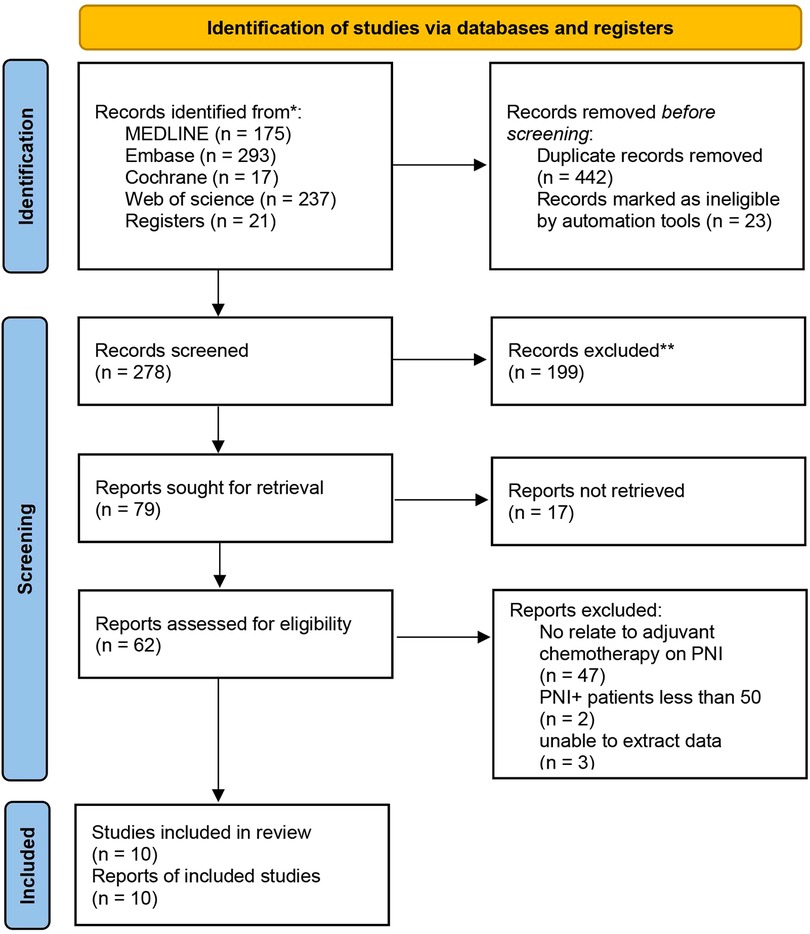
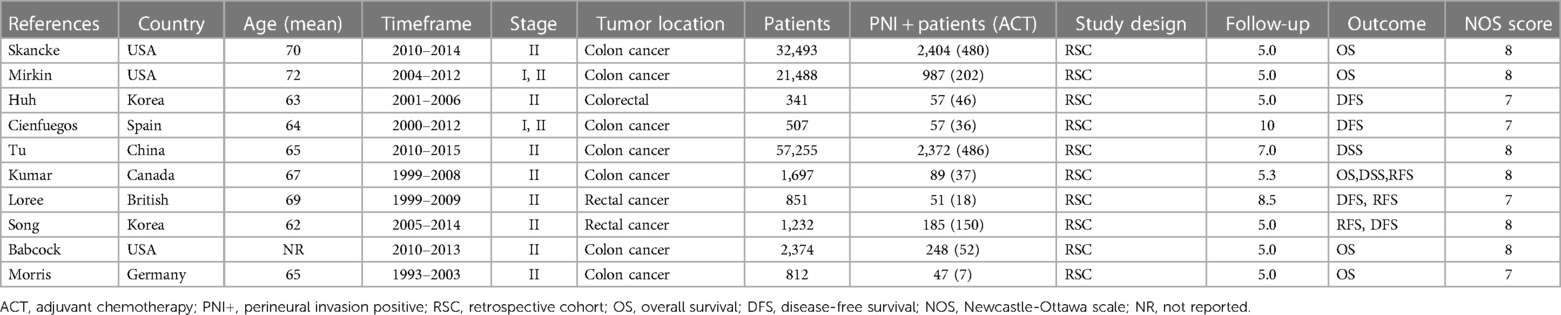
We conducted electronic searches of MEDLINE, Embase, Cochrane Library, and Web of Science, which yielded 743 studies. An additional 21 studies were identified from reference lists. After removing 442 duplicate records, 322 studies underwent title and abstract screening, of which 199 were excluded as Records excluded. The full texts of the remaining 123 studies were assessed; 17 studies could not be retrieved and 52 further studies were excluded based on predefined criteria. Ultimately, 10 studies met the inclusion criteria and were included in the systematic review and meta-analysis, comprising data on 118,529 patients in total. The study selection process is outlined in the PRISMA flow diagram (Figure 1). All patients underwent curative-intent resection of their CRC prior to ACT. Some studies also analyzed the high-risk factors after colon cancer surgery. Table 1 summarizes the 10 retrospective cohort studies included in the systematic review. These studies involved 118,529 patients with stage II CRC who underwent surgery. The studies compared ACT vs. no chemotherapy and reported on outcomes including OS, DFS, and recurrence. Follow-up times ranged from 5 to 10 years. We assessed the quality of ten articles by using the NOS score since they were retrospective cohort studies. A total of seven articles scored 7 points and six articles scored 8 points, with the main loss being the study controls for confounding factors (Table 2).
Effect of adjuvant chemotherapy on perineural invasion
We evaluated the survival of node-negative CRC patients who received ACT compared to no chemotherapy. Across the 10 included studies, 6,196 patients had PNI, with 1,467 receiving ACT. The prevalence of PNI ranged from 5.2% to 11.3% based on tumor location. OS was analyzed in 6 studies comprising 3,794 PNI + patients, of whom 786 underwent ACT, as well as 54,177 PNI- patients, including 6,535 who received ACT (12, 15, 28–31). DFS was examined in 4 studies including 344 PNI + patients (with 62 receiving ACT) and 3,285 PNI- patients (with 1,191 receiving ACT) (29, 32–34). Two studies with 2,461 PNI + (262 ACT) and 55,257 PNI- (4,675 ACT) patients analyzed DSS (15, 35). Recurrence-free survival (RFS) was assessed in 2 studies: one with 108 PNI + patients (43 ACT) and another with 2,498 PNI- patients (471 ACT) (15, 29). RFS was improved with ACT in PNI + patients (HR 0.79, 95% CI 0.42–1.46, I2 = 0%), but RFS data were unavailable for PNI- patients (Table 1).
We compared OS, DFS and DSS between patients who received ACT and those who observation only, stratified by PNI status. For patients with node-negative CRC and PNI+, ACT was associated with significantly improved OS compared to observation (HR 0.52, 95% CI 0.40–0.69). There was moderate heterogeneity between the 3 included studies (I2 = 41.3%, p = 0.130). In the PNI- subgroup, ACT also conferred an OS benefit over observation (HR 0.52, 95% CI 0.27–0.78). However, there was substantial heterogeneity between the 2 studies in this analysis (I2 = 77.1%, p = 0.013). ACT appeared to improve OS regardless of PNI status. The OS benefit with ACT was similar between PNI + and PNI- patients (Figure 2).
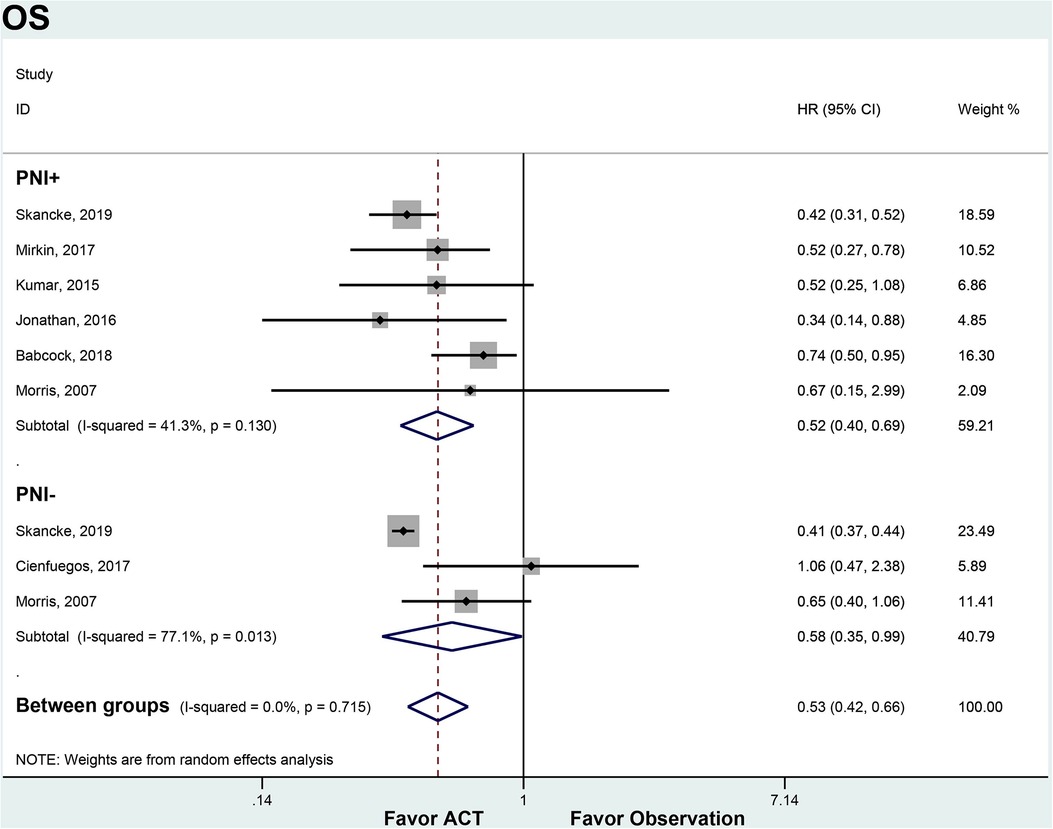
Figure 2. ACT versus observation-only patients stratified by PNI, OS. Diamond represents the pooled effect estimate of the overall analysis. Data are represented as HRs with 95% CIs. Inter-study heterogeneity quantified by I2 with significance p < 0.10. HR, hazard ratio; OS, overall survival; ACT, adjuvant chemotherapy; PNI, perineural invasion.
Among PNI + patients, ACT significantly improved DFS compared to observation alone (HR 0.53, 95% CI 0.35–0.82). There was no heterogeneity between the 4 studies in this subgroup (I2 = 0%, p = 0.797). In the PNI- subgroup, ACT did not provide a DFS benefit over observation (HR 1.13, 95% CI 0.72–1.77). No significant heterogeneity was found between the 2 PNI- studies (I2 = 0%, p = 0.328). ACT appeared to improve DFS in node-negative CRC patients with PNI, but not in those without PNI (Figure 3).
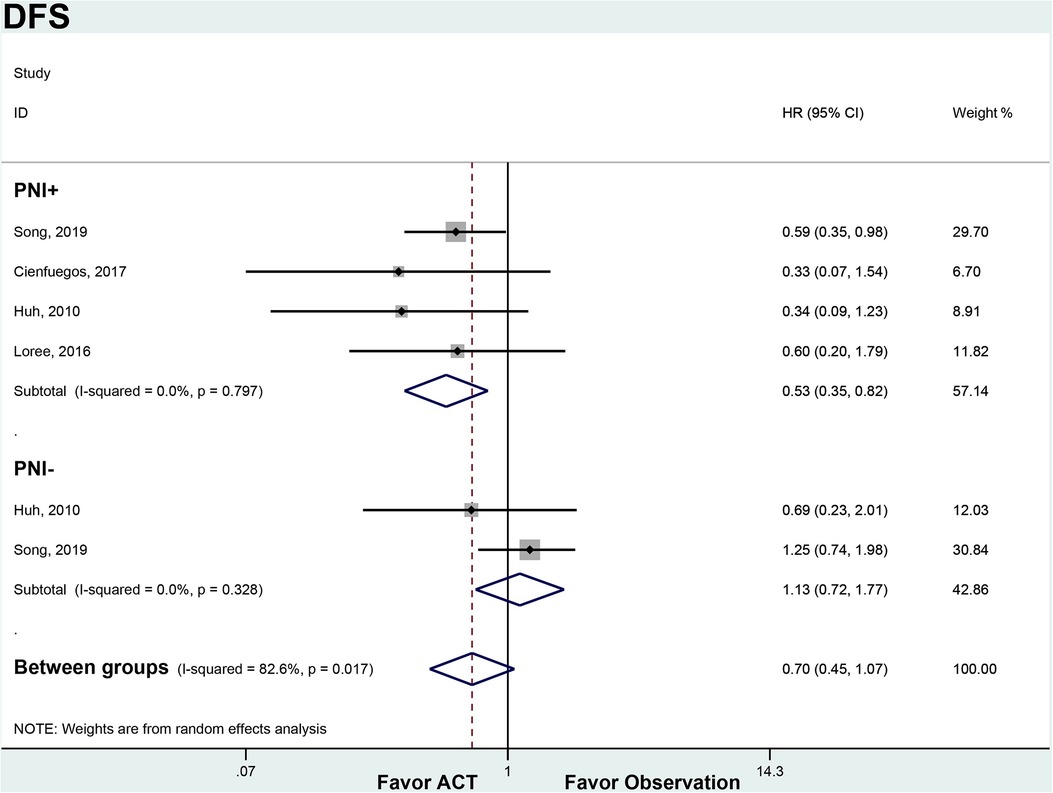
Figure 3. ACT versus observation-only patients stratified by PNI, DFS. Diamond represents the pooled effect estimate of the overall analysis. Data are represented as HRs with 95% CIs. Inter-study heterogeneity quantified by I2 with significance p < 0.10. HR, hazard ratio; DFS, disease-free survival; ACT, adjuvant chemotherapy; PNI, perineural invasion.
In the PNI + subgroup, ACT was associated with improved DSS compared to observation (HR 0.76, 95% CI 0.58–0.99). There was no heterogeneity between the 2 studies (I2 = 0%, p = 0.980). For PNI- patients, ACT also showed a trend towards improved DSS over observation that did not reach statistical significance (HR 0.76, 95% CI 0.57–1.00). Only 1 study was available for this subgroup analysis (Figure 4).
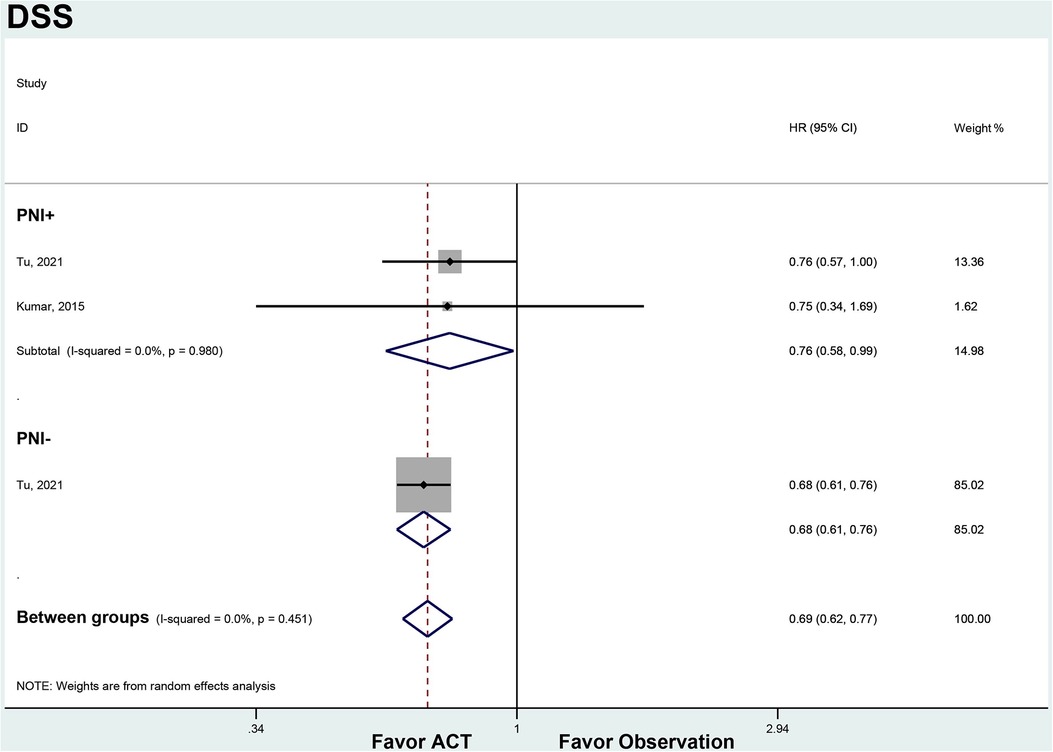
Figure 4. ACT versus observation-only patients stratified by PNI, DFS. Diamond represents the pooled effect estimate of the overall analysis. Data are represented as HRs with 95% CIs. Inter-study heterogeneity quantified by I2 with significance p < 0.10. HR, hazard ratio; DSS, disease-specific survival; ACT, adjuvant chemotherapy; PNI, perineural invasion.
Effect of perineural invasion on survival
Five studies involving 91,828 patients provided data on the impact of PNI on survival (28, 32–35). In three studies, PNI was found to decrease DFS (HR = 1.94, 95% CI = 1.52–2.47, p < 0.001). There was no significant heterogeneity between studies (I2 = 0.00%, p < 0.001). There were two studies analyzing the OS (28, 34). The OS decreased in the presence of PNI (HR = 1.75, 95% CI = 0.96–3.17). Significant heterogeneity was observed between studies (I2 = 89.8%, p = 0.002) (Figure 5).
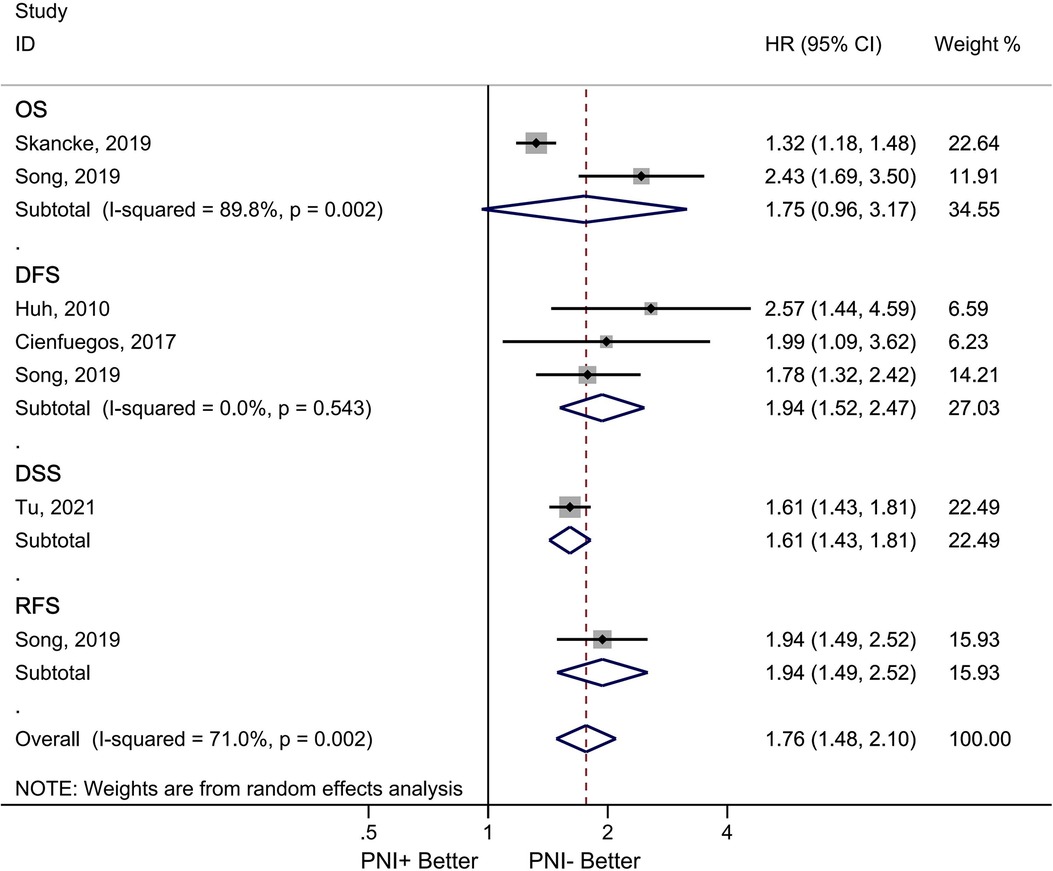
Figure 5. Association between PNI and survival in node negative colorectal cancer patients. Diamond represents the pooled effect estimate of the overall analysis. Data are represented as HRs with 95% CIs. Inter-study heterogeneity quantified by I2 with significance p < 0.10, HR, hazard ratio; ACT, adjuvant chemotherapy; PNI, perineural invasion; OS, overall survival; DFS, disease free survival overal; DSS, disease-specific survival; RFS, recurrence-free survival; PNI, perineural invasion.
Sensitivity analysis
Fixed effects and random effects models were compared to analyze prognosis (OS) in patients with PNI who were treated with ACT.
We analyzed the prognosis (OS) of patients with PNI who received ACT by comparing fixed effect and random effect models. OS did not differ significantly between the two models (fixed effect model: HR = 0.51, 95% CI = 0.43–0.61, random effect model: HR = 0.52, 95% CI = 0.40–0.69). In the sensitivity analysis, we arbitrarily deleted the OS and DFS literature, which did not affect the results of this study (Figure 6).
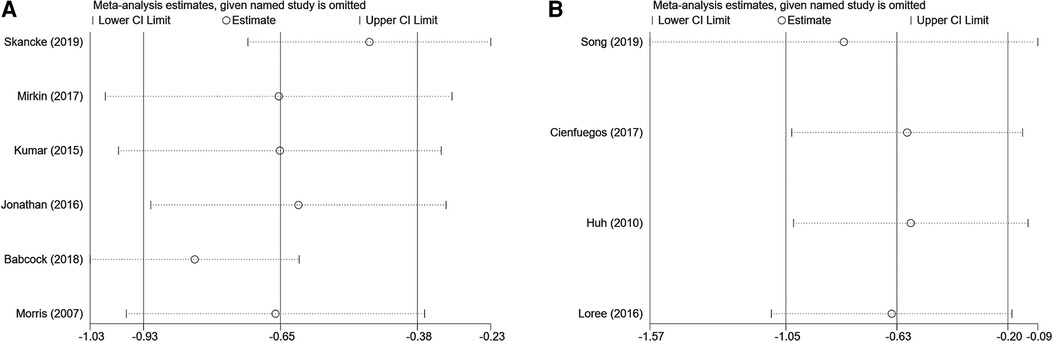
Figure 6. Sensitivity analysis of overall high-risk factors receiving adjuvant hemotherapy on OS (A) and disease-free survival (B).
Publication bias
Our analysis included ten studies, but the subgroup studies were relatively few because they assessed different outcomes. There is an inherent risk of public bias in all reviews. According to Egger and Begg tests (Egger test: p = 0.189; Begg test: p = 0.308), DFS analysis did not detect a significant publication bias. In addition, the DFS analysis found no evidence of publication bias (Egger test: p = 0.925; Begg's test: p = 1.00).
Discussion
This systematic review and meta-analysis examined the efficacy of ACT for node-negative CRC stratified by PNI status. Our results suggest that chemotherapy improves overall and DFS in patients with PNI, but may not affect DFS in those without PNI.
OS was significantly improved with ACT vs. observation in the PNI + subgroup (HR 0.52, 95% CI 0.40–0.69), consistent with prior studies showing a survival benefit for high-risk stage II patients receiving chemotherapy (36, 37). A recent cohort study of 500 colon cancer patients also found the addition of oxaliplatin to standard 5-FU chemotherapy prolonged OS and DFS selectively in the subgroup with PNI (33). The survival gain seen with chemotherapy in PNI + patients may be due to eradication of occult micrometastases not detectable on standard pathology (38). Interestingly, we also observed an OS benefit with chemotherapy in the PNI- subgroup (HR 0.52, 95% CI 0.27–0.78), although prior analyses have been conflicting (10, 39). The reason for improved OS with chemotherapy even for lower risk PNI- patients is unclear and warrants investigation.
DFS was significantly improved by chemotherapy in the PNI + subgroup (HR 0.53, 95% CI 0.35–0.82) but not in the PNI- subgroup (HR 1.13, 95% CI 0.72–1.77). These findings align with other studies demonstrating PNI is an independent prognostic factor for DFS (40). A potential explanation is that PNI + tumors are more aggressive and prone to early micrometastases or local recurrence after surgery that is eradicated by chemotherapy (41). The lack of DFS benefit with chemotherapy in PNI- patients highlights the need for risk-stratified treatment approaches to avoid over-treatment (42). Recent data suggest molecular profiling may help further stratify risk in node negative CRC (43).
This study has several limitations. The pooled sample size was relatively small for PNI subgroup analyses, particularly for secondary outcomes like DFS and DSS, warranting cautious interpretation. Publication bias remains a concern given the limited number of studies. There was heterogeneity between studies that may relate to differences in chemotherapy regimens, follow-up times, and underlying study populations. The retrospective observational nature of the included studies also has inherent biases compared to prospective trials. And this systematic review included studies published over a long timespan, ranging from 1993 to 2015. The inclusion of literature covering many decades could introduce bias, as changes in cancer treatments, staging modalities, and other factors over time may impact outcomes. Despite these limitations, this systematic review provides a comprehensive synthesis of current evidence regarding efficacy of ACT in early stage CRC with vs. without PNI.
ACT appears to improve survival outcomes primarily in node-negative CRC patients with PNI. PNI may be an important factor to guide chemotherapy decisions in this population. Additional well-designed prospective studies are needed to clarify the risk-benefit ratio of adjuvant treatment based on PNI status. Future research should also examine how emerging prognostic factors and individualized risk prediction models can optimize personalized adjuvant therapy for early stage CRC.
Conclusion
ACT improved OS and DSS in node-negative CRC patients regardless of PNI status. But DFS benefit with chemotherapy was observed only in patients with PNI. Overall, chemotherapy appears most beneficial for survival outcomes in node-negative patients with PNI, but may also confer some advantage in those without invasion.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author contributions
HY: Conceptualization, Investigation, Writing – original draft. JS: Conceptualization, Data curation, Writing – original draft. NL: Formal Analysis, Validation, Writing – original draft. XX: Software, Visualization, Writing – review & editing. WY: Conceptualization, Data curation, Writing – original draft. WH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the Taizhou Municipal Science and Technology Bureau of Zhejiang, China (grant number: 23ywa23).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. (2023) 73(3):233–54. doi: 10.3322/caac.21772
2. Lebeck Lee CM, Ziogas IA, Agarwal R, Alexopoulos SP, Ciombor KK, Matsuoka LK, et al. A contemporary systematic review on liver transplantation for unresectable liver metastases of colorectal cancer. Cancer. (2022) 128(12):2243–57. doi: 10.1002/cncr.34170
3. Mothes H, Bauschke A, Schuele S, Eigendorff E, Altendorf-Hofmann A, Settmacher U. Surgery for colorectal cancer in elderly patients: how can we improve outcome. J Cancer Res Clin Oncol. (2017) 143(9):1879–89. doi: 10.1007/s00432-017-2438-y
4. Miyamoto Y, Hiyoshi Y, Sawayama H, Tokunaga R, Baba H. Precision medicine for adjuvant chemotherapy of resected colorectal cancer. Ann Gastroenterol Surg. (2020) 4(6):635–45. doi: 10.1002/ags3.12397
5. Shibutani M, Nagahara H, Fukuoka T, Iseki Y, Hirakawa K, Ohira M. Efficacy of adjuvant chemotherapy according to the classification of recurrence risk based on systemic inflammatory markers in patients with liver metastases of colorectal cancer. Anticancer Res. (2019) 39(9):5039–45. doi: 10.21873/anticanres.13695
6. Chung SS, Ali SI, Cash BD. The present and future of colorectal cancer screening. Gastroenterol Hepatol (N Y). (2022) 18(11):646–53.36866031
7. Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for medicare beneficiaries with stage II colon cancer. J Clin Oncol. (2002) 20(19):3999–4005. doi: 10.1200/JCO.2002.11.084
8. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International multicentre pooled analysis of B2 colon cancer trials (IMPACT B2) investigators. J Clin Oncol. 1999 17(5): 1356–63. doi: 10.1200/JCO.1999.17.5.1356
9. Gertler R, Rosenberg R, Schuster T, Friess H. Defining a high-risk subgroup with colon cancer stages I and II for possible adjuvant therapy. Eur J Cancer. (2009) 45(17):2992–9. doi: 10.1016/j.ejca.2009.07.008
10. Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. (2007) 370(9604):2020–9. doi: 10.1016/S0140-6736(07)61866-2
11. Weiss JM, Gupta S, Burke CA, Axell L, Chen LM, Chung DC, et al. NCCN guidelines® insights: genetic/familial high-risk assessment: colorectal, version 1.2021. J Natl Compr Canc Netw. (2021) 19(10):1122–32.34666312
12. Mirkin KA, Hollenbeak CS, Mohamed A, Jia Y, El-Deiry WS, Messaris E. Impact of perineural invasion on survival in node negative colon cancer. Cancer Biol Ther. (2017) 18(9):740–5. doi: 10.1080/15384047.2017.1323602
13. Liu LL, Xiang ZL. Adjuvant chemotherapy improves survival in high-risk stage II colon cancer: a retrospective cohort study. Therap Adv Gastroenterol. (2022) 15:17562848221137758. doi: 10.1177/17562848221137758
14. O’Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. (2011) 29(25):3381–8. doi: 10.1200/JCO.2010.34.3426
15. Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gill S, Woods R, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer. (2015) 121(4):527–34. doi: 10.1002/cncr.29072
16. Cho JR, Lee KW, Oh HK, Kim JW, Kim JW, Kim DW, et al. Effectiveness of oral fluoropyrimidine monotherapy as adjuvant chemotherapy for high-risk stage II colon cancer. Ann Surg Treat Res. (2022) 102(5):271–80. doi: 10.4174/astr.2022.102.5.271
17. Paolo M, Cruz J, George C, Pales C, Min Kim K, Wan Kim Y. Adjuvant chemotherapy for high-risk stage II and stage III colon cancer: timing of initiation and optimal duration. J BUON. (2018) 23(3):568–73.30003720
18. Sinicrope FA, Chakrabarti S, Laurent-Puig P, Huebner L, Smyrk TC, Tabernero J, et al. Prognostic variables in low and high risk stage III colon cancers treated in two adjuvant chemotherapy trials. Eur J Cancer. (2021) 144:101–12. doi: 10.1016/j.ejca.2020.11.016
19. Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. (1985) 94(4 Pt 1):426–7. doi: 10.1177/000348948509400420
20. Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID. Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am J Surg Pathol. (2016) 40(1):103–12. doi: 10.1097/PAS.0000000000000518
21. Rebuzzi SE, Pesola G, Martelli V, Sobrero AF. Adjuvant chemotherapy for stage II colon cancer. Cancers (Basel). (2020) 12(9):2584. doi: 10.3390/cancers12092584
22. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
23. Lin L, Chu H, Murad MH, Hong C, Qu Z, Cole SR, et al. Empirical comparison of publication bias tests in meta-analysis. J Gen Intern Med. (2018) 33(8):1260–7. doi: 10.1007/s11606-018-4425-7
24. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17(24):2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24%3C2815::AID-SIM110%3E3.0.CO;2-8
25. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
26. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50(4):1088–101. doi: 10.2307/2533446
27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
28. Skancke M, Arnott SM, Amdur RL, Siegel RS, Obias VJ, Umapathi BA. Lymphovascular invasion and perineural invasion negatively impact overall survival for stage II adenocarcinoma of the colon. Dis Colon Rectum. (2019) 62(2):181–8. doi: 10.1097/DCR.0000000000001258
29. Loree JM, Kennecke HF, Renouf DJ, Lim HJ, Vickers MM, Speers CH, et al. Effect of adjuvant chemotherapy on stage II rectal cancer outcomes after preoperative short-course radiotherapy. Clin Colorectal Cancer. (2016) 15(4):352–9.e1. doi: 10.1016/j.clcc.2016.04.003
30. Babcock BD, Aljehani MA, Jabo B, Choi AH, Morgan JW, Selleck MJ, et al. High-risk stage II colon cancer: not all risks are created equal. Ann Surg Oncol. (2018) 25(7):1980–5. doi: 10.1245/s10434-018-6484-8
31. Morris M, Platell C, McCaul K, Millward M, van Hazel G, Bayliss E, et al. Survival rates for stage II colon cancer patients treated with or without chemotherapy in a population-based setting. Int J Colorectal Dis. (2007) 22(8):887–95. doi: 10.1007/s00384-006-0262-y
32. Cienfuegos JA, Martínez P, Baixauli J, Beorlegui C, Rosenstone S, Sola JJ, et al. Perineural invasion is a major prognostic and predictive factor of response to adjuvant chemotherapy in stage I–II colon cancer. Ann Surg Oncol. (2017) 24(4):1077–84. doi: 10.1245/s10434-016-5561-0
33. Huh JW, Kim HR, Kim YJ. Prognostic value of perineural invasion in patients with stage II colorectal cancer. Ann Surg Oncol. (2010) 17(8):2066–72. doi: 10.1245/s10434-010-0982-7
34. Song JH, Yu M, Kang KM, Lee JH, Kim SH, Nam TK, et al. Significance of perineural and lymphovascular invasion in locally advanced rectal cancer treated by preoperative chemoradiotherapy and radical surgery: can perineural invasion be an indication of adjuvant chemotherapy. Radiother Oncol. (2019) 133:125–31. doi: 10.1016/j.radonc.2019.01.002
35. Tu J, Yao Z, Wu W, Ju J, Xu Y, Liu Y. Perineural invasion is a strong prognostic factor but not a predictive factor of response to adjuvant chemotherapy in node-negative colon cancer. Front Oncol. (2021) 11:663154. doi: 10.3389/fonc.2021.663154
36. Yang Y, Lu Y, Tan H, Bai M, Wang X, Ge S, et al. The optimal time of starting adjuvant chemotherapy after curative surgery in patients with colorectal cancer. BMC Cancer. (2023) 23(1):422. doi: 10.1186/s12885-023-10863-w
37. Sargent D, Sobrero A, Grothey A, O'Connell MJ, Buyse M, Andre T, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. (2009) 27(6):872–7. doi: 10.1200/JCO.2008.19.5362
38. Delattre JF, Selcen Oguz Erdogan A, Cohen R, Shi Q, Emile JF, Taieb J, et al. A comprehensive overview of tumour deposits in colorectal cancer: towards a next TNM classification. Cancer Treat Rev. (2022) 103:102325. doi: 10.1016/j.ctrv.2021.102325
39. Morris EJ, Maughan NJ, Forman D, Quirke P. Who to treat with adjuvant therapy in dukes B/stage II colorectal cancer? The need for high quality pathology. Gut. (2007) 56(10):1419–25. doi: 10.1136/gut.2006.116830
40. Kim YI, Kim CW, Kim JH, Kim J, Ro JS, Lee JL, et al. Clinical implication of perineural and lymphovascular invasion in rectal cancer patients who underwent surgery after preoperative chemoradiotherapy. Dis Colon Rectum. (2022) 65(11):1325–34. doi: 10.1097/DCR.0000000000002219
41. Yang Y, Huang X, Sun J, Gao P, Song Y, Chen X, et al. Prognostic value of perineural invasion in colorectal cancer: a meta-analysis. J Gastrointest Surg. (2015) 19(6):1113–22. doi: 10.1007/s11605-015-2761-z
42. Dotan E, Cohen SJ. Challenges in the management of stage II colon cancer. Semin Oncol. (2011) 38(4):511–20. doi: 10.1053/j.seminoncol.2011.05.005
Keywords: perineural invasion, adjuvant chemotherapy, node negative, colorectal cancer, retrospective cohort
Citation: Ying H, Shao J, Liao N, Xu X, Yu W and Hong W (2023) The effect of adjuvant chemotherapy on survival in node negative colorectal cancer with or without perineural invasion: a systematic review and meta-analysis. Front. Surg. 10:1308757. doi: 10.3389/fsurg.2023.1308757
Received: 7 October 2023; Accepted: 31 October 2023;
Published: 16 November 2023.
Edited by:
Francesk Mulita, General University Hospital of Patras, GreeceReviewed by:
Georgios-Ioannis Verras, Epsom and St Helier University Hospitals NHS Trust, United KingdomAngelis Peteinaris, University of Patras, Greece
Christos Pitros, General University Hospital of Patras, Greece
© 2023 Ying, Shao, Liao, Xu, Yu and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwen Hong YW55d2F5QHdtdS5lZHUuY24=
†These authors have contributed equally to this work
 Hongan Ying1,†
Hongan Ying1,† Weiwen Hong
Weiwen Hong