
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 08 January 2024
Sec. Thoracic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1305326
 Gongmin Rim1,‡
Gongmin Rim1,‡ Hyung Joo Park1*†‡
Hyung Joo Park1*†‡ Seungyoun Kang1,‡
Seungyoun Kang1,‡ Jin Yong Jeong2*†
Jin Yong Jeong2*† Jungmin Koo3,‡
Jungmin Koo3,‡ Il-Tae Jang1,‡
Il-Tae Jang1,‡ Saemi Bae1,‡
Saemi Bae1,‡
Introduction: Conventional postoperative pain management using an intravenous (IV) patient-controlled approach or thoracic epidural analgesia is suboptimal following minimally invasive repair of the pectus excavatum (MIRPE). Recently, cryoanalgesia has gained popularity owing to its superior pain control outcomes compared to those associated with conventional methods. However, because of its invasiveness, additional instrumentation requirement, and limited effect at early postoperative periods, we hypothesized that serratus anterior plane block (SAPB) could be an effective method for post-repair pain management and a possibly superior alternative.
Methods: We conducted a retrospective cohort study of pediatric patients who had undergone MIRPE between March 2022 and August 2023. We compared the efficacy of pain control in three groups among 74 patients: Group N (conventional pain management, n = 24), Group C (cryoanalgesia, n = 24), and Group S (SAPB, n = 26). Group N received IV patient-controlled analgesia (PCA) and a subcutaneous local anesthetic infusion. Group C received bilateral cryoanalgesia on the fourth and seventh intercostal nerves using a cryoprobe at −80°C for 2 min during the operation and IV-PCA postoperatively. Group S received continuous bilateral SAPB with 0.25% ropivacaine and IV-PCA. The pain levels were measured using the visual analog scale (VAS; resting and dynamic), and the total IV rescue analgesic consumption was determined.
Results: The three groups had similar baseline characteristics. Group S showed significantly less pain throughout the immediate postoperative course, resting VAS score at 3 h (Group N, 7.21 vs. Group C, 5.75 vs. Group S, 3.81; p < 0.001), and prominent less total IV rescue analgesic consumption (Group N, 116.16 mg vs. Group C, 52.75 mg vs. Group S, 16.61 mg; p < 0.001).
Conclusion: SAPB resulted in better postoperative pain control than that associated with cryoanalgesia and conventional pain management after pectus excavatum repair, As it was effective in the immediate postoperative period, achieving a VAS score of <4 points (moderate pain) at 3 h postoperatively, it may play an important role and replace invasive cryoanalgesia in the management of pain after pectus surgery.
Advancements in minimally invasive surgical repair of the pectus excavatum (PE) in the last two decades have enabled significantly improved patient outcomes by developing better surgical techniques and instrumentation (1, 2). However, postoperative pain caused by major chest wall remodeling remains a challenge and optimal solutions are lacking. When elevating the depressed chest wall by the pectus bar(s), constant and excessive pressure is placed on the rib cage, triggering intense postoperative pain (3, 4). Conventional postoperative pain management for the PE repair includes intravenous (IV) patient-controlled analgesia (PCA), thoracic epidural analgesia, and continuous local anesthetic infusion (ON-Q PainBuster; B Braun, Hessen, Germany), all of which have limitations in optimal pain management.
Although earlier data have suggested that thoracic epidural analgesia offers maximum benefit in this setting (5–7), new evidence for equal efficacy of IV-PCA has emerged (8, 9). Ultimately, both approaches are suboptimal solutions for post-repair pain, each presenting its own disadvantages. A major concern in thoracic epidural analgesia is the development of catheter-related complications, ranging from minor issues (dislodgement or kinking) to severe problems (neurologic damage). Using opioids for IV-PCA is a problem because it may induce opioid-related side effects, such as nausea, vomiting, and even respiratory depression (9). Cryoanalgesia has recently gained popularity owing to its superior pain control outcomes compared to those associated with conventional methods (10–12). However, cryoanalgesia requires a thoracoscopic approach, single-lung ventilation, intrathoracic procedures, and additional instrumentation, which are invasive and require more time and cost (13). As a better alternative, we adopted serratus anterior (SA) plane block (SAPB), a technique involving local anesthetic infusion through an extrathoracic catheter (14, 15). Therefore, we hypothesized that SAPB would be a superior method for pain management compared to that associated with conventional treatment and cryoanalgesia to help alleviate immediate postoperative pain and reduce opioid use after minimally invasive PE repair.
This retrospective analysis enrolled 74 patients out of 126 patients who underwent pectus excavatum (PE) repair surgery between March 2022 and August 2023, excluding those who met the following exclusion criteria. (1) Patients with a history of prior pectus excavatum repair resulting in recurrence. (2) Patients with a history of chronic pain or psychological disorders. (3) Patients with incomplete medical records, particularly with regard to pain scores. Notably, Group C and Goup N's subset of patients were published previously (13).
All pectus repairs were performed by the corresponding author (H.J.P). Demographic data, medications administered, surgical and medical histories, and perioperative data [i.e., operative time, pain level, opioid use, complications, and length of hospital stay (LOS)] were collected through patient interviews and electronic medical records. The total IV rescue analgesic consumption was determined using approximate morphine milligram equivalent (MME) (16, 17). The patients were divided into groups according to the pain control modality as follows: Group N, conventional pain management (n = 24); Group C, cryoanalgesia (n = 24); and Group S, SAPB (n = 26) for the evaluation and comparison of efficacy and adverse reactions.
The severity of postoperative pain was determined by the designated investigator using a visual analog scale (VAS) at various intervals (1, 3, 6, 12, 24, 48, and 72 h) postoperatively. Patients were instructed to score their pain levels on a scale from 0 (no pain) to 10 (worst pain) points (Figure 1) in both the resting (VAS-R) and dynamic (VAS-D) states, VAS-R in the supine position, and VAS-D in the upright position when coughing. VAS scores <4 indicated tolerable pain control state (Figure 1). The postoperative pain scale was assessed by an independent registered nurse.
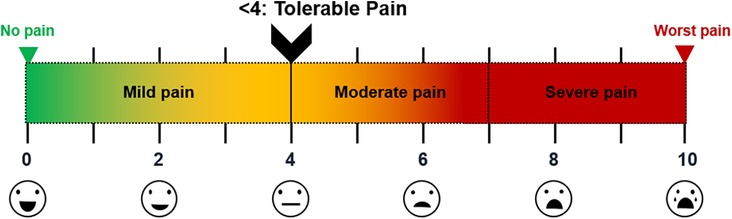
Figure 1. VAS scores of postoperative pain Illustration of pain levels; scores <4 points viewed as tolerable (mild pain). VAS, visual analog scale.
We measured the total IV rescue analgesic consumption at postoperative intervals (6, 12, 24, 48, and 72 h), LOS, complications (pneumothorax, wound complications, reoperation, pectus bar dislocation, bleeding necessitating transfusion, and neurological or cardiac issues), total operative time, and block time of cryoanalgesia or SAPB.
All pectus repair procedures were performed by a single surgeon at a single center and the surgical techniques were identical, except for the nerve block procedure. Patients were positioned supine with both arms freely suspended in overhead slings to avoid arm stretching. Bilateral 1.5-cm skin incisions were made at the midaxillary line, forming pockets at the subcutaneous layer.
The principal operative technique included total craned lifting of the sternum, pectoscope (PrimeMed, Seoul, Republic of Korea) visualization/dissection, multiple bar placement with bridge plate fixation (PrimeMed), and flarebuster/magic string technique using No. 5 Ethibond strings (Ethicon Inc., Somerville, NJ, USA) (18, 19).
Before repair, the depressed sternum was fully elevated to ensure that the pectus bars were accurately positioned without any effort or risk of injury to other organs (20). Sternal pre-lifting was made using the Easy crane system (PrimeMed) over the level of target chest wall height in all cases. By doing this, the chest wall depression was elevated with crane power, eliminating the need for pectus bar turnover power. Notably, the crane was set up with sternal wiring (1) or sternal screws, which is our novel system (21, 22).
Our surgical policy for correcting deformities focused on remodeling the entire chest wall; not just raising the depressed portion of the wall but also covering the entire anterior chest wall between both anterior axillary lines to achieve anatomic integrity of the transformed chest wall.
Cryoanalgesia (intercostal nerve cryoablation) was administered before PE repair. A double-lumen endotracheal tube was used for selective ventilation to avoid lung injury and to maintain a clear view of the target intercostal nerves during the ablation period. A Cardioblate CryoFlex Surgical Ablation Console (Medtronic Inc., Minneapolis, MN, USA) was used (Figure 2A). The cryoprobe was applied to the fourth to seventh intercostal nerves (T4–T7) bilaterally under video-thoracoscopic assistance and cooled to −80°C using Argon gas for 2 min (Figure 2B).
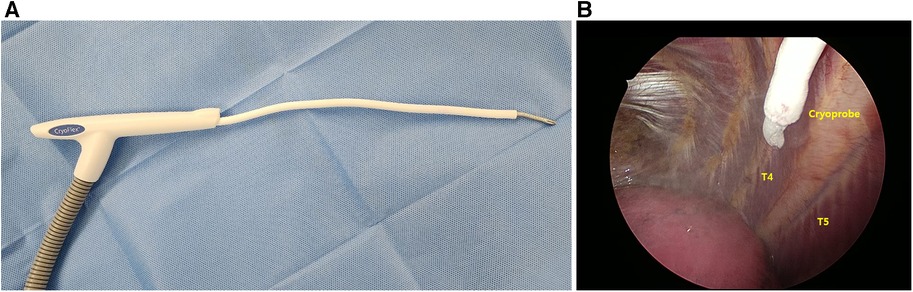
Figure 2. Intraoperative cryoanalgesia. (A) Cardioblate cryoFlex probe (Medtronic, Inc) with malleable tip; and (B) thoracoscope-assisted cryoablation of T5, right side (T4–T7 treated at −80°C for 2 min bilaterally).
SAPB was performed before PE repair. After the patient was in the supine position, a high-frequency linear ultrasound transducer was placed anterior to the midaxillary line at the level of the fourth or fifth ribs on the side of the block (Figure 3A) (23–25). After identification of the SA and latissimus dorsi (LD), the block needle (20 gauge -BD Perisafe™ Modified Tuohy Point Epidural Needles, BD Inc., Eschborn, Germany) was advanced between the interfacial plane of SA and LD using ultrasound in-plane technique. First, 5–10 ml of saline was injected to open the interfacial space between the SA and LD and, then, 30 mL of 0.25% ropivacaine (maximum dose of ropivacaine: 3 mg/kg) with 1:200000 epinephrine and adjuvants of 5 mg dexamethasone and 50 mcg Fentanyl to increase the quality and efficacy of local anesthecis under sono guide bilaterally was injected bilaterally (Figure 3B). It blocks the lateral cutaneous branch of the 2nd–9th intercostal nerves and the long thoracic nerve that covers the area of the anterior and lateral chest walls. After PE repair, for continuous SAPB, infusion catheters (Painfusion, Baxter Inc., Deerfield, IL, USA) were placed in the SA planes on both sides under ultrasound-guide. The elastomeric infusion pump was connected to the catheter, and 0.3% ropicavaine was delivered in a flow of 5 ml/h continuously (Figure 3C).
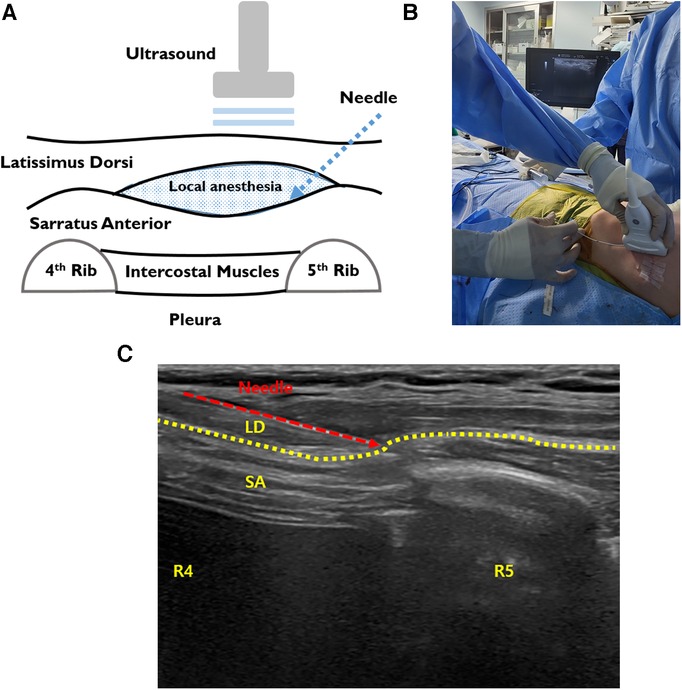
Figure 3. Intraoperative SAPB. (A) Illustration of SAPB, injecting local anesthetic agents between the SA and LD. (B) The procedure of ultrasound-guided SAPB before repair. (C) Bilateral ultrasound-guided painfusor catheter placement for continuous SAPB after PE repair. LD, latissimus dorsi; SA, serratus anterior; SAPB, serratus anterior plane block.
All study participants received a standardized pain regimen according to the institutional protocol. General anesthesia was induced using IV lidocaine (1–2 mg/kg; maximum, 100 mg) and propofol (2–4 mg/kg; maximum, 150 mg), with rocuronium (0.6–1 mg/kg; maximum, 60 mg) or vecuronium (0.1 mg/kg; maximum, 10 mg) to facilitate endotracheal intubation. Moreover, 1.2–1.5% sevoflurane was also used for maintenance in a mixture of 50% air and 50% O2 with a bispectral index less than 60.
The conventional postoperative pain management protocol includes: (1) IV-PCA, (2) subcutaneous local anesthetic infusion, (3) shots of nonsteroidal analgesics on demand, and (4) oral basal analgesics.
IV-PCA was initiated at the recovery room in all patients in the three groups using pumps delivering fentanyl (15 mcg/kg) in 100-ml normal saline (basal rate, 0.5 ml/h; bolus dose, 1 ml; lockout time, 10 min). The orally administered postoperative analgesics for both groups were ibuprofen (10–15 mg/kg, every 6 h) and acetaminophen (10–15 mg/kg, every 6 h). For breakthrough pain, ketorolac (0.5 mg/kg, every 6 h) and pethidine (0.5 mg/kg, every 6 h) were administered as IV rescue analgesics on an as-needed basis. IV rescue analgesia was administered based on the patient's request for relief from breakthrough pain, without any direct involvement from the investigator. The total rescue analgesic consumption was determined by conversion to oral MMEs. Patients with postoperative emesis or nausea received ondansetron (0.1–0.15 mg/kg; maximum, 4 mg).
For subcutaneous infusion of local anesthetic agents (ON-Q PainBuster), catheters (7.5 or 15 cm) were placed bilaterally at the posterior axillary lines after repair. A 240-ml reservoir released 0.3% ropivacaine at a fixed rate of 5 ml/h. The catheters were removed at 2–3 days postoperatively.
Patients in Group C received video thoracoscopy-assisted bilateral cryoanalgesia (T4–T7), besides the conventional pain management protocol. Patients in Group S received bilateral SAPB and a continuous local anesthetic catheter using a conventional pain management protocol.
Normally distributed continuous data, skewed data, and categorical data are presented as means [standard deviation (SD)] values, medians (interquartile ranges), and numbers, respectively. Descriptive statistics (number of individuals, mean, SD, median, minimum, maximum, and quartile range) and categorical data were conveyed as subject numbers and percentages, checked for normal distribution and relations, and assessed using analysis of variance or the Kruskal–Wallis test. All computations were performed using standard software (SPSS v. 25.0; IBM Corp., Armonk, NY, USA).
Among the 74 enrolled patients, there were no group differences in age, height, weight, body mass index, sex, American Society of Anesthesiologists class, or PE type. The baseline characteristics of the study groups are presented in Table 1.
Perioperative clinical and radiologic characteristics are summarized in Table 2.
All groups underwent surgical PE repair under crane application using a pectoscope, multiple pectus bars (parallel, cross, or XI shaped), and the flarebuster/magic string technique. The pectus bar size and number of pectus bars did not differ significantly between the groups. However, there were no single-bar repairs in any group.
Pain score assessments, whether resting (VAS-R) or dynamic (VAS-D), were performed at the anterior (VAS-R-A, VAS-D-A) and lateral (VAS-R-L, VAS-D-L) chest walls (Tables 3, 4). The mean VAS score was significantly lower in Group S for the entire 72-h postoperative period, with resting scores (VAS-R-A, VAS-R-L) being <4 points for the 3 h (3.81 points) and 24-h (3.57 points) periods, respectively. However, the VAS-R-A and VAS-R-L scores for Group N were >4 points during the full 72-h postoperative period, and the resting scores (VAS-R-A, VAS-R-L) were <4 points at 48 h (3.17 points) and 24 h (3.61 points) (Figure 4A). In Group S, the dynamic scores were <4 points at 24 h, respectively, whereas the VAS-D were >4 points during the entire postoperative period in Group N. The dynamic scores were <4 points at postoperative 72 h in Group C (Figure 4B).
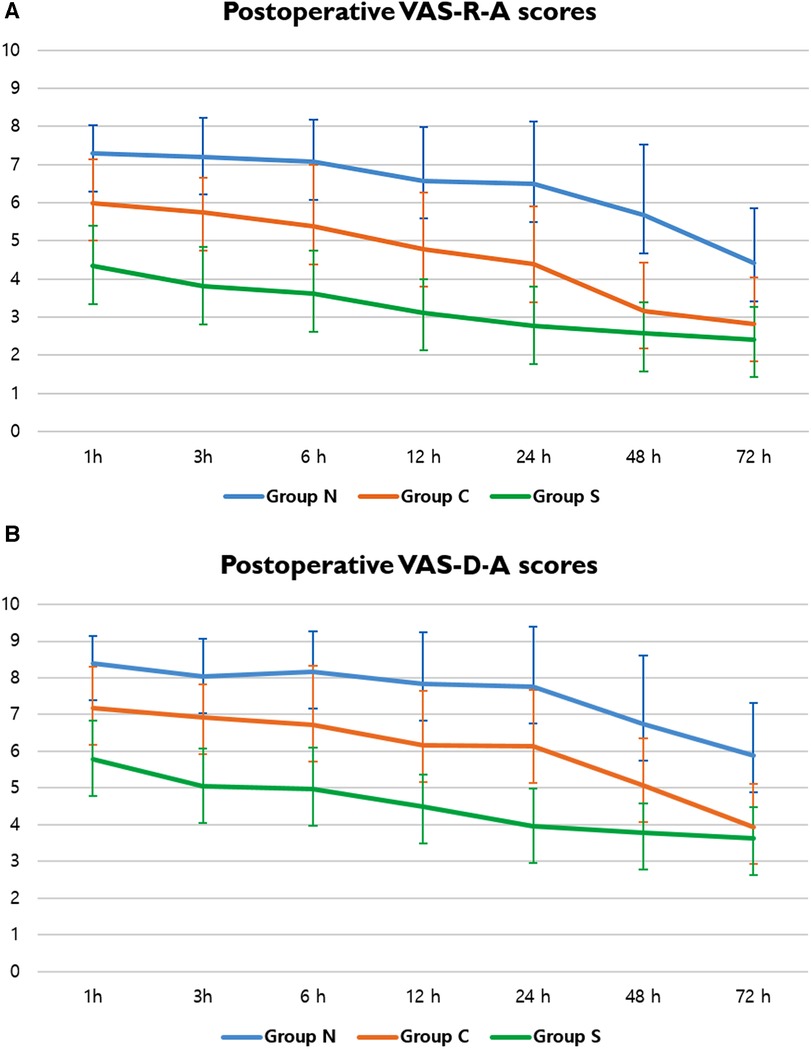
Figure 4. VAS scores of postoperative pain. (A) Postoperative VAS-R-A scores in Groups N, C, and S; and (B) postoperative VAS-D-A scores in Groups N, C, and S. A, anterior chest wall; VAS, visual analog scale; VAS-D, dynamic VAS score; VAS-R, resting VAS score.
The total IV analgesic consumption in each group administered for breakthrough pain after surgical repair was converted to oral MME.; fentanyl, pethidine, and ketorolac served as IV rescue analgesics. Overall, there were significant differences in total analgesic consumption between the three groups; Group S showed significantly lower MME at 72 h postoperatively (Group N, 116.16; Group C, 52.75; Group S, 16.61; p < 0.001) (Table 5, Figure 5).
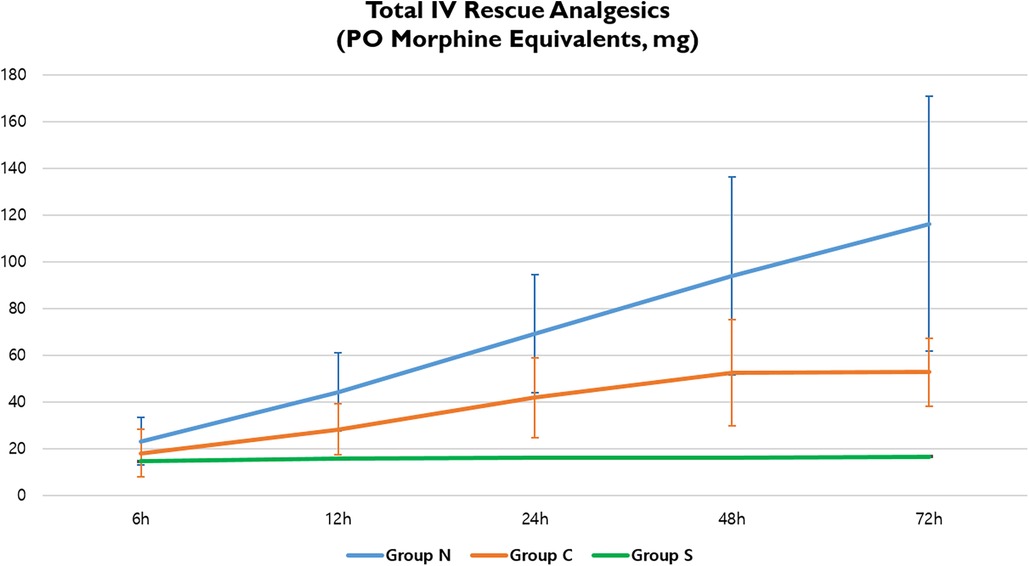
Figure 5. Total IV rescue analgesic consumption in three groups. IV, intravenous; PO, postoperative.
Owing to the nerve block time requirements, the mean operative time in Group C (159.42 min) was significantly longer than that in Group N (125.96 min). However, Group S had the shortest operative time, even when a nerve block was performed (110.96 min; p = 0.001). The mean cryoanalgesia time for Group C was 30.33 min and the SAPB time for Group S was 17.77 min (p < 0.001).
The LOS was not significantly different among the three groups. In Group S, patients stayed for 4.61 days on average, compared with 5.22 and 4.83 days in Groups N and C, respectively (p = 0.057). The postoperative complications were not significantly different among the three groups. Four patients in Groups S, C, and N developed postoperative pneumothorax (p = 0.85), which required no intervention and resolved spontaneously. One patient in Group N had a postoperative wound seroma that was treated with antibiotics (p = 0.36). One patient in Group S had postoperative pneumonia, which was treated with antibiotics (p = 0.40), and one in Group S had postoperative thoracic outlet syndrome that required no intervention and resolved spontaneously (p = 0.45; Table 6). There were no catheter related complications, such as infection, dislocation and obstruction.
Postoperative pain after PE repair remains challenging. Despite recent advances in surgical techniques and postoperative management, pain after PE repair remains a challenge. Conventionally, IV-PCA has been well established as the first line of postoperative pain control, and on-demand bolus shots of opioids or nonsteroidal analgesics have been used (26). Additionally, local intercostal nerve blocks and paravertebral blocks were also used (26, 27).
Thoracic epidural analgesia is considered one of the most effective methods for pain management in adults undergoing thoracic procedures; however, complications may range from minor catheter issues (kinking or dislodgement) to serious neurological consequences (8, 28). When considering the efficacy or problems related to the modalities of pain management, current pain control methods remain suboptimal.
Recently, cryoanalgesia has been investigated and proven to be relatively superior to conventional pain control methods (10, 12, 29). The cryoprobe is rapidly cooled to the target temperature and applied directly to the targeted intercostal nerve, inducing axonotmesis and reversible transient axonal disruption, which allows for several weeks to months of analgesia (28–35). However, this requires invasive and time-consuming procedures, including single-lung ventilation with double-lumen endotracheal intubation, videoscopy-assisted intrathoracic procedures, and expensive cryo equipment (34, 35). Conversely, SAPB is a new regional block technique for obtaining thoracic local anesthetic analgesia between T2 and T9 (23, 24). SAPB was originally proposed for breast surgery; however, its application has been extended and is highlighted in thoracic surgery, especially in video-assisted thoracic surgery (23, 29). The nerve block can cover the anterior and lateral chest walls, which is the operative field for pectus repair. SAPB involves blocking the intercostal nerve branch with local anesthetic infusion through an extrathoracic catheter. Catheter placement can be conveniently performed using a sonography-guided subcutaneous approach at the operating table after repair.
Bilateral single-injection SAPB in patients undergoing minimally invasive repair of the pectus excavatum decreases pain and opioid consumption compared to those associated with IV-PCA alone during the early postoperative period (14, 31).
However, in our study, we tested the efficacy of continuous SAPB for postoperative pain control and compared the results with those of cryointercostal ablation. For meticulous evaluation of SAPB, we measured the VAS scores in the resting and dynamic states and at dual locations (anterior and lateral chest wall) and identified significant comparative reductions in postoperative resting and dynamic, dual locations (anterior and lateral chest wall).
Assuming a VAS score <4 (mild pain) as the target level for tolerable pain, SAPB improved pain control after PE repair, lowering VAS scores (VAS-R and VAS-D) to this level at 3–24 h postoperatively, whereas cryoanalgesia only reached the level at 48–72 h postoperatively.
The average block time for cryoanalgesia (mean, 30.33 min) exceeded that for SAPB (mean, 17.77 min). Theoretically, VATS intercostal nerve cryoablation requires a minimum of 20 min, as 2 min of each intercostal nerve at the bilateral T4–T7 level is required for the procedure. In contrast, SAPB catheterization is a subcutaneous approach with bedside sonographic guidance.
It is clear that cryoanalgesia added extra time to the procedure due to the need for nerve freezing at bilateral multiple levels. In this study, the reason for the shorter operation time in Group N compared to Group S, which requires a block time, is not apparent. However, we believe that the duration of the procedure can be influenced by various factors. In our clinical practice, it often involves additional time for reshaping the pectus bars or, in some instances, modifying the bar placement strategy when it is considered unsuitable for optimal repair.
Other studies have reported significant reductions in LOS and opioid consumption with cryoanalgesia compared to those associated with conventional pain management alone (including thoracic epidural analgesia) (28, 32, 33). However, in our previous study, we did not find total IV rescue analgesics, and LOS was significantly reduced after cryoanalgesia (13).
We are very hopeful of using SAPB for pain management because it is economic, less invasive, and technically straightforward. Moreover, as SAPB takes effect immediately on its infusion, while cryoanalgesia has some latency of the effect, we can handle severe pain upon awakening of the patient from general anesthesia. The pain is most intense during right after the surgery but gradually decreases and becomes bearable as the patient recovers. Rarely we observed persistent pain beyond a few weeks after the operation. Hence, our focus was on relieving the acute pain during the immediate postoperative period, from 6 to 48 h. In this study, we aimed to confirm the effectiveness of pain control during this critical time. The local anesthetic infusion takes effect immediately upon administration, precisely when it is needed most. Although its effect is temporary and only lasts during the infusion, we did not observe any continued, significant pain after discharge or in the subsequent recovery period. Based on our findings, SAPB could be a better option than using cryoanalgesia or IV-PCA alone for controlling acute pain in the early postoperative period, and it has the potential to replace the costly and invasive cryoanalgesia technique.
To our knowledge, this is the first study to investigate the efficacy of continuous SAPB in postoperative pain management by comparing the cryoanalgesic and conventional groups.
However, this study had several limitations. First, the sample size examined was small and the duration was relatively short. Second, the study was retrospective in nature. Third, additional information, such as a longer follow-up outcome, is necessary. Our study was focused on immediate postoperative periods (up to 72 h), it has limitation on evaluation of the patient's longer-term condition after discharge. Fourth, since the preoperative Haller index varied between the three groups, this may have influenced postoperative pain outcomes. Fifth, the different bar patterns of each group may have affected postoperative outcomes. Due to our repair policy shifted towards remodeling the entire anterior chest wall in recent cases, predominantly utilizing the XI fashion approach, which includes cross bars and an upper horizontal bar with three bars in total. While this approach may appear more complex due to the use of three bars (two for cross and three for XI), we hypothesize that it may result in less postoperative pain when combined with SAPB. Sixth, We did not take additional steps, such as injecting a local anesthetic into the intercostal nerves along with cryoablation, which could have served to mitigate the delayed effect of cryo alone. Seventh, We did not do preoperative percutenaous application of ultrasound guided cryoablation due to resource limitations, despite its demonstrated benefits in previous research. Nevertheless, it's worth recognizing that distinct patterns of pectus bars could have influenced postoperative outcomes in each group.
To confirm the validity of these preliminary findings and to establish SAPB as the standard method of pain management after pectus excavatum repair, additional prospective research is required.
After PE repair, continuous SAPB improved postoperative pain control in both resting and dynamic states. Moreover, the pain intensity reduced to a mild level (VAS score, 0–4 points) immediately postoperatively, and the total IV rescue analgesic consumption diminished. Our study suggests that SAPB could be more effective than the conventional procedure and even cryoanalgesia for immediate postoperative pain relief. However, our results are confined to the initial 72 h post-surgery, specifically within the in-hospital postoperative period. Further study is required to prove the long term efficacy of pain control of SAPB after PE repair.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by The Gangnam Nanoori Institutional Review Board (IRB) (IRB number: NR-IRB 2023-006). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
GR: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. HP: Conceptualization, Supervision, Writing – review & editing. SK: Conceptualization, Methodology, Writing – review & editing. JJ: Supervision, Writing – review & editing. JK: Data curation. IJ: Project administration, Supervision, Writing – review & editing. SB: Investigation, Methodology, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Park HJ, Jeong JY, Jo WM, Shin JS, Lee IS, Kim KT, et al. Minimally invasive repair of pectus excavatum: a novel morphology-tailored, patient-specific approach. J Thorac Cardiovasc Surg. (2010) 139:379–86. doi: 10.1016/j.jtcvs.2009.09.003
2. Park HJ, Kim KS, Lee S, Jeon HW. A next-generation pectus excavatum repair technique: new devices make a difference. Ann Thorac Surg. (2015) 99:455–61. doi: 10.1016/j.athoracsur.2014.08.026
3. Kelly RE, Goretsky MJ, Obermeyer R, Kuhn MA, Redlinger R, Haney TS, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg. (2010) 252:1072–81. doi: 10.1097/SLA.0b013e3181effdce
4. Nuss D, Obermeyer RJ, Kelly RE. Nuss bar procedure: past, present and future. Ann Cardiothorac Surg. (2016) 5:422–33. doi: 10.21037/acs.2016.08.05
5. Muhly WT, Maxwell LG, Cravero JP. Pain management following the Nuss procedure: a survey of practice and review. Acta Anaesthesiol Scand. (2014) 58:1134–9. doi: 10.1111/aas.12376
6. Weber T, Mätzl J, Rokitansky A, Klimscha W, Neumann K, Deusch E. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patient-controlled analgesia after minimally invasive pectus excavatum repair. J Thorac Cardiovasc Surg. (2007) 134:865–70. doi: 10.1016/j.jtcvs.2007.05.050
7. Soliman IE, Apuya JS, Fertal KM, Simpson PM, Tobias JD. Intravenous versus epidural analgesia after surgical repair of pectus excavatum. Am J Ther. (2009) 16:398–403. doi: 10.1097/MJT.0b013e318187de3e
8. Sujka JA, Dekonenko C, Millspaugh DL, Doyle NM, Walker BJ, Leys CM, et al. Epidural versus PCA pain management after pectus excavatum repair: a multi-institutional prospective randomized trial. Eur J Pediatr Surg. (2020) 30:465–71. doi: 10.1055/s-0039-1697911
9. Heo MH, Kim JY, Kim JH, Kim KW, Lee SI, Kim KT, et al. Epidural analgesia versus intravenous analgesia after minimally invasive repair of pectus excavatum in pediatric patients: a systematic review and meta-analysis. Korean J Anesthesiol. (2021) 74:449–58. doi: 10.4097/kja.21133
10. Morikawa N, Laferriere N, Koo S, Johnson S, Woo R, Puapong D. Cryoanalgesia in patients undergoing Nuss repair of pectus excavatum: technique modification and early results. J Laparoendosc Adv Surg Tech A. (2018) 28:1148–51. doi: 10.1089/lap.2017.0665
11. Cadaval Gallardo C, Martínez J, Bellía-Munzon G, Nazar M, Sanjurjo D, Toselli L, et al. Thoracoscopic cryoanalgesia: a new strategy for postoperative pain control in minimally invasive pectus excavatum repair. (Crioanalgesia toracoscópica: nueva estrategia para el control del dolor postoperatorio en cirugía del pectus excavatum). Cir Pediatr. (2020) 33:11–5.32166917
12. Arshad SA, Hatton GE, Ferguson DM, Li LT, Austin MT, Tsao K. Cryoanalgesia enhances recovery from minimally invasive repair of pectus excavatum resulting in reduced length of stay: a case-matched analysis of NSQIP-pediatric patients. J Pediatr Surg. (2021) 56:1099–102. doi: 10.1016/j.jpedsurg.2021.03.017
13. Rim GM, Kim HK, Koo JM, Park HJ. A randomized controlled trial of cryoanalgesia for pain management following pectus excavatum repair: a single-center, single-blind, parallel design study. Eur J Pediatr Surg. (2023). PMID: 37364610. doi: 10.1055/a-2117-4628 [Epub ahead of print].37364610
14. Liu X, Song T, Xu HY, Chen X, Yin P, Zhang J. The serratus anterior plane block for analgesia after thoracic surgery: a meta-analysis of randomized controlled trails. Medicine (Baltimore). (2020) 99:e20286. doi: 10.1097/md.0000000000020286
15. Hu NQ, He QQ, Qian L, Zhu JH. Efficacy of ultrasound-guided serratus anterior plane block for postoperative analgesia in patients undergoing breast surgery: a systematic review and meta-analysis of randomised controlled trials. Pain Res Manag. (2021) 2021:7849623. doi: 10.1155/2021/7849623
16. Dowell D RK, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain. (2022). p. 1–95.
17. Chang SC, Ma CC, Lee CT, Hsieh SW. Pharmacoepidemiology of chronic noncancer pain patients requiring chronic opioid therapy: a nationwide population-based study. Acta Anaesthesiol Taiwan. (2015) 53:89–94. doi: 10.1016/j.aat.2015.04.002
18. Park HJ, Kim KS. The sandwich technique for repair of pectus carinatum and excavatum/carinatum complex. Ann Cardiothorac Surg. (2016) 5:434–9. doi: 10.21037/acs.2016.08.04
19. Park HJ. A technique for complex pectus excavatum repair: the cross-bar technique for grand canyon type deformity (park classification). Ann Cardiothorac Surg. (2016) 5:526–7. doi: 10.21037/acs.2016.08.01
20. Park HJ. Minimally invasive surgery for pectus excavatum: park technique. J Clin Anal Med. (2011) 2:84–90. doi: 10.4328/JCAM.518
21. Park HJ, Rim G. Development of a screw-crane system for pre-lifting the sternal depression in pectus excavatum repair: a test of mechanical properties for the feasibility of a new concept. J Chest Surg. (2021) 54:186–90. doi: 10.5090/jcs.21.008
22. Baytar MS, Yılmaz C, Karasu D, Baytar Ç. Comparison of ultrasonography guided serratus anterior plane block and thoracic paravertebral block in video-assisted thoracoscopic surgery: a prospective randomized double-blind study. Korean J Pain. (2021) 34:234–40. doi: 10.3344/kjp.2021.34.2.234
23. Chen JQ, Yang XL, Gu H, Chai XQ, Wang D. The role of serratus anterior plane block during in video-assisted thoracoscopic surgery. Pain Ther. (2021) 10:1051–66. doi: 10.1007/s40122-021-00322-4
24. Fenikowski D, Tomaszek L. Intravenous morphine infusion versus thoracic epidural infusion of ropivacaine with fentanyl after the ravitch procedure-a single-center cohort study. Int J Environ Res Public Health. (2022) 19:11291. doi: 10.3390/ijerph191811291
25. Vogt A, Stieger DS, Theurillat C, Curatolo M. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth. (2005) 95:816–21. doi: 10.1093/bja/aei250
26. Jaroszewski DE, Temkit M, Ewais MM, Luckritz TC, Stearns JD, Craner RC, et al. Randomized trial of epidural vs. subcutaneous catheters for managing pain after modified Nuss in adults. J Thorac Dis. (2016) 8:2102–10. doi: 10.21037/jtd.2016.06.62
27. Torre M, Mameli L, Bonfiglio R, Guerriero V, Derosas L, Palomba L, et al. A new device for thoracoscopic cryoanalgesia in pectus excavatum repair: preliminary single center experience. Front Pediatr. (2020) 8:614097. doi: 10.3389/fped.2020.614097
28. Evans PJ. Cryoanalgesia. The application of low temperatures to nerves to produce anaesthesia or analgesia. Anaesthesia. (1981) 36(11):1003–13. doi: 10.1111/j.1365-2044.1981.tb08673.x
29. Bassett FH 3rd, Kirkpatrick JS, Engelhardt DL, Malone TR. Cryotherapy-induced nerve injury. Am J Sports Med. (1992) 20:516–8. doi: 10.1177/036354659202000505
30. Whittaker DK. Degeneration and regeneration of nerves following cryosurgery. Br J Exp Pathol. (1974) 55:595–600.4447794
31. Tore Altun G, Arslantas MK, Corman Dincer P, Aykac ZZ. Ultrasound-guided serratus anterior plane block for pain management following minimally invasive repair of pectus excavatum. J Cardiothorac Vasc Anesth. (2019) 33(9):2487–91. doi: 10.1053/j.jvca.2019.03.063
32. Keller BA, Kabagambe SK, Becker JC, Chen YJ, Goodman LF, Clark-Wronski JM, et al. Intercostal nerve cryoablation versus thoracic epidural catheters for postoperative analgesia following pectus excavatum repair: preliminary outcomes in twenty-six cryoablation patients. J Pediatr Surg. (2016) 51:2033–8. doi: 10.1016/j.jpedsurg.2016.09.034
33. Graves C, Idowu O, Lee S, Padilla B, Kim S. Intraoperative cryoanalgesia for managing pain after the Nuss procedure. J Pediatr Surg. (2017) 52:920–4. doi: 10.1016/j.jpedsurg.2017.03.006
34. Harbaugh CM, Johnson KN, Kein CE, Jarboe MD, Hirschl RB, Geiger JD, et al. Comparing outcomes with thoracic epidural and intercostal nerve cryoablation after Nuss procedure. J Surg Res. (2018) 231:217–23. doi: 10.1016/j.jss.2018.05.048
Keywords: minimally invasive repair of pectus excavatum, pain control, pectus excavatum, serratus anterior plane block, cryoanalgesia
Citation: Rim G, Park HJ, Kang S, Jeong JY, Koo J, Jang I-T and Bae S (2024) Serratus anterior plane block for acute pain management after pectus excavatum repair. Front. Surg. 10:1305326. doi: 10.3389/fsurg.2023.1305326
Received: 1 October 2023; Accepted: 23 November 2023;
Published: 8 January 2024.
Edited by:
Marcelo Jimenez, University of Salamanca, SpainReviewed by:
Dawn Jaroszewski, Mayo Clinic Arizona, United States© 2024 Rim, Park, Kang, Jeong, Koo, Jang and Bae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyung Joo Park aHlqcGFya2tvcmVhQGdtYWlsLmNvbQ== Jin Yong Jeong amVvbmc3NEBjYXRob2xpYy5hYy5rcg==
†These authors have contributed equally to this work
‡ORCID Gongmin Rim orcid.org/0000-0002-0982-7851 Hyung Joo Park orcid.org/0000-0003-0886-0817 Seungyoun Kang orcid.org/0009-0003-9004-484X Jungmin Koo orcid.org/0000-0002-3465-8113 Il-Tae Jang orcid.org/0000-0002-2121-0221 Saemi Bae orcid.org/0009-0004-6040-3212
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.