
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 19 December 2023
Sec. Surgical Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1303128
This article is part of the Research Topic Translational Research in Surgical Applications and Spinal Tumors View all 9 articles
 Martin Vychopen1,2*
Martin Vychopen1,2* Felix Arlt1,2
Felix Arlt1,2 Florian Wilhelmy1,2
Florian Wilhelmy1,2 Clemens Seidel2,3
Clemens Seidel2,3 Alonso Barrantes-Freer2,4
Alonso Barrantes-Freer2,4 Erdem Güresir1,2
Erdem Güresir1,2 Johannes Wach1,2
Johannes Wach1,2
Objective: Spinal meningiomas (SM) account for 25%–46% of all primary spinal tumors and show an excellent long-term disease control in case of complete resection. Therefore, the postoperative functional outcome is of high importance. To date, reports on dorsally located SM are scarce. Moreover, the impact of radiomics shape features on the functional outcome after surgery for primary dorsal SMs has not been analyzed yet.
Methods: We retrospectively performed an analysis of shape-based radiomic features in 3D slicer software and quantified the tumor volume, surface area, sphericity, surface area to volume ratio and tumor canal ratio. Subsequently, we evaluated the correlation between the radinomic parameters and the postoperative outcome according to Modified Japanese Orthopedic Association (mJOA) score.
Results: Between 2010 and 2022, we identified 24 Females and 2 Males operated on dorsal SMs in our institutional database. The most common SM localization was thoracic spine (n = 20), followed by cervical (n = 4), and lumbar (n = 2). The univariate analysis and the receiver operating characteristic (ROC) analysis showed a strong diagnostic performance of sphericity in the prediction of postoperative functional outcome based on mJOA score (AUC of 0.79, sphericity cut-of value 0.738; p = 0.01). Subsequently, the patients were divided into two groups (mJOA improved vs. mJOA stable/worsened). Patients with improved mJOA score showed significantly higher sphericity (0.79 ± 0.1 vs. 0.70 ± 1.0; p = 0.03). Finally, we divided the cohort based on sphericity (<0.738 and ≥0.738). The group with higher sphericity exhibited a significantly higher positive mJOA difference 3 months postoperatively (16.6 ± 1.4 vs. 14.8 ± 3.7; p = 0.03).
Conclusion: In our study investigating primary sporadic dorsal SMs, we demonstrated that a higher degree of sphericity may be a positive predictor of postoperative improvement, as indicated by the mJOA score.
Spinal meningioma (SM) accounts for 25%–46% of all primary spinal tumors, while comprising 12% of all meningiomas (1–5). Notably, 90% of SMs are intradural, 5% are extradural, and the remaining 5% have both intra- and extradural components. SMs show a female predominance, and most cases are sporadic, despite known associations with neurofibromatosis type 2 or prior ionizing radiation exposure (6–8). SMs are further classified as either anterior/anterolateral to or posterior/posterolateral to the dentate ligament (9). In view of the reported high amount (95.7%) of World Health Organization (WHO grade) 1 meningiomas achieving excellent long-term tumor control after complete resection, preserving neurological function is of paramount importance (10).
SMs located anteriorly to the denticulated ligament are considered more challenging to resect and pose a risk factor for perioperative neurological deterioration (11, 12). In contrast, only a limited number of studies have focused exclusively on dorsally located SMs, despite a large-scale bicentric study reported comparable postoperative functional outcomes between ventrally and dorsally located SMS (8, 13). Recently, radiomics of the preoperative meningioma shape were observed to be significantly associated with the WHO grade of cranial meningiomas and with the postoperative cranial nerve functioning after surgery for medial sphenoid wing meningiomas (14, 15).
However, the impact of radiomics based shape features on postoperative neurological functioning in surgery for solely dorsally located SM has not been analyzed so far. The present retrospective investigation examines the role of shape features with regard to postoperative morbidity and neuropathological characteristics.
This study retrospectively reviews consecutive patients with primary sporadic SM who had undergone surgical resection between 2010 and 2022. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Medical Faculty of the University of Leipzig (No. 159/23-ek). Informed consent for scientific use of anonymized data was signed by all patients. Patients with dorsal SMs located below the foramen magnum were included. Patients with anterior foramen magnum meningiomas, craniocervical meningiomas with intracranial extension, ventrally located SMs, recurrent SM after radiotherapy, and patients with a neurofibromatosis type II were excluded due to the differences regarding clinical signs, histopathology, and therapy (3, 7, 12, 16, 17). Primary sporadic SMs localized posterior to the dentate ligament were considered as dorsal SM and included in this analysis (9).
To assess the patient characteristics, we analyzed the demographic data, laboratory tests on admission, comorbidities, preoperative neurological status according to modified Japanese Orthopedic Association (mJOA) score and retrospectively reviewed the patient charts to determine the modified McCormick Scale (mMCs). We also examined the operation protocols to evaluate the duration, blood loss, the use of the drains and the extent of the tumor resection. To define the extent of resection, we used the Simpson classification system in line with the European Association of Neuro-Oncology (EANO) (18). The histological reports were analyzed for WHO Grade, Molecular Immunology Borstel (MIB)-1 index and the number of mitotic figures. Finally, we reviewed the outpatient reports to assess the postoperative outcome.
Image analysis of preoperative MRI scans using T1-weighted contrast enhanced and T2-weighted images was performed. We quantified the shape features of the lesion using the segment statistics including tumor volume, tumor surface area, sphericity, tumor surface area to volume ratio, and tumor canal ratio (formula: (tumor volume/spinal canal volume) × 100) (19). The Hakon-Wadell method was used to calculate sphericity (20). The following formula was used to determine the sphericity: Sphericity = π1/3(6 × tumor volume)2/3/surface area (20). The value of sphericity ranges from 0 to 1 with 1 indicating a perfectly spherical shape. The reviewer of the images was blinded to clinical data of the patients.
The medical image analysis and visualization was performed using 3D slicer software (version 5.2.1, Surgical Planning Laboratory, Harvard University, USA) (21). The segmentation of the lesion was performed in a semi-automatic fashion using the “Fast Marching” method according to Pichon et al. (22). For detailed information, see Figure 1.

Figure 1. (A,D) illustrates a regularly shaped dorsal primary sporadic SM with the shape-based radiomic features tumor volume, surface area, sphericity, and surface-area-to-volume ratio, whereas (B,E) show an irregularly shaped dorsally located primary sporadic SM. Illustratory cases of tumor segmentation with 3D-Slicer (version 5.2.1, Surgical Planning Laboratory, Harvard University, US). (C,F) show a regularly shaped dorsal primary sporadic SM with the 3D volume and the corresponding axial T1-gadolinium enhanced sequence.
Indications for surgery included pain, neurological dysfunctions, and spinal cord compression. Surgical management was dependent on the site of dural attachment of the tumor, tumor size, and the affected segments. The usual workflow involved laminectomy, dural opening and en-bloc or piecemeal resection of the tumor. After removing the tumor, the dural attachement was coagulated using bipolar coagulation forceps and dura suture was supported with TachoSil(®) (Takeda Austria GmbH, Linz, Austria).
Paraffin sections were stained with hematoxylin and eosin (H&E). Immunohistochemical investigation was performed on the specimen using the Molecular Immunology Borstel-I (MIB-I, DAKO, Glostrup, Denmark) antibody to detect the Ki67 antigen and calculate the MIB-I index by the average method as previously described (30).
Data were organized and analyzed using SPSS for Windows (version 29.0; IBM Corp., Armonk, NY, USA). Kolmogorov-Smirnov (KS) test were performed to investigate distributions. Normally distributed data are reported as the mean with the standard deviation (SD). Receiver-operating characteristic (ROC) curves were constructed to analyze the diagnostic value of the quantitative shape-based feature sphericity in association with the postoperative course of the mJOA following surgery for dorsal SM (23). Cut-off values for sphericity were determined using the ROC analysis. Preoperative demographics, tumor volume, tumor surface area, sphericity, surface-to-volume ratio, histopathological, and immunohistochemical features were compared between patients with worsened and stable or improved mJOA at 3-months after surgery using Fisher's exact test (two-sided) for categorical values and independent t-test for continuous values. Violin-Plots displaying sphericity among those SM patients with worsened and stable or improved mJOA at 3-months after surgery were created using Prism 8 for macOS (Version 8.4.3, GraphPad Software, San Diego, CA, USA).
Patient characteristic showed an unequal distribution of sex with 24 Females and 2 Males. The median age was 67.5 (IQR 57.3–80.0). The mJOA score preoperatively amounted to 15.0 (IQR 13.0–17.0) and the median KPS was 80 (IQR 70–90). The median preoperative mMCs was 2.0 (IQR: 1.0–4.0).
Most of the meningiomas were localized in thoracic part of the spine (n = 20), followed by cervical (n = 4) and lumbar (n = 2). The median tumor volume was 2.9 cm3 (IQR 1.4–3.9), with median surface area of 11.7 cm2 (8.5–16.1). The surface to volume ratio was 0.47 (IQR 0.40–0.56), and sphericity was 0.71 (IQR 0.66–0.80). Two SMs showed signs of calcification, and only one SM was cystic.
Postoperative histological examination revealed 21 WHO Grade 1 SMs and 5 WHO Grade 2 SMs. The median MIB-1 index was 4.25 (IQR 3.0–5.25) and the median number of mitotic figures was 3 (IQR 2–8.75).
According to operation protocols, Simpson Grade I resection was achieved in 1 case, Simpson Grade II resection in 23 cases and Simpson Grade IV resection in 2 cases. For detailed information, please refer to Table 1.
After performing the radiomic analysis with 3-D Slicer (Version 4.10), we conducted a ROC analysis of sphericity and mJOA difference (preoperative compared to 3 months postoperative). The analysis revealed an area under curve (AUC) of 0.79 with 75% sensitivity and 92.3% specificity and sphericity cut-off value of 0.738 (p = 0.01). For details on ROC-Curve analysis, refer to Figure 2.
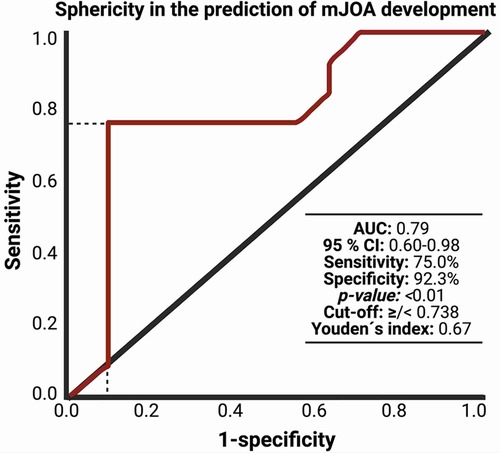
Figure 2. Receiver-operating characteristic curve (ROC) illustrating the ability of sphericity in the association with mJOA development within 3 months after surgery for dorsal primary sporadic spinal meningioma.
Subsequently, we divided the cohort into two groups according to postoperative mJOA score. We compared patients who showed mJOA improvement (delta: ≥1) to patients with stable or worse postoperative mJOA score (delta: <1). There was a significantly higher sphericity in patients in the improvement group (0.79 ± 0.1 vs. 0.70 ± 1.0; p = 0.03). We found no difference in age, sex, preoperative KPS and mJOA, tumor size, tumor area, surface to volume ratio, Simpson Grade, WHO grade, MIB-1 index and Mitotic figure count. For detailed information, refer to Table 2 and Figure 3.
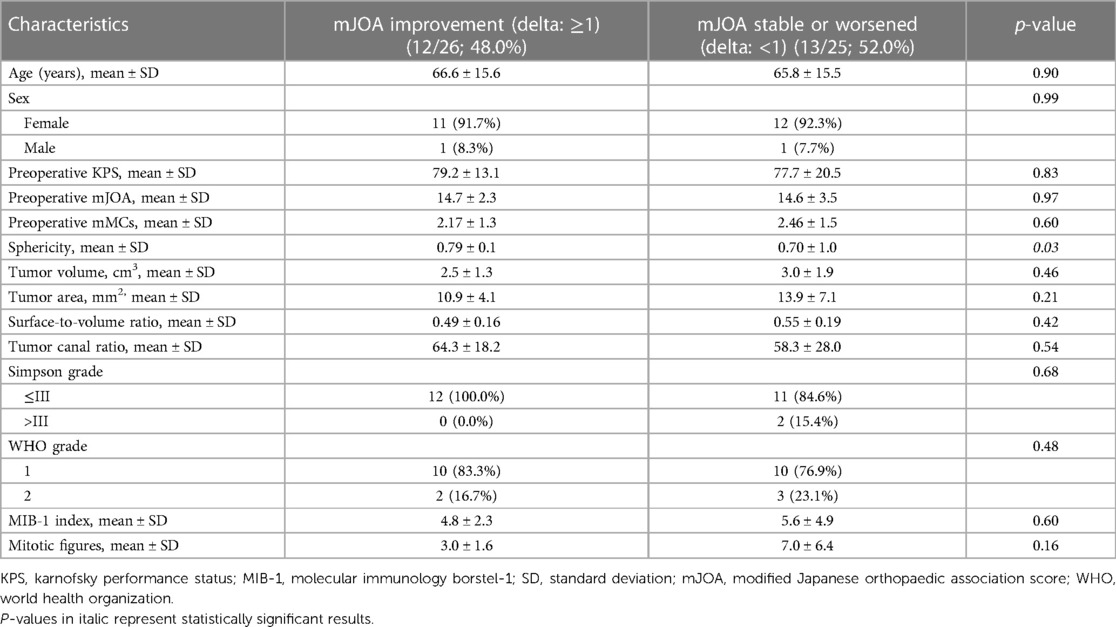
Table 2. Comparison of patient characteristics in groups with improvement of mJOA or worsening/stable mJOA after 3 months compared with baseline mJOA after surgery for dorsal primary sporadic spinal meningioma [using Fisher's exact test (two-sided) and independent t-test].
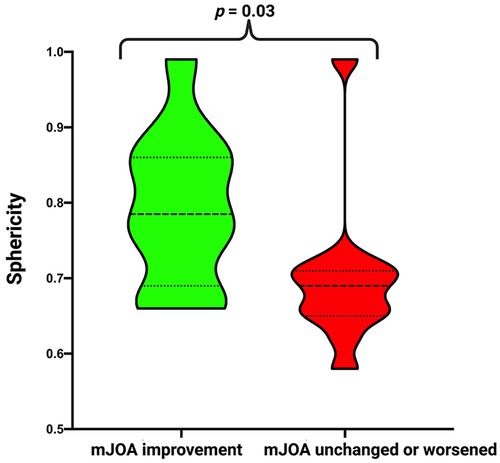
Figure 3. Truncated violin-plots displaying sphericity in patients with mJOA improvement (green) and with stable/worsened mJOA (red). Median values are presented by the thick black lines (p-values of the Student's t-test).
Finally, we divided the cohort into two groups according to postoperative mJOA score. We compared patients who showed mJOA improvement (delta: ≥ 1) to patients with stable or worse postoperative mJOA score (delta: <1). There was a significantly higher sphericity in those patients, who showed an improvement of the functional outcome at 3-months after surgery (0.79 ± 0.1 vs. 0.70 ± 1.0; p = 0.03). Patients with a low sphericity also had a significantly higher mMCs at 3 months (2.47 +/−1.56 vs. 1.50 +/−0.71, p = 0.047) and a poorer outcome if the intraindividual delta (mMCs 3 months vs. mMCs preoperative: 0.13 +/− 1.12 vs. −0.80 +/− 0.79, p = 0.03) was assessed. The baseline functioning displayed by the preoperative mJOA or mMCs did not differ between those with low or high sphericity. We found no differences in age, sex, preoperative KPS and mJOA, tumor size, tumor area, surface to volume ratio, Simpson Grade, WHO grade, MIB-1 index and mitotic figure count among those with decreased or normal sphericity. For detailed information, refer to Table 3 and Figure 3.

Table 3. Baseline clinical, pathologic, and imaging characteristics in dorsal spinal meningioma patients with a decreased and normal sphericity [using Fisher's exact test (two-sided) and independent t-test].
The present investigation analyzed shaped radiomic features based on shape to determine their association with postoperative neurological outcome and neuropathological characteristics in primary sporadic dorsal SMs. Our findings revealed that reduce sphericity, indicative of a more irregular SM shape, is significantly associated with worsened postoperative neurological outcome. However, we did not observe any significant association between shape and neuropathological factors.
Generally, resection of primary sporadic dorsal SM is considered a safe procedure, and the majority of patients experience significant improvement in their neurological outcomes. According to a recent systematic review, 65.2% of SM patients showed improvement, while 28.8% remained unchanged in terms of neurological functioning (10). However, the literature reports a range from 0% to 15.2% of patients experiencing postoperative worsening of their neurological functions (10). Poor postoperative neurological functioning is of paramount importance concerning health-related quality of life and cost-effectiveness, as individuals with worse neurological functioning might require prolonged hospital treatment, postoperative rehabilitation programs, and experience delayed return to work (24). Several factors have been described as predictive of the postoperative neurological status of SM patients. For instance, basic demographic feature such as older age, preoperative degree and duration of symptoms have been identified as risk factors for an unfavorable neurological outcome (24–28). However, in the present anatomic subgroup of dorsal primary sporadic SM, no heterogeneous distribution of age, preoperative functioning and symptom duration has been observed among SM patients with improved, worsened, or stable neurological functioning. These predictors have been described in different SM cohorts, and there have been various time endpoints used for determining postoperative functioning. In a retrospective single institutional study by Viereck et al. (29), patients with intradural extramedullary spinal tumors were followed-up for more than 12 months using the generic EQ-5D questionnaire. The study found that mobility and self-care worsened within 1 month following surgery, but significantly improved between the 3-to 12-month follow-up period. Therefore, using the 3-month time endpoint for evaluating the course of the mJOA score in the present study appears to be reliable. We found that patients with a low sphericity reflecting those patients with an irregularly shaped SM had a poorer neurological recovery at 3-months according to both mJOA score and mMCs. Furthermore, we found that the baseline neurological functioning did not differ between those a worsened, stable, or improved neurological function at 3-months after surgical resection. The preoperative neurological functioning has been described as an important predictor of outcome (10, 25). However, most of these studies do not exclusively focus on the dorsally located SMs.
The present manuscript represents the first analysis of the shape-based radiomic feature, sphericity, investigating its correlation with neurological functioning. It was observed that irregularly shaped dorsal primary sporadic SMs were significantly associated with worsened functional outcomes at 3-months after surgery. Meningiomas are often categorized into regularly or irregularly shaped tumors based on the characteristics of their margins and infiltrative growth patterns. The irregular shape is assumed to be caused by significant variations in the rate of cell proliferation within different subregions of the tumor, with certain areas displaying notably accelerated growth rates (30, 31). Hence, the shape of meningiomas has been proposed as a potential indicator of meningioma grade (32). Sphericity, a measurable shape characteristic derived from magnetic resonance images, has been observed to correlate with survival outcomes in glioblastoma (33). In an analysis involving 303 patients who underwent surgical removal of non-skull or skull base meningiomas classified as WHO grades 1–3, it was revealed that decreased sphericity significantly predicted both local meningioma regrowth and survival. In a previous institutional series, we could also identify sphericity as a surrogate parameter regarding postoperative cranial nerve functioning after surgery for medial sphenoid wing meningioma (15). Furthermore, an irregular shape was found to be significantly associated with increased proliferative activity, as reflected by the MIB-1 index, in medial sphenoid wing meningiomas (15). However, in the present series, we could not observe a correlation between the shape of primary dorsal SMs and the number of mitotic figures or the MIB-1 index. The strong correlation between meningioma shape and WHO grading of 126 cranial tumors has been found by Popadic et al. (14). However, this investigation exclusively focused on cranial meningiomas. Therefore, the association between shape and WHO grading in spinal meningiomas has not been confirmed yet. To further investigate and address the correlation between spinal meningioma shape and molecular characteristics driving proliferation large-scale cohorts are needed. Moreover, the impact of quantitative shape measures, such as sphericity, on progression-free survival in SMs, needs to be investigated in a large-scale cohort.
We exclusively investigated this shape-based quantitative feature regarding the outcome in dorsally located primary sporadic SM because ventral dural attachment is frequently analyzed and suggested as a potential risk factor for worsened outcome (1, 11, 12). Furthermore, in dorsally located primary sporadic SMs, division of the dentate ligament and lateralization of the myelon are not necessary. These crucial steps in the surgical approach of the resection of ventrally located SMs might be essential confounders in a small cohort investigating shape-based radiomics in primary sporadic SMs regarding functional outcome (34). Hence, shape-based radiomic features seem to be an important factor when informing patients and their relatives about the risks and potential outcomes after surgery for dorsally located primary sporadic SM. Future large-scale studies will need to externally validate our findings. Additionally, further studies should evaluate whether shape-related features are also significant in relation to progression-free survival and postoperative follow-up imaging.
The main limitation of the present study is the retrospective nature and single-center design, with limited sample size. However, the present study considered highly selective inclusion criteria in order to focus the investigation on a precise anatomical subgroup of SMs. Further large-scale studies will have to evaluate our findings using multivariable analyses and investigate whether those quantitative shape features can be correlated with intraoperative findings such as the attachments to arachnoid webs or invasive growth of SMs.
The present study shows the correlation of sphericity with the postoperative outcome of the patients with dorsally attached primary sporadic spinal meningioma. This shape-based radiomic parameter could potentially serve as a valuable supporting parameter in indication of the operative resection and preoperative risk-evaluation.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethic committee of University of Leipzig. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The present research includes only retrospective data. Therefore, our ethic committee agreed that no informed consent for this retrospective study is necessary.
MV: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. FA: Writing – review & editing. FW: Writing – review & editing. CS: Writing – review & editing. AB-F: Writing – review & editing. EG: Supervision, Writing – review & editing. JW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The graphical abstract in this article was created using BioRender.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1303128/full#supplementary-material
1. Aboul-Enein HA, Khidr WM, Abdeen KM, Madawi AA. Surgical management of ventrally based lower cervical (subaxial) meningiomas through the lateral approach: report on 16 cases. Clin Neurol Neurosurg. (2015) 139:152–8. doi: 10.1016/j.clineuro.2015.10.008
2. Alafaci C, Grasso G, Granata F, Salpietro FM, Tomasello F. Ossified spinal meningiomas: clinical and surgical features. Clin Neurol Neurosurg. (2016) 142:93–7. doi: 10.1016/j.clineuro.2016.01.026
3. Antinheimo J, Haapasalo H, Haltia M, Tatagiba M, Thomas S, Brandis A, et al. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J Neurosurg. (1997) 87:610–4. doi: 10.3171/jns.1997.87.4.0610
4. Barber SM, Konakondla S, Nakhla J, Fridley JS, Xia J, Oyelese AA, et al. Oncologic benefits of dural resection in spinal meningiomas: a meta-analysis of simpson grades and recurrence rates. J Neurosurg Spine. (2019) 8:1–11. doi: 10.3171/2019.8.SPINE19859
5. Bassiouni H, Ntoukas V, Asgari S, Sandalcioglu EI, Stolke D, Seifert V. Foramen magnum meningiomas: clinical outcome after microsurgical resection via a posterolateral suboccipital retrocondylar approach. Neurosurgery. (2006) 59:1177–85. doi: 10.1227/01.NEU.0000245629.77968.27
6. Bret P, Lecuire J, Lapras C, Deruty R, Dechaume JP, Assaad A. Intraspinal meningiomas. A series of 60 cases. Neurochirurgie. (1976) 22(1):5–22.958566
7. Ciappetta P, Domenicucci M, Raco A. Spinal meningiomas: prognosis and recovery factors in 22 cases with severe motor deficits. Acta Neurol Scand. (1988) 77(1):27–30. doi: 10.1111/j.1600-0404.1988.tb06969.x
8. Cohen-Gadol AA, Zikel OM, Koch CA, Scheithauer BW, Krauss WE. Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg. (2003) 98(3 Suppl.):258–63. doi: 10.3171/spi.2003.98.3.0258
9. Onken J, Obermüller K, Staub-Bartelt F, Meyer B, Vajkoczy P, Wostrack M. Surgical management of spinal meningiomas: focus on unilateral posterior approach and anterior localization. J Neurosurg Spine. (2018) 30(3):308–13. doi: 10.3171/2018.8.SPINE18198
10. El-Hajj VG, Pettersson-Segerlind J, Fletcher-Sandersjöö A, Edström E, Elmi-Terander A. Current knowledge on spinal meningiomas epidemiology, tumor characteristics and non-surgical treatment options: a systematic review and pooled analysis (part 1). Cancers. (2022) 14(24):6251. doi: 10.3390/cancers14246251
11. Takami T, Naito K, Yamagata T, Yoshimura M, Arima H, Ohata K. Posterolateral approach for spinal intradural meningioma with ventral attachment. J Craniovertebr Junction Spine. (2015) 6(4):173–8. doi: 10.4103/0974-8237.167862
12. Tola S, De Angelis M, Bistazzoni S, Chiaramonte C, Esposito V, Paolini S. Hemilaminectomy for spinal meningioma: a case series of 20 patients with a focus on ventral- and ventrolateral lesions. Clin Neurol Neurosurg. (2016) 148:35–41. doi: 10.1016/j.clineuro.2016.06.015
13. Wang X, Wang J, Wang L, Lin Y, Yang M, Chen X, et al. Surgical resection of dorsal spinal meningiomas with the inner Dura layer-an improved preservation technique of spinal Dura in 40 cases. World Neurosurg. (2022) 160:e250–5. doi: 10.1016/j.wneu.2021.12.118
14. Popadic B, Scheichel F, Pinggera D, Weber M, Ungersboeck K, Kitzwoegerer M, et al. The meningioma surface factor: a novel approach to quantify shape irregularity on preoperative imaging and its correlation with WHO grade. J Neurosurg. (2021) 138(6):1535–41. doi: 10.3171/20215.JNS204223
15. Wach J, Naegeli J, Vychopen M, Seidel C, Barrantes-Freer A, Grunert R, et al. Impact of shape irregularity in medial sphenoid wing meningiomas on postoperative cranial nerve functioning, proliferation, and progression-free survival. Cancers. (2023) 15(12):3096. doi: 10.3390/cancers15123096
16. Faraji AH, Tonetti DA, Flickinger JC, Engh JA. Alteration of the Ki-67 proliferative index following surgical resection with or without radiation therapy of intracranial meningiomas. Cureus. (2017) 9:e1873. doi: 10.7759/cureus.1873
17. Samii M, Klekamp J, Carvalho G. Surgical results for meningiomas of the craniocervical junction. Neurosurgery. (1996) 39:1086–95. doi: 10.1097/00006123-199612000-00003
18. Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO Guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. (2016) 17(9):e383–91. doi: 10.1016/s1470-2045(16)30321-7
19. Baro V, Moiraghi A, Carlucci V, Paun L, Anglani M, Ermani M, et al. Spinal meningiomas: influence of cord compression and radiological features on preoperative functional Status and outcome. Cancers. (2021) 13(16):4183. doi: 10.3390/cancers13164183
20. Wadell H. Volume, shape, and roundness of quartz particles. J Geol. (1935) 43:250–80. doi: 10.1086/624298
21. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. (2012) 30(9):1323–41. doi: 10.1016/j.mri.2012.05.001
22. Pichon E, Tannenbaum A, Kikinis R. A statistically based flow for image segmentation. Med Image Anal. (2004) 8(3):267–74. doi: 10.1016/j.media.2004.06.006
23. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. (1982) 143(1):29–36. doi: 10.1148/radiology.143.1.7063747
24. Kilinc F, Setzer M, Marquardt G, Keil F, Dubinski D, Bruder M, et al. Functional outcome and morbidity after microsurgical resection of spinal meningiomas. Neurosurg Focus. (2021) 50:E20. doi: 10.3171/2021.2.FOCUS201116
25. Schwake M, Adeli A, Sporns P, Ewelt C, Schmitz T, Sicking J, et al. Spinal meningiomas—risks and potential of an increasing age at the time of surgery. J Clin Neurosci. (2018) 57:86–92. doi: 10.1016/j.jocn.2018.08.030
26. Wach J, Banat M, Schuss P, Güresir E, Vatter H, Scorzin J. Age at diagnosis and baseline myelomalacia sign predict functional outcome after spinal meningioma surgery. Front Surg. (2021) 8:682930. doi: 10.3389/fsurg.2021.682930
27. Kobayashi K, Ando K, Matsumoto T, Sato K, Kato F, Kanemura T, et al. Clinical features and prognostic factors in spinal meningioma surgery from a multicenter study. Sci Rep. (2021) 11(1):11630. doi: 10.1038/s41598-021-91225-z
28. Gilard V, Goia A, Ferracci FX, Marguet F, Magne N, Langlois O, et al. Spinal meningioma and factors predictive of post-operative deterioration. J Neurooncol. (2018) 140(1):49–54. doi: 10.1007/s11060-018-2929-y
29. Viereck MJ, Ghobrial GM, Beygi S, Harrop JS. Improved patient quality of life following intradural extramedullary spinal tumor resection. J Neurosurg Spine. (2016) 25(5):640–5. doi: 10.3171/2016.4.SPINE151149
30. Liu H, Zhou J, Li W, Liu G. Comparative analysis of the magnetic resonance imaging features between anaplastic meningioma and atypical meningioma. J Craniofac Surg. (2016) 27:e229–233. doi: 10.1097/SCS.0000000000002361
31. Yao Y, Xu Y, Liu S, Xue F, Wang B, Qin S, et al. Predicting the grade of meningiomas by clinical-radiological features: a comparison of precontrast and postcontrast MRI. Front Oncol. (2022) 12:1053089. doi: 10.3389/fonc.2022.1053089
32. Yan PF, Yan L, Hu TT, Xiao DD, Zhang Z, Zhao HY, et al. The potential value of preoperative MRI texture and shape analysis in grading meningiomas: a preliminary investigation. Transl Oncol. (2017) 10:570–7. doi: 10.1016/j.tranon.2017.04.006
33. Sanghani P, Ti AB, Kam King NK, Ren H. Evaluation of tumor shape features for overall survival prognosis in glioblastoma multiforme patients. Surg Oncol. (2019) 29:178–83. doi: 10.1016/j.suronc.2019.05.005
Keywords: functional outcome, shape, spinal meningioma, sphericity, surgery
Citation: Vychopen M, Arlt F, Wilhelmy F, Seidel C, Barrantes-Freer A, Güresir E and Wach J (2023) Association of quantitative radiomic shape features with functional outcome after surgery for primary sporadic dorsal spinal meningiomas. Front. Surg. 10:1303128. doi: 10.3389/fsurg.2023.1303128
Received: 27 September 2023; Accepted: 1 December 2023;
Published: 19 December 2023.
Edited by:
Patricia Sullivan, Rhode Island Hospital, United StatesReviewed by:
Mahmoud M. Taha, Zagazig University, Egypt© 2023 Vychopen, Arlt, Wilhelmy, Seidel, Barrantes-Freer, Güresir and Wach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Vychopen bWFydGluLnZ5Y2hvcGVuQG1lZGl6aW4udW5pLWxlaXB6aWcuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.