
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 11 January 2024
Sec. Surgical Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1292031
This article is part of the Research Topic Robotic and Video-Assisted Surgery for Cancer Treatment View all 10 articles
Background: The effect of robotic surgery (RS) for rectal cancer after neoadjuvant therapy is still controversial, and a comprehensive search and analysis of the current relevant evidence is necessary. Our study aimed to evaluate the efficacy of RS for rectal cancer after neoadjuvant therapy compared with conventional laparoscopic surgery (LS).
Methods: Up to August 23, 2023, Embase, PubMed, Web of Science, and Cochrane databases were searched for studies of RS for rectal cancer after neoadjuvant therapy. Odds ratio (OR) or mean difference (MD) was used to calculate the effect sizes using RevMan 5.3.
Results: A total of 12 studies reporting on 11,686 participants were included. Compared with LS, RS increased the operative time (MD 35.16 min; 95% CI: 16.24, 54.07), but it did significantly reduce the risk of the conversion to open surgery (OR 0.46, 95% CI 0.40, 0.53) and improved the TME incomplete rate (OR 0.40, 95% CI 0.17, 0.93). Moreover, there were no difference in total postoperative complications (OR 1.13, 95% CI 0.84, 1.52), circumferential resection margin positivity (OR 0.90, 95% CI 0.63, 1.27), distal margin positive (OR 0.60, 95% CI 0.29, 1.22), blood loss (MD −11.57 ml; 95% CI: −39.09, 15.94), length of hospital stay (MD −0.08 days; 95% CI: −1.26, 1.10), mortality (OR 0.59, 95% CI 0.29, 1.21), lymph node harvested (MD 0.69.; 95% CI: −0.43, 1.82), and the time of first flatus (MD −0.47 days; 95% CI: −1.19, 0.25) between the two groups.
Conclusions: RS was associated with superiority over LS in reducing the risk of the conversion to open surgery and improving TME incomplete rate, which suggested that RS could be an effective method for treating rectal cancer after neoadjuvant therapy.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=460084, PROSPERO (CRD42023460084).
Colorectal cancer is the third most common cancer and one of the most common causes of cancer death worldwide. About 40% of colorectal cancers occur in the rectum (1, 2). Surgical resection is the main treatment strategy for rectal cancer. Compared with open surgery, laparoscopic minimally invasive surgery has the advantages of shorter operation time, faster postoperative recovery and fewer complications, and is becoming more and more popular among surgeons (3, 4). However, there are some limitations of the laparoscopic surgery (LS), such as anatomical limitations of the narrow pelvis, amplification of the handle tremor, and unstable images provided by the hand-held camera of the assistant (4). Preoperative chemoradiotherapy can effectively reduce the tumor stage, circumferential resection margin positive rate and local recurrence rate (5). However, neoadjuvant chemoradiotherapy can cause tissue edema and fibrosis, which will lead to blurred dissection plane and increase the difficulty of surgery (6).
Robotic surgery (RS) is expected to overcome these limitations by providing a 3D view of the environment, flexible operating instruments and a stable camera platform (4, 5). A large number of studies have explored the application of RS in rectal cancer surgery, but there are limited studies on the application of RS in patients receiving neoadjuvant therapy. In addition, several current studies provide conflicting evidence. Yamanashi et al. (7) demonstrated that RS reduced the incidence of postoperative complications and shortened the length of postoperative hospital stay in patients with neoadjuvant rectal cancer compared with LS. However, the study by Chen et al. (8) found that the incidence of postoperative complications in the robotic group was similar to that in the laparoscopic group. In addition, RS resulted in increased operative time and blood loss.
To resolve the conflicting results of current studies and overcome the lack of high-quality evidence, we conducted a comprehensive literature search and conducted a meta-analysis of currently published data to evaluate the differences in postoperative short-term outcomes between RS and LS in rectal cancer patients after neoadjuvant therapy.
A systematic search for the current meta-analysis was conducted on material published from inception, to August 23, 2023, in the Embase, Web of Science, PubMed, and Cochrane Library databases, using the following search string: (rectal cancer OR rectum cancer OR rectal tumor OR rectum tumor OR rectum neoplasm OR rectal neoplasm) AND (laparoscopic OR laparoscopy OR laparoscope) AND (robotic OR robotics OR robotic-assisted OR robot-assisted) AND (neoadjuvant OR neoadjuvant chemoradiotherapy OR neoadjuvant chemotherapy OR neoadjuvant radiotherapy) (Table 1). No language restrictions were imposed. The list of references for the relevant reviews and included studies were also searched.
The prespecified PICO criteria were: (1) patients: patients undergoing rectal cancer surgery after neoadjuvant therapy, (2) intervention: intervention with robotic rectal resection, (3) comparator: compare with laparoscopic rectal resection, (4) outcome: the outcomes included any of the following: postoperative complications, surgical margins, operative time, length of hospital stay, the time of first flatus and defecation, mortality, lymph node harvested, blood loss, conversion rate, and total mesorectal excision (TME) completeness, (5) study design: randomized controlled trials (RCTs) and comparative non-randomized studies (NRS). Primary outcome indicator was the incidence of postoperative complications, and the secondary outcome were postoperative complications, surgical margins, operative time, length of hospital stay, the time of first flatus and defecation, mortality, lymph node harvested, blood loss, conversion rate, and TME completeness.
Exclusions contain: review, conference abstracts, case reports, letters to the editor, technical reports, single arm studies, and non-human studies.
Data, including study type, country, sample size, age, neoadjuvant therapy, sex, the first author, year of publication, intervention type, control groups, and outcomes were extracted from each study. If relevant data could not be extracted from the literature, we tried to contact the corresponding author of the study to obtain relevant information.
Methodological quality of RCTs was assessed using the Cochrane Collaboration's risk-of-bias tool (9): (1) the randomizing process, (2) allocation concealment, (3) participant and operator blinding, (4) blinding of outcome assessment, (5) incomplete data, (6) selective reporting, and (7) other biases. the Newcastle–Ottawa scale (NOS) was used to evaluate the quality of NRS. The score of NOS is 9 points. Scores ≥ 6 were considered high quality studies, and scores < 6 were classified as low quality studies. Two authors (Ye and Zhang) independently conducted literature retrieval, study selection, data extraction, and quality assessment of the methodology included in the study. When there were inconsistencies between the two authors, they were discussed and resolved by a third author (Ye, Dong and Zhang).
Odds ratio (OR) with 95% confidence intervals (CI) were calculated for qualitative variables and mean difference (MD) for quantitative outcomes. If median with range or interquartile was reported, mean and SD were calculated using the formula reported by Wan et al. (10) and Luo et al. (11). Heterogeneity between studies was assessed using the I2 statistic (12). The random effects model was selected when I² was >50%. Otherwise, the fixed-effect model was selected. To explore the robustness of the results, one-study exclusion test was used to examine the impact of each study on the pooled effect size. Analysis was conducted using Review 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration 2014; Copenhagen, Denmark). P < 0.05 was considered significant.
We initially identified 755 articles. 561 were retained after 194 duplicates were excluded. 471 articles were excluded by reading titles and abstracts, and the remaining 90 articles were evaluated for full text. Finally, twelve trials (5–8, 13–20) were eligible and included for meta-analysis (Figure 1).
All the 12 included studies were NRS, and three studies (7, 8, 18) adopted propensity score matching. Twelve studies (5–8, 13–20) with a total of 11,686 participants (RS = 3,954, LS = 7,732) were published between 2013 and 2023. Three of the studies were conducted in China (6, 8, 15), three (13, 18, 20) in South Korea, two (16, 17) in the United States, two (5, 7) in Japan, and the remaining two in Turkey (14) and Switzerland (19), respectively. The quality assessment scores of the included studies are summarized in Table 2, with all studies scoring between six and nine.
A total of 1,096 participants in nine studies (5, 6, 8, 13–15, 18–20) mentioned postoperative complications. The combined results suggested that the total postoperative complications were comparable between the RS and LS groups, with low heterogeneity (OR 1.13, 95% CI 0.84, 1.52; Heterogeneity: I2 = 0%, P = 0.49) (Figure 2).
Incidence of conversion to open surgery was reported in nine studies (7, 13–20). Compared with LS, RS was associated with a lower risk of conversion to open surgery (OR 0.46, 95% CI 0.40, 0.53) (Figure 3). No significant heterogeneity (I2 = 0%, P = 0.70) was observed between studies.
Postoperation mortality data were recorded in seven studies (6, 7, 13, 16, 17, 19, 20), five of which had no deaths in the RS and LS groups. The pooled results showed no significant difference in mortality between the RS and LS groups (OR 0.59, 95% CI 0.29, 1.21; Heterogeneity: I2 = 0%, P = 0.63) (Figure 4).
Ten studies (5–8, 13–15, 18–20) described the operative time. Compared with LS, RS significantly prolonged the operation time (MD 35.16 min; 95% CI: 16.24, 54.07; Heterogeneity: I2 = 73%, P < 0.0001) between studies (Figure 5A). The pooled data from seven studies (5–8, 13, 15, 20) showed that the blood loss in RS was similar to that in LS (MD −11.57 ml; 95% CI: −39.09, 15.94; Heterogeneity: I2 = 75%, P = 0.0006) (Figure 5B).
A total of 341 participants in three studies (14, 19, 20) reported TME incomplete, and RS significantly reduced the risk of TME incomplete (OR 0.40, 95% CI 0.17, 0.93, P = 0.03) (Figure 6A). In addition, circumferential resection margin (CRM) positivity was comparable (OR 0.90, 95% CI 0.63, 1.27, P = 0.54) between the RS and LS groups (Figure 6B). Distal margin positive rates were reported in two studies (8, 18), and the pooled results showed no significant difference in the rate of distal margin positive rates between the RS and LS groups (OR 0.60, 95% CI 0.29, 1.22, P = 0.16) (Figure 6C). There was no significant difference in the number of nodes dissection between RS and LS (MD 0.69.; 95% CI: −0.43, 1.82, P = 0.23) (Figure 6D).
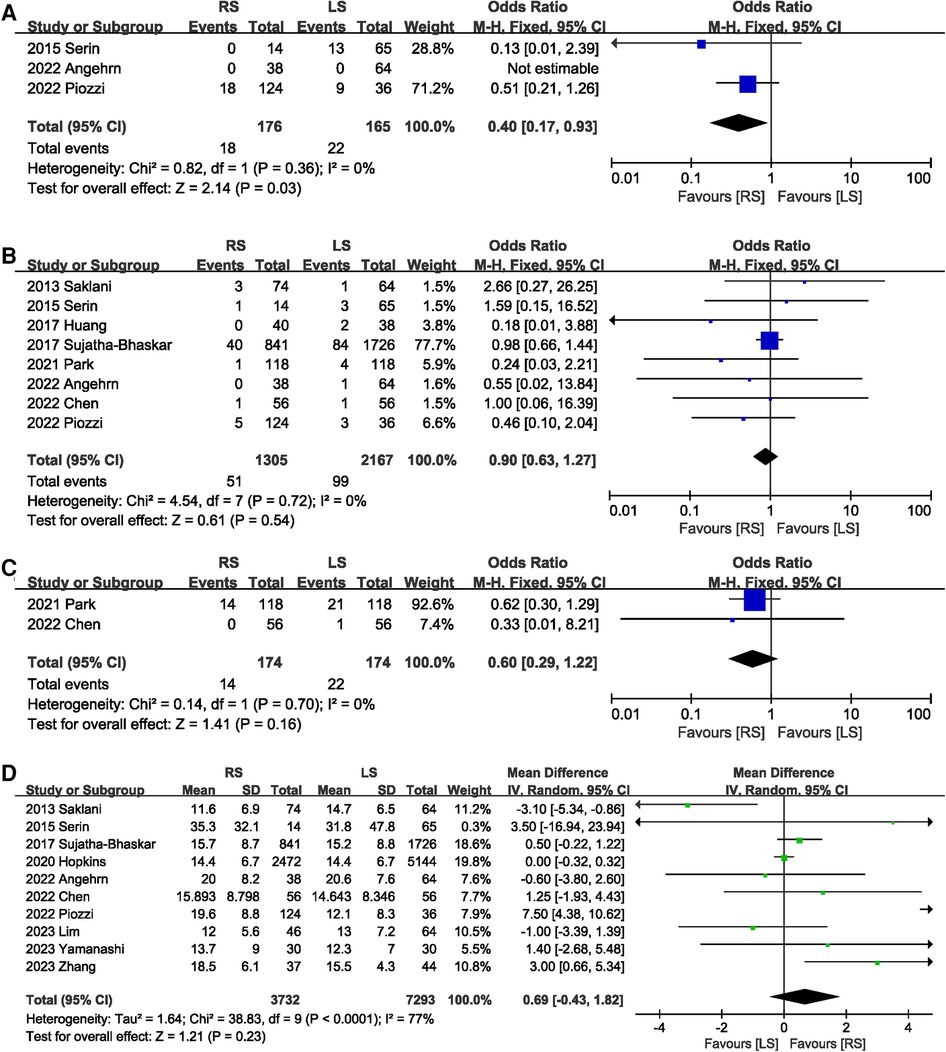
Figure 6. Forest plot of (A) TME incomplete, (B) CRM positivity, (C) distal margin positive rates, and (D) the number of nodes dissection.
A total of 536 participants in five studies (6, 13–15, 20) reported the time to first flatus. The overall effect indicated that RS did not shorten the time to first flatus (MD −0.47 days; 95% CI: −1.19, 0.25), with significant heterogeneity (I2 = 89%, P < 0.00001) between studies (Figure 7A). Only one study described the first bowel movement time. This study found that RS shortened the time to the first bowel movement. Length of hospital stay was mentioned in nine (6–8, 13–15, 17, 19, 20) studies. No significant difference in the length of hospital stay was observed between the RS and LS groups (MD −0.08 days; 95% CI: −1.26, 1.10) (Figure 7B).
The results of the sensitivity analysis showed that the total effect size of postoperative complications, CRM positivity, distal margin positive rates, operative time, length of hospital stay, the time of first flatus and defecation, lymph node harvested, and conversion rate were not affected by the elimination of any one study. The total effect size for the blood loss changed when the study by Chen et al. (8) (MD −24.27 ml; 95% CI: −38.42, −10.13, P = 0.0008) was excluded.
Our results showed that RS significantly increased the operation time, but it reduced the risk of conversion to open surgery, and improved the completeness of TME compared with LS in patients with rectal cancer after neoadjuvant therapy. In addition, there were no significant differences in postoperative morbidity, mortality, length of hospital stay, blood loss, time to first exhaust, CRM positive rate, lymph node detection rate, and distal margin positive rates between RS and LS.
Due to the lack of obvious clinical symptoms, most patients with rectal cancer are not clearly diagnosed until they have developed to locally advanced stage (21). Patients with advanced rectal cancer often require neoadjuvant therapy. Although neoadjuvant therapy can effectively reduce the tumor stage and local recurrence rate, it is associated with an increase in postoperative complications (21, 22). Previous evidence has shown that the incidence of postoperative complications in patients with rectal cancer treated with neoadjuvant therapy is as high as 21% (23). Similarly, the overall incidence of postoperative complications in our study was 25%. Postoperative complications not only increase the economic burden of patients, but also compromise their oncologic outcomes (21). Theoretically, RS has good maneuvability in the limited pelvic space and provides a good field of view, which may be expected to reduce postoperative morbidity. However, the results of our meta-analysis suggest that robot-assisted surgery does not reduce the risk of postoperative complications and mortality. This is consistent with findings from several recent large RCTs (24–26).
The conversion rate in minimally invasive surgery not only reflects the complexity of surgical techniques, but also has important clinical significance. Previous evidence suggests that conversion to open surgery is associated with an increased risk of postoperative morbidity and local recurrence (7, 27). Conversion rates for laparoscopic rectal cancer surgery range from 9% to 16%, with rates as high as 25% among patients receiving neoadjuvant therapy (7). In a narrow pelvis, the 3D view of the RS system is able to allow the surgeon to clearly identify anatomical structures and gaps to minimize tissue damage caused by the dissection procedure (3). Therefore, RS may be a potential strategy to reduce the rate of conversion to open surgery. The failure of the ROLARR trial to confirm a benefit of RS in reducing conversion to open surgery may be related to the inclusion of patients with higher rectal cancers and the low proportion of patients who received neoadjuvant therapy (25). However, a recently published large RCT showed that RS (10/586) significantly reduced the conversion rate of mid-low rectal cancer surgery compared with LS (23/585) (28). In addition, several recently published meta-analyses have also suggested that RS is associated with lower rates of conversion to open surgery (3, 29). This evidence is consistent with our results. Considering the potential impact of conversion to open surgery on long-term prognosis, future studies are warranted to further explore the impact of RS on the long-term prognosis of rectal cancer patients undergoing neoadjuvant therapy.
A large number of previous studies have shown that RS prolongs the operation time compared with LS (3, 4, 30). Our study also showed that RS was associated with longer operative time, but intraoperative blood loss was comparable between the RS and LS groups. The prolonged operation time may be related to the following two aspects. On the one hand, LS has been widely and skillfully used by surgeons, while RS is relatively a new surgical procedure, and some surgeons may not be as proficient in RS as LS. On the other hand, the setup of surgical instruments for RS takes a lot of time (30). When the time to assemble the machine was excluded, there was no significant difference in the duration of surgery between the two groups (31). With the popularization of RS, the difference in operative time is expected to gradually decrease.
The introduction of TME has improved the oncologic prognosis of patients with rectal cancer. At present, TME has become the gold standard for rectal cancer surgery, and complete TME can reduce the risk of postoperative recurrence (32). Our results suggest that RS improves the TME completeness rate, which may help improve the prognosis of patients with rectal cancer. Similarly, a meta-analysis of 12 studies showed that RS was a better way to achieve complete TME than traditional LS (33). The number of harvested lymph nodes not only determines the accuracy of postoperative staging, but also is related to the prognosis of patients (34). In addition, positive CRM increased the risk of tumor recurrence (35). Our study showed no significant difference between robotic progenitor and laparoscopic groups in the number of lymph nodes harvested and CRM positive rate. These results indicate that RS can achieve pathological results that are noninferior to LS in patients with rectal cancer.
Several studies have suggested that RS may be associated with faster gastrointestinal function recovery and shorter hospital stay (3, 36). The time to first flatus and defecation are the key indicators to evaluate the recovery of intestinal function. Of the 12 studies we included, only 5 studies assessed the time to first flatus and 1 study reported the time to first defecation. The pooled results showed that the time to first flatus was comparable between the RS and LS groups. Due to the limited number of included studies, more studies are needed to evaluate the effects of robots on gastrointestinal function recovery.
Our study has the following strengths. On the one hand, we performed a comprehensive database search without language and time constraints. On the other hand, we included the most recent data, making the pooled results more convincing.
Meanwhile, our meta-analysis has several limitations. First, the studies we included were all retrospective studies, which have the inherent bias of retrospective studies. Second, some measures may have been underpowered to draw firm conclusions. Finally, we included some studies with small samples, which may have potential bias.
In conclusion, based on evidence from NRS, this meta-analysis indicates that RS prolonged the procedure time, but significantly improved the rate of conversion to open surgery and the completeness of TME. In addition, RS and LS were comparable in terms of postoperative morbidity, mortality, length of hospital stay, blood loss, time to first exhaust, CRM positive rate, lymph node detection rate, and distal margin positive rates. In the future, high-quality prospective studies are needed to further explore the impact of RS on rectal cancer patients after neoadjuvant therapy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
YZ: Conceptualization, Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. BD: Conceptualization, Data curation, Formal Analysis, Software, Writing – original draft. GL: Data curation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft. WY: Conceptualization, Data curation, Formal Analysis, Resources, Software, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Seow W, Dudi-Venkata NN, Bedrikovetski S, Kroon HM, Sammour T. Outcomes of open vs laparoscopic vs robotic vs transanal total mesorectal excision (TME) for rectal cancer: a network meta-analysis. Tech Coloproctol. (2023) 27(5):345–60. doi: 10.1007/s10151-022-02739-1
3. Yao Q, Sun QN, Ren J, Wang LH, Wang DR. Comparison of robotic-assisted versus conventional laparoscopic surgery for mid-low rectal cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. (2023) 149:15207–17. doi: 10.1007/s00432-023-05228-6
4. Wang L, Zhang Z, Gong L, Zhan Y, Li M, Li S, et al. A systematic review and Bayesian network meta-analysis: short-term and long-term outcomes of three surgery procedures following neoadjuvant chemoradiotherapy for rectal cancer. J Laparoendosc Adv Surg Tech A. (2019) 29(5):663–70. doi: 10.1089/lap.2018.0069
5. Lim S, Nagai Y, Nozawa H, Kawai K, Sasaki K, Murono K, et al. Surgical outcomes of robotic, laparoscopic, and open low anterior resection after preoperative chemoradiotherapy for patients with advanced lower rectal cancer. Surg Today. (2023) 53(1):109–15. doi: 10.1007/s00595-022-02537-0
6. Zhang L, Shi F, Hu C, Zhang Z, Liu J, Liu R, et al. Comparison of robotic versus laparoscopic lateral lymph node dissection for advanced lower rectal cancer: a retrospective study at two institutions. Surg Endosc. (2023) 37(6):4403–13. doi: 10.1007/s00464-023-09925-8
7. Yamanashi T, Miura H, Tanaka T, Watanabe A, Yokoi K, Kojo K, et al. Short-term outcomes of robot-assisted versus conventional laparoscopic surgery for mid and low rectal cancer after neoadjuvant chemoradiotherapy: a propensity score-matched analysis. J Robot Surg. (2023) 17(3):959–69. doi: 10.1007/s11701-022-01498-3
8. Chen TC, Liang JT. Robotic versus laparoscopic surgery for rectal cancer after neoadjuvant chemoradiotherapy: a propensity-score matching analysis. J Formos Med Assoc. (2022) 121(8):1532–40. doi: 10.1016/j.jfma.2021.10.025
9. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration (2011). Available online at: https://training.cochrane.org/handbook (accessed January 05, 2024).
10. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
11. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27(6):1785–805. doi: 10.1177/0962280216669183
12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
13. Saklani AP, Lim DR, Hur H, Min BS, Baik SH, Lee KY, et al. Robotic versus laparoscopic surgery for mid-low rectal cancer after neoadjuvant chemoradiation therapy: comparison of oncologic outcomes. Int J Colorectal Dis. (2013) 28(12):1689–98. doi: 10.1007/s00384-013-1756-z
14. Serin KR, Gultekin FA, Batman B, Ay S, Kapran Y, Saglam S, et al. Robotic versus laparoscopic surgery for mid or low rectal cancer in male patients after neoadjuvant chemoradiation therapy: comparison of short-term outcomes. J Robot Surg. (2015) 9(3):187–94. doi: 10.1007/s11701-015-0514-3
15. Huang YM, Huang YJ, Wei PL. Outcomes of robotic versus laparoscopic surgery for mid and low rectal cancer after neoadjuvant chemoradiation therapy and the effect of learning curve. Medicine (Baltimore). (2017) 96(40):e8171. doi: 10.1097/md.0000000000008171
16. Sujatha-Bhaskar S, Jafari MD, Gahagan JV, Inaba CS, Koh CY, Mills SD, et al. Defining the role of minimally invasive proctectomy for locally advanced rectal adenocarcinoma. Ann Surg. (2017) 266(4):574–81. doi: 10.1097/sla.0000000000002357
17. Hopkins MB, Geiger TM, Bethurum AJ, Ford MM, Muldoon RL, Beck DE, et al. Comparing pathologic outcomes for robotic versus laparoscopic surgery in rectal cancer resection: a propensity adjusted analysis of 7616 patients. Surg Endosc. (2020) 34(6):2613–22. doi: 10.1007/s00464-019-07032-1
18. Park SY, Lee SM, Park JS, Kim HJ, Choi GS. Robot surgery shows similar long-term oncologic outcomes as laparoscopic surgery for mid/lower rectal cancer but is beneficial to ypT3/4 after preoperative chemoradiation. Dis Colon Rectum. (2021) 64(7):812–21. doi: 10.1097/dcr.0000000000001978
19. Angehrn FV, Schneider R, Wilhelm A, Daume D, Koechlin L, Fourie L, et al. Robotic versus laparoscopic low anterior resection following neoadjuvant chemoradiation therapy for stage II-III locally advanced rectal cancer: a single-centre cohort study. J Robot Surg. (2022) 16(5):1133–41. doi: 10.1007/s11701-021-01351-z
20. Piozzi GN, Rusli SM, Lee TH, Baek SJ, Kwak JM, Kim J, et al. Robotic approach may be associated with a lower risk of lung metastases compared to laparoscopic approach for mid-low rectal cancer after neoadjuvant chemoradiotherapy: a multivariate analysis on long-term recurrence patterns. Int J Colorectal Dis. (2022) 37(9):2085–98. doi: 10.1007/s00384-022-04249-w
21. Tang G, Pi F, Wei Z, Li X. Effect of glutamine on short-term surgical outcomes in rectal cancer patients receiving neoadjuvant therapy: a propensity score matching study. Nutr Cancer. (2023) 75(4):1254–62. doi: 10.1080/01635581.2023.2189046
22. Couwenberg AM, Burbach JPM, van Grevenstein WMU, Smits AB, Consten ECJ, Schiphorst AHW, et al. Effect of neoadjuvant therapy and rectal surgery on health-related quality of life in patients with rectal cancer during the first 2 years after diagnosis. Clin Colorectal Cancer. (2018) 17(3):e499–512. doi: 10.1016/j.clcc.2018.03.009
23. Zhang X, Ma S, Guo Y, Luo Y, Li L. Total neoadjuvant therapy versus standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis of 15 trials. PLoS One. (2022) 17(11):e0276599. doi: 10.1371/journal.pone.0276599
24. Park JS, Lee SM, Choi GS, Park SY, Kim HJ, Song SH, et al. Comparison of laparoscopic versus robot-assisted surgery for rectal cancers: the COLRAR randomized controlled trial. Ann Surg. (2023) 278(1):31–8. doi: 10.1097/sla.0000000000005788
25. Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. Jama. (2017) 318(16):1569–80. doi: 10.1001/jama.2017.7219
26. Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam BH, et al. Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg. (2018) 267(2):243–51. doi: 10.1097/sla.0000000000002321
27. Gouvas N, Georgiou PA, Agalianos C, Tzovaras G, Tekkis P, Xynos E. Does conversion to open of laparoscopically attempted rectal cancer cases affect short- and long-term outcomes? A systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A. (2018) 28(2):117–26. doi: 10.1089/lap.2017.0112
28. Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y, et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. (2022) 7(11):991–1004. doi: 10.1016/s2468-1253(22)00248-5
29. Flynn J, Larach JT, Kong JCH, Rahme J, Waters PS, Warrier SK, et al. Operative and oncological outcomes after robotic rectal resection compared with laparoscopy: a systematic review and meta-analysis. ANZ J Surg. (2023) 93(3):510–21. doi: 10.1111/ans.18075
30. Ohtani H, Maeda K, Nomura S, Shinto O, Mizuyama Y, Nakagawa H, et al. Meta-analysis of robot-assisted versus laparoscopic surgery for rectal cancer. In Vivo. (2018) 32(3):611–23. doi: 10.21873/invivo.11283
31. Gavriilidis P, Wheeler J, Spinelli A, de'Angelis N, Simopoulos C, Di Saverio S. Robotic vs laparoscopic total mesorectal excision for rectal cancers: has a paradigm change occurred? A systematic review by updated meta-analysis. Colorectal Dis. (2020) 22(11):1506–17. doi: 10.1111/codi.15084
32. Wilder FG, Burnett A, Oliver J, Demyen MF, Chokshi RJ. A review of the long-term oncologic outcomes of robotic surgery versus laparoscopic surgery for colorectal cancer. Indian J Surg. (2016) 78(3):214–9. doi: 10.1007/s12262-015-1375-8
33. Milone M, Manigrasso M, Velotti N, Torino S, Vozza A, Sarnelli G, et al. Completeness of total mesorectum excision of laparoscopic versus robotic surgery: a review with a meta-analysis. Int J Colorectal Dis. (2019) 34(6):983–91. doi: 10.1007/s00384-019-03307-0
34. Tonini V, Birindelli A, Bianchini S, Cervellera M, Bacchi Reggiani ML, Wheeler J, et al. Factors affecting the number of lymph nodes retrieved after colo-rectal cancer surgery: a prospective single-centre study. Surgeon. (2020) 18(1):31–6. doi: 10.1016/j.surge.2019.05.002
35. Wang X, Cao G, Mao W, Lao W, He C. Robot-assisted versus laparoscopic surgery for rectal cancer: a systematic review and meta-analysis. J Cancer Res Ther. (2020) 16(5):979–89. doi: 10.4103/jcrt.JCRT_533_18
Keywords: robotic surgery, conventional laparoscopic surgery, rectal cancer, neoadjuvant therapy, meta-analysis
Citation: Zhang Y, Dong B, Li G and Ye W (2024) Short-term outcomes of robotic vs. laparoscopic surgery for rectal cancer after neoadjuvant therapy: a meta-analysis. Front. Surg. 10:1292031. doi: 10.3389/fsurg.2023.1292031
Received: 10 September 2023; Accepted: 28 December 2023;
Published: 11 January 2024.
Edited by:
Gianluca Rompianesi, University of Naples Federico II, ItalyReviewed by:
Michele Manigrasso, University of Naples Federico II, Italy© 2024 Zhang, Dong, Li and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Ye d2VpeWUyMDExMjAyM0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.