
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 06 November 2023
Sec. Visceral Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1290706
This article is part of the Research TopicColorectal Surgery and Proctology: Past, Present, and FutureView all 9 articles
 Francesco Pata1,2*
Francesco Pata1,2* Luigi M. Bracchitta3
Luigi M. Bracchitta3 Bruno Nardo1
Bruno Nardo1 Gaetano Gallo4
Gaetano Gallo4 Giancarlo D’Ambrosio5,†
Giancarlo D’Ambrosio5,† Salvatore Bracchitta6,†
Salvatore Bracchitta6,†
Introduction: Around 20% of population in western countries is under anticoagulant treatment. However, there is paucity of evidence about the treatment of HD in patients under anticoagulant/antiplatelet therapy, although both suspension and continuation in the perioperative period may increase the risk of severe complications. The aim of this pilot study was to confirm the feasibility and safety of sclerobanding (Combined Rubber Band Ligation with 3% Polidocanol Foam Sclerotherapy), an office-based procedure, for the treatment of second-and third-degree HD in patients under anticoagulant/antiplatelet therapy without suspension.
Materials and methods: Patients affected by second-third-degree haemorrhoids unresponsive to conservative treatment and under anticoagulant/antiplatelet were enrolled between November 2019 and October 2021. Postoperative complications, readmission, mortality and reintervention during the follow-up were evaluated.
Results: Fifty-one patients were recruited, 23 female (45.1%) and 28 male (54.9%), with an average age of 65 years ± 11.4 SD (range 42–90). Twenty-seven patients (52.9%) had II-degree haemorrhoidal disease, and 24 (47.1%) had grade III-degree. The most frequently taken medications were dual antiplatelet therapy (51%) and new oral anticoagulants (NOACs) (21.6%). The mean follow-up was 23 months. No intraoperative complications were recorded. The rate of complications in the first postoperative month was 13.7%, represented by mild complications: 6 cases of moderate to severe pain and 1 case (2%) of thrombosis of a residual haemorrhoidal nodule, all regressing after conservative therapy. No severe complications were reported. Postoperative complications were not statistically significantly associated with the number of nodules treated (1, 2, or 3), the disease grade (2nd vs. 3rd) or the specific anticoagulant/antiplatelet regimen. During follow-up, 2 patients (4%) required a new procedure for recurrent bleeding: one an infrared photocoagulation as outpatient, and another a haemorrhoidectomy after 3 months. No cases of intraoperative or postoperative mortality occurred.
Conclusions: Sclerobanding is a safe and effective technique in treating intermediate-grade haemorrhoidal disease in patients at high risk on anticoagulant/antiplatelet therapy. Sclerobanding is repeatable, usually does not require anaesthesia, and is cost-effective. Observational multicentre studies with a larger number of patients and controlled clinical trials will be needed to confirm these results.
Approximately 1/5 of the United States population is on anticoagulant treatment (1), with 52% of them being treated with acetylsalicylic acid in the 45–75 year age group (2). The most common indications for therapy include primary prevention of ischemic heart disease and stroke, aortic-coronary stent placement, and treatment of deep venous thrombosis (3). In the ROCKET AF trial, a randomized control trial (RCT) comparing Rivaroxaban with Warfarin for the prevention of stroke and embolism in Atrial Fibrillation, 4.8% of patients experienced gastrointestinal bleeding episodes, with 29% being of rectal origin (4).
Haemorrhoidal disease is one of the most common causes of lower gastrointestinal bleeding (5), often self-limiting, but in some cases leading to severe anaemia requiring hospitalization, operative treatment, and/or transfusions (6). Treating haemorrhoidal disease in patients on anticoagulants represents a therapeutic challenge: on one hand, continuation of anticoagulant therapy is associated with a higher risk of postoperative bleeding; on the other hand, suspension itself can lead to new cardiovascular, neurological or thrombotic events, due to a rebound hypercoagulability phenomenon (7–10). Suspending antiplatelet therapy in secondary prevention may result in a new coronary or cerebrovascular event in 4.1% and 4.9% of cases, respectively (11, 12).
The use of office-based techniques, not requiring anaesthesia and hospitalization, mitigates but does not eliminate this risk. Rubber band ligation (RBL), reported as the most effective ambulatory technique for the treatment of haemorrhoidal disease, may rarely be associated with severe late bleeding (13), often occurring between the 10th and 14th postoperative day, and this risk is increased in patients on anticoagulant therapy (14, 15).
However, there is a gap in the literature: the few published studies on anticoagulant and antiplatelet-treated patients for haemorrhoidal disease are mostly case reports, case-series or single-centre retrospective studies with selection biases that reduce their external validity. The failure to conduct larger studies, after the initial preliminary cases reported, raises doubts about the clinical relevance of some of them (16, 17). Furthermore, the growing cohort of patients on new oral anticoagulants (NOACs) has been underinvestigated so far, despite the evidence of a higher incidence of rectal bleeding compared to warfarin (4).
The aim of this study was to assess the feasibility and safety of a novel technique named Sclerobanding (Combined Rubber Band Ligation with 3% Polidocanol Foam Sclerotherapy) on high-risk patients, with II or III degree haemorrhoidal disease, under anticoagulant/antiplatelet treatment, without suspending such therapy in the perioperative period.
Between November 2019 and October 2021, 51 patients underwent Sclerobanding at the Coloproctology Centre of Clinica del Mediterraneo Hospital in Ragusa, Italy. Inclusion criteria were:
- Bleeding II or III degree haemorrhoidal disease unresponsive to conservative treatment;
- Anticoagulant or antiplatelet therapy;
- High surgical risk (ASA 4) and/or high risk of thrombotic complications in case of therapy suspension, as assessed by the referring specialist;
- Increased risk of morbidity/mortality in case of anaemia as consequence of the recurrent bleeding;
Exclusion criteria were age under 18 years, pregnancy and concomitant anorectal pathologies;
The study was conducted according with the IDEAL Stage 2a framework.
Perioperative demographic data and postoperative complications (first postoperative days) were recorded in a local database. Each patient consented to the procedure after a discussion of indications, risks, and rationale of the Sclerobanding and the signature of a consent form. The Goligher classification was used to stage the disease. All procedures were performed by the same colorectal surgeon. Patients were seen at one week, one month, three months, six months, one year, and then every six months. The follow-up assessment included an interview to evaluate symptoms potentially related to complications or a possible recurrence (anal bleeding), inspection of the anorectal region in the Sims position, and digital rectal examination. An anoscopy was performed starting at the 3-month follow-up visit.
The primary aim of the study was to evaluate the feasibility and safety of Sclerobanding. The primary outcomes included postoperative morbidity, measured as the incidence of intraoperative adverse events and the complication rate within the first month, as well as the 30-day readmission rate. Secondary outcomes comprised the rate of reintervention during follow-up and patient satisfaction. The secondary objective was to investigate potential differences in postoperative complications based on the disease grade (second vs. third degree), the number of nodules treated during the procedure, and the anticoagulant regimen.
Patient satisfaction with the procedure was assessed using a visual analogue scale (VAS, 0-10) during the final follow-up visit.
The Sclerobanding technique has been described in the literature in 2021 (18, 19). No antibiotics is administered before the procedure. The patient is placed in a lithotomy position, and each haemorrhoidal nodule to be treated is ligated at the base above the dentate line to reduce the risk of postoperative pain, using a rubber band applied with a dedicated ligator. Subsequently, 2 or 3 ml of 3% polidocanol foam (obtained by mixing 2 ml of 3% polidocanol oil with 8 ml of air through two silicone-free syringes, one of 5 ml and one of 10 ml, connected by a connector) are injected into the ligated nodule. After the procedure, the patient is monitored for one hour and discharged with a dedicated phone contact number in case of need. Anaesthesia is typically not required; however, in some cases, patients may request local anesthesia. Postoperatively, analgesics and stool softeners are prescribed to minimize discomfort, postoperative pain, and the risk of bleeding. Patients are advised to abstain from alcohol consumption and smoking for at least the first month following the procedure.
Data are presented as means ± 1 standard deviation if normally distributed, range, or percentage. Fisher's exact test (alpha 0.05) was used to compare the rate of complications between patient subgroups (second vs. third degree; 1 vs. 2 vs. 3 nodules treated; complications according with different anticoagulant/antiplatelet regimens). A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using Microsoft® Excel® 2016 (Microsoft Corporation, Redmond, WA, USA), and the online calculators https://www.socscistatistics.com/tests/fisher/default2.aspx and http://vassarstats.net/fisher2×4.html for Fisher's exact test.
Fifty-one consecutive patients were recruited, 23 female (45.1%) and 28 male (54.9%), with an average age of 65 years ± 11.4 SD (range 42–90). Twenty-seven patients (52.9%) had II-degree haemorrhoidal disease, and 24 (47.1%) had grade III-degree. Table 1 presents clinical, perioperative, and follow-up data for the patients included in the study.
Figures 1, 2 show the degree of the disease and the number of nodules treated per patient.
The most common comorbidities leading to anticoagulant therapy were ischemic heart disease with previous stent placement and atrial fibrillation (Figure 3), while the most frequently taken medications were dual antiplatelet therapy (51%) and new oral anticoagulants (NOACs) (21.6%), as illustrated in Figure 4.
The mean follow-up was 23 months. No intraoperative complications occured. The rate of complications in the first postoperative month was 13.7%, represented only by mild complications, particularly 6 cases of moderate to severe pain with partial response to analgesic therapy and 1 case (2%) of thrombosis of a residual haemorrhoidal nodule, all regressing after conservative therapy (Table 1). No severe complications or significant bleeding were reported. Postoperative complications were not statistically significantly associated with the number of nodules treated (1, 2, or 3), the grade of disease (2nd vs. 3rd degree) or the anticoagulant treatment (Tables 2–4).
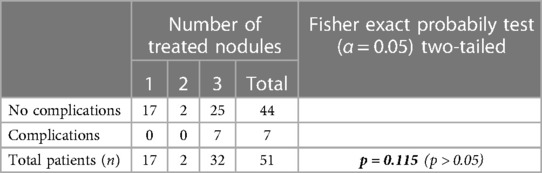
Table 2. Comparison of the complication rate according with the number of hemorrhoidal nodules treated during the procedure using the Fisher's exact test. A p-value < 0.05 was considered statistically significant.
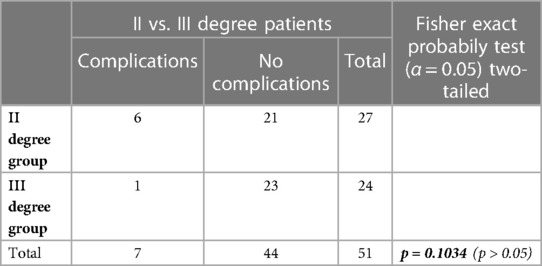
Table 3. Comparison of the complication rate according with the HD Degree of the patients included in the study using the Fisher's exact test. A p-value < 0.05 was considered statistically significant.
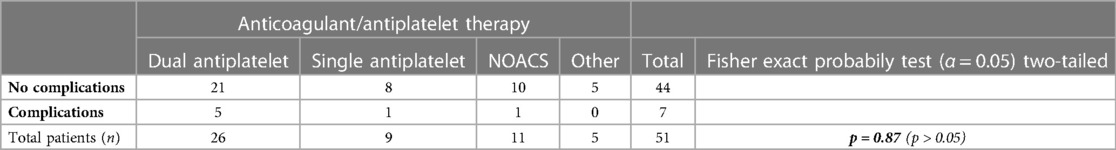
Table 4. Comparison of the complication rate according with the anticoagulant/antiplatelet regimen of the patients included in the study using the Fisher's exact test. A p-value < 0.05 was considered statistically significant.
Only one patient (2%), who was taking a single antiplatelet medication (acetylsalicylic acid), required local anesthesia with 2% Mepivacaine (Carbocaine), and experienced an uneventful postoperative recovery.
During the follow-up, 2 patients (4%) required a new procedure for recurrent bleeding: one was treated with infrared photocoagulation on an outpatient basis, and the second required an open haemorrhoidectomy after 3 months. No cases of intraoperative or postoperative mortality occurred.
Forty patients (78.43%) expressed a high level of satisfaction, rating 8–10 the procedure in a VAS scale (0-10) (Figure 5).
Our study shows that Sclerobanding is a feasible technique in patients on anticoagulant/antiplatelet treatment with a high safety profile, considering the absence of significant bleeding, intraoperative accidents, or severe postoperative complications. In addition to the feasibility and safety of the method, which were already demonstrated in a previous study on non-anticoagulated patients (20), it offers simplicity of execution, a short learning curve, cost-effectiveness (the procedure costs approximately 30 euros), repeatability in case of recurrence, and it usually does not require anaesthesia.
The complication rate was 13.7%, but it was represented only by 1 case of internal haemorrhoidal thrombosis [detected under sedation with an evaluation under anaesthesia (EUA) to reduce the patient's severe discomfort and treated conservatively], and 6 cases of moderate to severe pain, all regressed within 20 days. The absence of severe complications is the major advantage of the method.
The possibility of not discontinuing the anticoagulant treatment in the perioperative period contributes further to the safety profile, preventing the occurrence of serious thrombotic complications related to therapy suspension and rebound hypercoagulability, without an increased bleeding risk. This is particularly important since most experts suggest suspending anticoagulant or antiplatelet drugs at least one week before and 1–2 weeks after a surgical or an office-based procedure to reduce the risk of severe late bleeding, as described several times in the literature (21–23).
In an observational study of 1,269 proctologic interventions, the frequency of postoperative bleeding was 5%, with episodes occurring up to the 18th postoperative day, requiring transfusions and/or haemostasis in the operating theatre in a quarter of patients (24). The frequency rose to 33% in patients who had temporarily suspended NOAC therapy in favour of low-molecular-weight heparins. Considering that proctologic interventions require no or minimal hospitalization, the possibility of late bleeding events in these patients at home represents an additional risk. In particular, the use of clopidogrel seems to entail an additional risk compared to other anticoagulants. Nelson et al. (25), in a sample of 364 patients on anticoagulant or antiplatelet therapy undergoing rubber band ligation for haemorrhoids, reported 23 cases (3.7%) of postoperative bleeding, but only 6 severe cases (0.9%) requiring hospitalization or blood transfusion. However, 6 of these cases occurred in the 18 patients taking clopidogrel, 3 of which presented severe bleeding. Such data have been confirmed by other studies (23, 26). In our study, the use of clopidogrel alone or in dual antiplatelet therapy with acetylsalicylic acid (the largest group of patients) did not result in an increased risk of bleeding.
In a 4-year retrospective study, Martin et al. (27) investigated 111 patients necessitating hospitalization due to post-proctologic surgery bleeding. The study revealed significant associations between postoperative bleeding and factors such as hemorrhoid surgery, anticoagulant treatment (particularly direct oral anticoagulants), and an ASA score of 3. Notably, active smoking exhibited a protective effect, consistent with previous findings by other authors (28), likely attributed to smoking-induced vasoconstriction at the surgical site.
However, active smoking is a well-documented adverse factor on both wound healing and cardiovascular system, particularly in patients with other risk factors. Consequently, we recommended all treated patients to discontinue active smoking, although the compliance with this recommendation was not assessed.
Our study primarily included subjects on dual antiplatelet therapy and on NOAC therapy, at higher risk of postoperative bleeding, while most published studies focus on single antiplatelet therapy (acetylsalicylic acid and clopidogrel) or oral anticoagulant therapy (OAC).
Atallah et al. (1), in a retrospective study involving 106 subjects, reported 36 patients on anticoagulant/antiplatelet therapy, who underwent dearterialization with mucopexy (THD) without discontinuing ongoing therapy except for the day of the procedure. No statistically significant differences in postoperative complications were recorded, and postoperative bleeding events were similar between patients on anticoagulant therapy and healthy patients (19.4% vs. 15.7%, odds ratio 1.295, 95% CI 0.455–3.688, p = 0.785).
However, the study was limited by the heterogeneity of the anticoagulant drugs used, with the majority of patients (25 out of 36, or 69.4%) being solely on acetylsalicylic acid therapy, often at a low-dose regimen (81 mg/day), thereby posing challenges for generalizing these findings. The procedure also required regional or general anaesthesia, which can potentially lead to additional complications.
In another prospective study (29), including 73 patients with bleeding disorders who underwent sclerotherapy alone with 3% polidocanol foam, the complication rate was 9.6%, but with only 6 patients under dual antiplatelet therapy and 31 patients (42.4%) requiring a second or third session to treat the bleeding in a 1-year follow-up.
Regarding the grade of disease, the type of anticoagulant treatment and the number of nodules treated, no statistically significant differences were detected between subgroups. However, all complications occurred in the subgroup with 3 nodules treated during the same procedure. Thus, the lack of statistical significance might be attributed to the small sample size.
During the pandemic and in the post-pandemic period, the ability to treat the majority of patients with haemorrhoidal disease on an outpatient basis offers several advantages. This approach helps to preserve healthcare resources, such as hospital beds, operating rooms, and anaesthesiologists, which can then be directed toward critical care needs (30, 31). It also contributes to the reduction of waiting lists for other surgical conditions (20). Moreover, this outpatient approach can have a substantial impact on cost savings, the organization of the healthcare system, and the overall well-being of the patient. Patients benefit from avoiding hospital admissions and the associated risk of severe nosocomial infections (32).
In addition, the positive assessment provided by the patients further underscores the feasibility and the high acceptance rate of the technique.
The present study has inherent limitations due to its design and the small sample size. However, the sample size reflects a highly selected group of patients. We did not conduct any symptom evaluation scoring before and after the procedure. Nevertheless, the primary objective of the study was to assess the safety of the procedure and the absence of major complications, as outlined in the methods section. The procedure was primarily indicated to control recurrent bleeding, which could have had catastrophic consequences for this group of patients, rather than specifically addressing other symptoms. Therefore, the consideration for a new treatment during the follow-up period was linked to suboptimal results in controlling recurrent bleeding and the potential effects thereof.
The safety and feasibility results obtained in a highly selected group of high-risk patients and the potential benefits of the systematic use of the method represent a starting point for planning further multicentre studies with larger samples and longer follow-ups, necessary to confirm these promising preliminary results.
Sclerobanding is a safe and effective technique in treating intermediate-grade haemorrhoidal disease (2nd and 3rd degree according to Goligher) in patients at high risk on anticoagulant/antiplatelet therapy. Sclerobanding is repeatable, usually does not require anaesthesia, and is cost-effective. Observational multicentre studies with a larger number of patients and controlled clinical trials will be needed to confirm these results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was conducted according to the Declaration of Helsinki. As it was a descriptive analysis of anonymized data and because each patient consented for the procedure and for the use of his/her anonymized data for scientific purposes, ethical review approval was waived for this study and not necessary according with the local rules. However, an internal IRB approval (106-107/22 by Clinica del Mediterraneo, Ragusa, Italy) was obtained. The studies were conducted in accordance with the local legislation and institutional requirements.
FP: Conceptualization, Investigation, Writing – original draft. LB: Data curation, Investigation, Writing – review & editing, Methodology, Writing – original draft. BN: Supervision, Writing – review & editing. GG: Supervision, Visualization, Writing – review & editing. GD: Conceptualization, Supervision, Writing – review & editing. SB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The present study was also a part of the PhD thesis defended by FP on February 16, 2023, at Sapienza University in Rome, Italy.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Atallah S, Maharaja GK, Martin-Perez B, Burke JP, Albert MR, Larach SW. Transanal hemorrhoidal dearterialization (THD): a safe procedure for the anticoagulated patient? Tech Coloproctol. (2016) 20(7):461–6. doi: 10.1007/s10151-016-1481-z
2. Anita Soni (2005) Aspirin use among the adult U.S. noninstitutionalized population, with and without indicators of heart disease. Statistical Brief #179. Available at: http://meps.ahrq.gov/mepsweb/data_files/publications/st179/stat179.pdf
3. Umerah Co, Momodu II. Anticoagulation. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2022). Available at: https://www.ncbi.nlm.nih.gov/books/NBK560651/
4. Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh EY, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol. (2015) 66(21):2271–81. doi: 10.1016/j.jacc.2015.09.024
5. Pata F, Sgró A, Ferrara F, Vigorita V, Gallo G, Pellino G. Anatomy, physiology and pathophysiology of haemorrhoids. Rev Recent Clin Trials. (2021) 16(1):75–80. doi: 10.2174/1574887115666200406115150
6. Ozdil B, Akkiz H, Sandikci M, Kece C, Cosar A. Massive lower gastrointestinal hemorrhage secondary to rectal hemorrhoids in elderly patients receiving anticoagulant therapy: case series. Dig Dis Sci. (2010) 55(9):2693–4. doi: 10.1007/s10620-009-1043-6
7. Cundiff DK. Clinical evidence for rebound hypercoagulability after discontinuing oral anticoagulants for venous thromboembolism. Medscape J Med. (2008) 10(11):258.19099008
8. Genewein U, Haeberli A, Straub PW, Beer JH. Rebound after cessation of oral anticoagulant therapy: the biochemical evidence. Br J Haemotol. (1996) 1:479–85. doi: 10.1046/j.1365-2141.1996.d01-1499.x
9. Harenberg J, Haas R, Zimmermann R. Plasma hypercoagulability after termination of oral anticoagulants. Thromb Res. (1983) 15:627–33. doi: 10.1016/0049-3848(83)90217-7
10. Sise HS, Moschos CB, Gauthier J, Becker R. The risk of interrupting long-term anticoagulant treatment a rebound hypercoagulable state following hemorrhage. Circulation. (1961) 1:1137–42. doi: 10.1161/01.CIR.24.5.1137
11. Ho PM, Peterson ED, Wang L, Magid DJ, Fihn SD, Larsen GC, et al. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA. (2008) 299(5):532–9. doi: 10.1001/jama.299.5.532
12. Maulaz AB, Bezerra DC, Michel P, Bogousslavsky J. Effect of discontinuing aspirin therapy on the risk of brain ischemic stroke. Arch Neurol. (2005) 62(8):1217–20. doi: 10.1001/archneur.62.8.1217
13. Jiang YD, Liu Y, Wu JD, Li GP, Liu J, Hou XH, et al. Massive gastrointestinal bleeding after endoscopic rubber band ligation of internal hemorrhoids: a case report. World J Clin Cases. (2022) 10(19):6656–63. doi: 10.12998/wjcc.v10.i19.6656
14. Marshman D, Huber PJ, Timmerman W, Simonton CT, Odom FC, Kaplan ER. Hemorrhoidal ligation. A review of efficacy. Dis Colon Rectum. (1989) 32:369–71. doi: 10.1007/BF02563683
15. Albuquerque A. Rubber band ligation of hemorrhoids: a guide for complications. World J Gastrointest Surg. (2016) 8(9):614–20. doi: 10.4240/wjgs.v8.i9.614
16. Garg P. Novel nonsurgical treatment of intractable bleeding in hemorrhoid patients on anticoagulants. Surg Innov. (2018) 25(4):423. doi: 10.1177/1553350618775527
17. Lawes DA, Palazzo FF, Clifton MA. The use of ligasure haemorrhoidectomy in patients taking oral anticoagulation therapy. Colorectal Dis. (2004) 6(2):111–2. doi: 10.1111/j.1463-1318.2004.00580.x
18. Bracchitta S, Bracchitta LM, Pata F. Combined rubber band ligation with 3% polidocanol foam sclerotherapy (ScleroBanding) for the treatment of second-degree haemorrhoidal disease: a video vignette. Colorectal Dis. (2021) 23(6):1585–6. doi: 10.1111/codi.15613
19. Pata F, Gallo G, Pellino G, Vigorita V, Podda M, Di Saverio S, et al. Evolution of surgical management of hemorrhoidal disease: an historical overview. Front Surg. (2021) 8:727059. doi: 10.3389/fsurg.2021.727059
20. Pata F, Bracchitta LM, D'Ambrosio G, Bracchitta S. Sclerobanding (combined rubber band ligation with 3% polidocanol foam sclerotherapy) for the treatment of second- and third-degree hemorrhoidal disease: feasibility and short-term outcomes. J Clin Med. (2021) 11(1):218. doi: 10.3390/jcm11010218
21. Corman ML. Colon and rectal surgery. 5th ed. Philadelphia: Lippincott Williams & Wilkins (2002). 192.
22. Gordon PH, Nivatongs S. Principles and practice of surgery for the colon, rectum and anus. 2nd St. Louis: Quality Medical Publishing (1999).
23. Beattie GC, Rao MM, Campbell WJ. Secondary haemorrhage after rubber band ligation of haemorrhoids in patients taking clopidogrel–a cautionary note. Ulster Med J. (2004) 73(2):139–41.15651778
24. Pigot F, Juguet F, Bouchard D, Castinel A, Vove JP. Prospective survey of secondary bleeding following anorectal surgery in a consecutive series of 1,269 patients. Clin Res Hepatol Gastroenterol. (2011) 35(1):41–7. doi: 10.1016/j.gcb.2010.10.001
25. Nelson RS, Ewing BM, Ternent C, Shashidharan M, Blatchford GJ, Thorson AG. Risk of late bleeding following hemorrhoidal banding in patients on antithrombotic prophylaxis. Am J Surg. (2008) 196(6):994–9. doi: 10.1016/j.amjsurg.2008.07.036
26. Pigot F, Juguet F, Bouchard D, Castinel A. Do we have to stop anticoagulant and platelet-inhibitor treatments during proctological surgery? Colorectal Dis. (2012) 14(12):1516–20. doi: 10.1111/j.1463-1318.2012.03063.x
27. Martin G, Chatellier G, Beaussier H, de Parades V. Secondary bleeding following proctological surgery: rare but potentially severe. J Visc Surg. (2021) 158(6):462–8. doi: 10.1016/j.jviscsurg.2020.11.007
28. Taieb S, Atienza P, Zeitoun JD, Taouk M, Bourguignon J, Thomas C, et al. Frequency and risk factors of severe postoperative bleeding after proctological surgery: a retrospective case-control study. Ann Coloproctol. (2022) 38(5):370–5. doi: 10.3393/ac.2021.00122.0017
29. Salgueiro P, Rei A, Garrido M, Rosa B, Oliveira AM, Pereira-Guedes T, et al. Polidocanol foam sclerotherapy in the treatment of hemorrhoidal disease in patients with bleeding disorders: a multicenter, prospective, cohort study. Tech Coloproctol. (2022) 26(8):615–25. doi: 10.1007/s10151-022-02600-5
30. Di Saverio S, Pata F, Gallo G, Carrano F, Scorza A, Sileri P, et al. Coronavirus pandemic and colorectal surgery: practical advice based on the Italian experience. Colorectal Dis. (2020) 22(6):625–34. doi: 10.1111/codi.15056.7
31. Gallo G, Picciariello A, Pietroletti R, Novelli E, Sturiale A, Tutino R, et al. Sclerotherapy with 3% polidocanol foam to treat second-degree haemorrhoidal disease: Three-year follow-up of a multicentre, single arm, IDEAL phase 2b trial. Colorectal Dis. (2023) 25(3):386–95. doi: 10.1111/codi.16380
32. Sikora A, Zahra F. Nosocomial infections. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2022) Available at: https://www.ncbi.nlm.nih.gov/books/NBK559312/
Keywords: hemorrhoids, anticoagulant, antiplatelet, hemorrhoidal disease, sclerobanding, haemorrhoids, sclerotherapy, rubber band ligation
Citation: Pata F, Bracchitta LM, Nardo B, Gallo G, D’Ambrosio G and Bracchitta S (2023) Sclerobanding in the treatment of second and third degree hemorrhoidal disease in high risk patients on antiplatelet/anticoagulant therapy without suspension: a pilot study. Front. Surg. 10:1290706. doi: 10.3389/fsurg.2023.1290706
Received: 7 September 2023; Accepted: 9 October 2023;
Published: 6 November 2023.
Edited by:
Roberto Peltrini, University of Naples Federico II, ItalyReviewed by:
Argyrios Ioannidis, Athens Medical Group, Greece© 2023 Pata, Bracchitta, Nardo, Gallo, D’Ambrosio and Bracchitta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Pata ZnJhbmNlc2NvLnBhdGFAZ21haWwuY29t
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.