- Department of Breast and Thyroid Surgery, Guiqian International Hospital, Guiyang, China
Chylous fistula is a common postoperative complication for the head and neck surgery, thoracic and upper gastrointestinal surgery, but it rarely happens after breast surgery. There are few reports of chylous fistula after breast benign tumor resection according to literature retrieval. To our acknowledgement, this is the first case report of chylous fistula after breast fibroadenoma resection.
Introduction
Chylous fistula usually occurs after radical neck dissection and thoracic surgery, but it seldom happens after breast surgery. Its incidence is 0.5%–8.3% in patients receiving radical neck dissection (1), 0.36%–0.84% in patients receiving breast cancer surgery (2). In this article, we reported a case of chylous fistula after right breast fibroadenoma resection without axillary dissection, and summarized the etiology, prevention and treatment strategy for the patient.
Case report
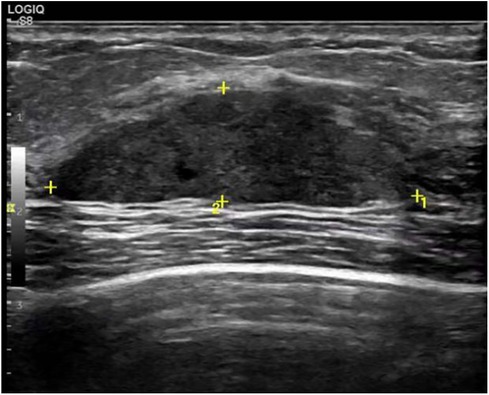
A 29-year-old woman was admitted to Guiqian International Hospital because of a palpable lump on the right breast for 3 months. She had no special medical history. High-frequency ultrasound showed a 3.90 cm × 1.21 cm × 4.22 cm solid phyma in the upper outer quadrant of the right breast, with clear boundary and regular shape, and no significant enlargement of axillary lymph nodes (Figure 1). The magnetic resonance imaging (MRI) of the breast indicated a 3.65 cm × 1.10 cm × 4.01 cm nodular lesion of abnormal enhancement at approximately 10 o'clock in the right breast, with clear boundary and slightly irregular shape (Figure 2). Breast phyma resection was applied under local infiltration anesthesia. Due to the large size of the lump and the wide range of residual cavity in the surgical area, closed suction drainage was placed in the surgical area to prevent fluid accumulation. Postoperative pathology confirmed the diagnosis of breast fibroadenoma, adenosis and metaplasia of the sweat gland. On postoperative day 2, 90 ml of milky liquid was observed in the closed suction drainage (Figure 3). Subsequently, a serologic test for celiac disease proved to be positive. Therefore, conservative treatment was given, including compressive bandage and a low-fat diet. Meanwhile, a new method of dressing change was used, i.e., repeated rinsing with hydrogen peroxide and physiological saline in the surgical area, and multiple local injections of hypertonic syrup and tuberculin pure protein derivatives from the drainage tube with a syringe, in order to promote local adhesion and reduce the risk of fluid accumulation. However, the outcome was not satisfactory and daily drainage was 50–120 ml/day for 11 days. After obtaining the consent of the patient, we performed a reoperation to explore the surgical area. During operation, we removed necrotic tissues, and carefully searched for the leakage, but unfortunately we couldn't find it. Therefore, we performed segmental ligation on fresh granulation tissue after removing necrotic tissue to achieve the goal of ligating potential leakage sites and further promote fibrosis of the tissue (Figure 4). Finally, the incision was closed, with one drainage tube replaced. After reoperation, daily drainage dropped to 30–75 ml/day for 10 days, suggesting that chylous leakage continued but with decreased volume. The color Doppler ultrasound showed a small amount of fluid accumulated in the surgical area (Figure 5) and then the drain tube was removed on postoperative day 11. After extubation, there was a little fluid in the surgical area, which was relieved by compressive bandage and a low-fat diet. On postoperative day 13, the patient was discharged uneventfully.
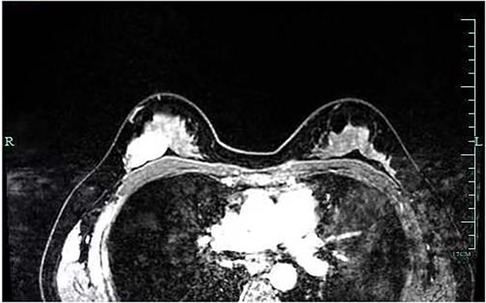
Figure 2. Breast MRI indicated a nodular lesion of abnormal enhancement, with clear boundary and slightly irregular shape.
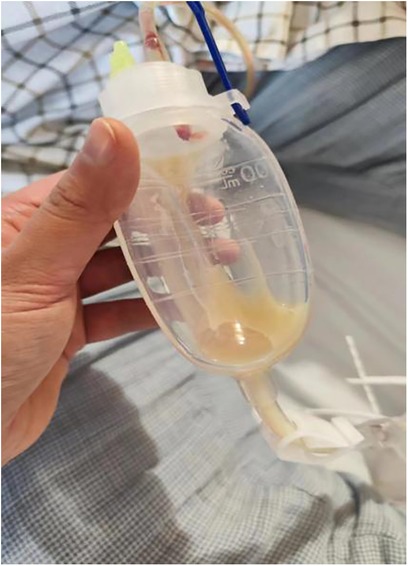
Figure 3. On postoperative day 2, there was milky fluid in the drainage tube, suggesting the existence of chylous fistula.
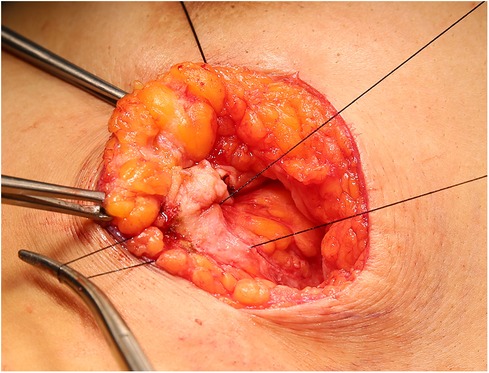
Figure 4. During operation, we performed segmental ligation on of fresh granulation tissue after removing necrotic tissue.
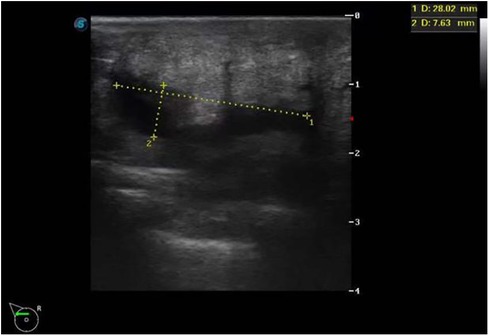
Figure 5. Color Doppler ultrasound indicated a small amount of fluid accumulated in the surgical area.
Discussion
Chylous fistula is a rare complication that, if manifested, can exacerbate the patients' pain and psychological distress, prolong hospital stay, and increase financial burden. Its diagnosis and treatment is challenging in clinic. The literature shows that chylous fistula is primarily observed after axillary dissection for left breast cancer (3–5), whereas chylous fistula after right axillary dissection is infrequent (2, 6, 7). A few cases of chylous fistula following axillary sentinel lymph node biopsy have been reported (6, 8). However, a comprehensive literature review indicates that chylous fistula is generally associated with breast surgery involving axillary procedures, with only a few isolated cases not involving such procedures (9–11). To the best of our knowledge, no case of chylous fistula following benign breast tumor resection without axillary dissection has been reported yet.
In anatomy, the thoracic duct starts from the cisterna chyli, enters the thoracic cavity through the aortic hiatus, ascends to the level of the fifth thoracic vertebra, inclines to the left, and ascends along the left front of the spine, to receive the lymph of the lower limbs, pelvis, abdomen, left upper limb, left chest, head and neck. The right lymphatic duct drains lymph from the right upper limb, right chest, head and neck. In some cases, the thoracic duct bifurcates into right and left branches during its course through the thoracic region: the left branch proceeds in the conventional manner, while the right branch terminates by joining the right subclavian vein, in conjunction with the right lymphatic duct (12). A documented case exists in the literature wherein the thoracic duct deviated from its usual course and persisted on the right side, ultimately terminated into the right internal jugular vein (13). The lateral and upper lymphatic vessels of the breast converge to form the subclavian trunk and neck trunk, and the right side flows into the right lymphatic duct and the left side into the thoracic duct. Based on their relationship with the breast parenchyma, the lymphatic vessels draining from the breast to the axilla can be classified into two groups: the deep system and the superficial system, each with its own independent lymphatic drainage pathways to the armpits. Suami et al. (14) reported that in the breast region, most of the lymph collectors run between the dermis and the breast tissue and there are also perforating lymphatic vessels in the breast gland itself. The lymphatic plexus under the areola, the dermis, subcutaneous lymphatic vessels, and lymphatic vessels in the gland compose a widely interconnected lymphatic network.
The underlying mechanism of axillary chylous fistula remains unclear yet. According to some studies, chylous fistula in the axilla can be caused by the injury of the abnormal branch of thoracic duct draining the left axilla (5), or the the subclavian duct in the right axilla (15). Langford et al.'s (12) study showed that the path of the thoracic duct from the tracheo-oesophageal groove to the venous angle remained constant, and in all 24 cadavers the duct approached the venous angle from behind and terminated within 1 cm of the venous angle, which was also previously reported by Parsons and Sargent (16) and by Van Pernis (17). The usual termination of the thoracic duct is single-sited, and the most common site is the jugulo-subclavian angle (12, 18). Therefore, we concluded that the injury to the thoracic duct or its branches may not result in chylous fistula, but injury to the subclavian duct or its tributary during axillary dissection can cause chylous fistula, which is consistent with the viewpoint of Singh et al. (15).
In this case, we only removed the breast fibroadenoma, without axillary lymph node dissection. Her chylorrhea may be attributed to an altered thoracic duct or cervical lymphatic duct injury. According to our experience of neck surgery, if the thoracic duct, the left cervical lymphatic duct, or its main branches, are directly injured intraoperatively, chylous leakage volume will be higher than 1,000 ml per day. The maximum daily drainage of this patient was 120 ml, so it suggested that the chylorrhea was caused by damage to the secondary branch of the left cervical lymphatic duct through the breast tissue, rather than the left cervical lymphatic duct or this primary branch.
Most of chylous fistula patients are treated by conservative management, consisting of a fat-free diet allowing only medium-chain fatty acids, adequate rest, closed drainage, and pressure dressings (15, 19). Although there is growing evidence that nutrition plays a role in the management of chylous fistula, it is hard to determine which dietary is more effective (20). Refractory cases of lymphatic and chylous fistula have been treated with octreotide (a long-acting synthetic analogue of somatostatin) and orlistat (a pancreatic lipase inhibitor) (21, 22).
Surgical intervention is preferred for patients with high output chylous fistula,, leakage persists for more than 2 weeks, daily drainage volume is continuously over 1 L for 1 week, or patients present metabolic complications (9, 15, 19).
Since this patient's chylorrhage had persisted for nearly 2 weeks, we scheduled a reoperation to find the damaged lymphatic vessels, but failed during operation. Unlike chylous leakage in the neck surgery, fluid volume was extremely limited. Finally, we removed the suspected necrotic tissue and re-sutured the mammary gland tissue to reduce the residual cavity.
Meanwhile, we adopted a new method of dressing change, to be specific, multiple local injections of hypertonic syrup and tuberculin pure protein were given to emanate aseptic inflammatory reactions, cause local adhesion and promote the healing. But the outcome was not satisfactory. Fortunately, the patient's drainage was slowly decreased, and she was eventually discharged on postoperative day 13.
Conclusion
We effectively managed chylous fistula following benign breast tumor resection through a carefully planned regimen. Timely diagnosis and surgical interventions are crucial in the treatment, especially for the cases that chylous output remains low but persists for a long time (e.g., 2 weeks). Furthermore, breast surgeons should have an in-depth understanding of breast lymphatic anatomy and beware of potential anatomical variations, which is essential for the identification and appropriate management of this complication.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Guiqian International Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HL: Data curation, Supervision, Writing – original draft, Writing – review & editing. LL: Writing – original draft, Writing – review & editing. XY: Writing – review & editing, Data curation, Methodology, Resources, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Park I, Her N, Choe JH, Kim JS, Kim JH. Management of chyle leakage after thyroidectomy, cervical lymph node dissection, in patients with thyroid cancer. Head Neck. (2018) 40(1):7–15. doi: 10.1002/hed.24852
2. Daggett JD, Watt AW, Smith PD. Chyle leak following right axillary lymph node dissection: a case report and review of current literature. Int J Surg Case Rep. (2016) 20:68–73. doi: 10.1016/j.ijscr.2015.12.044
3. Yin L, Chen PQ, Agyekum EA, Xiao XD, Qian XQ. Chylous leakage after breast conserving surgery and axillary clearance: case report and management strategies. Front Oncol. (2022) 29(12):878645. doi: 10.3389/fonc.2022.878645
4. Donkervoort SC, Roos D, Borgstein PJ. A case of chylous fistula after axillary dissection in breast-conserving treatment for breast cancer. Clin Breast Cancer. (2006) 7(2):171–2. doi: 10.3816/CBC.2006.n.030
5. Taylor J, Jayasinghe S, Barthelmes L, Barthelmes L, Chare M. Chyle leak following axillary lymph node clearance—a benign complication: review of the literature. Breast Care (Basel). (2011) 6(2):130–2. doi: 10.1159/000327507
6. Malik M, Ruiz DE, Annamalai A, Lee S, Karsif K. Chyle fistula after simple mastectomy and sentinel lymph node biopsy: a rare occurrence. Breast J. (2012) 18(5):488–90. doi: 10.1111/j.1524-4741.2012.01295.x
7. Cong MH, Liu Q, Zhou WH, Zhu J, Song CX, Tian XS. Six cases of chylous leakage after axillary lymph node dissection. Onkologie. (2008) 31(6):321–4. doi: 10.1159/000131218
8. Al-Ishaq Z, Gupta S, Collins MA, Sircar T. Chyle leak following an axillary sentinel lymph node biopsy for breast cancer in a patient with superior vena caval thrombosis—a case report and review of the literature. Ann R Coll Surg Engl. (2018) 100(6):e147–9. doi: 10.1308/rcsann.2018.0074
9. Long SR, Butterworth JA. Chyle leak following autologous breast reconstruction a rare complication of a deep inferior epigastric artery perforator flap. Ann Plast Surg. (2019) 82(2):193–5. doi: 10.1097/SAP.0000000000001639
10. Nakatsuka K, Karakawa R, Fuse Y, Yoshimatsu H, Yano T. Donor-site chyle leakage after breast reconstruction using a deep inferior epigastric artery perforator flap. Plast Reconstr Surg Glob Open. (2022) 10(10):e4612. doi: 10.1097/GOX.0000000000004612
11. Tam D, Scamp T, Tan J. Chyle leak after breast augmentation. Aesthe Surg J. (2009) 29(2):113–5. doi: 10.1016/j.asj.2009.01.015
12. Langford RJ, Daudia AT, Malins TJ. A morphological study of thoracic duct at the jugulo-subclavian junction. J Craniomaxillofac Surg. (1999) 27(2):100–4. doi: 10.1016/s1010-5182(99)80021-3
13. Gobblieb MI, Greenfield J. Variations in the terminal portion of the human thoracic duct. AMA Arch Surg. (1956) 73(6):955–9. doi: 10.1001/archsurg.1956.01280060055012
14. Suami H, Pan WR, Mann GB, Taylor GI. The lymphatic anatomy of the breast and its implications for sentinel lymph node biopsy: a human cadaver study. Ann Surg Oncol. (2008) 15(3):863–71. doi: 10.1245/s10434-007-9709-9
15. Singh M, Deo SVS, Shukla NK, Pandit A. Chylous fistula after axillary lymph node dissection: incidence, management, and possible cause. Clin Breast Cancer. (2011) 11(5):320–4. doi: 10.1016/j.clbc.2011.04.003
16. Parsons SG, Sargent PW. On the termination of the thoracic duct. Lancet. (1909) 1:1173–4. doi: 10.1016/S0140-6736(00)66967-2
18. Shimada K, Sato I. Morphological and histological analysis of the thoracic duct at the jugulo-subclavian junction in Japanese cadavers. Clin Anat. (1997) 10(3):163–72. doi: 10.1002/(SICI)1098-2353(1997)10:3%3C163::AID-CA2%3E3.0.CO;2-V
19. Farkas N, Wong J, Monib S, Thomson S. A systematic review of chyle leaks and their management following axillary surgery. Eur J Surg Oncol. (2020) 46(6):931–42. doi: 10.1016/j.ejso.2020.01.029
20. Steven BR, Carey S. Nutritional management in patients with chyle leakage: a systematic review. Eur J Clin Nutr. (2015) 69(7):776–80. doi: 10.1038/ejcn.2015.48
21. Delaney SW, Shi H, Shokrani A, Sinha UK. Management of chyle leak after head and neck surgery: review of current treatment strategies. Int J Otolaryngol. (2017) 2017:1–12. doi: 10.1155/2017/8362874
Keywords: chylous fistula, breast, fibroadenoma, surgery, case report
Citation: Liu H, Liu L and Yang X (2024) Chylous fistula after right breast fibroadenoma resection: a case report. Front. Surg. 10:1269301. doi: 10.3389/fsurg.2023.1269301
Received: 2 August 2023; Accepted: 8 December 2023;
Published: 4 January 2024.
Edited by:
Xiaowei Qi, Army Medical University, ChinaReviewed by:
Xiaodong Zheng, Chongqing University, ChinaEnock Adjei Agyekum, Jiangsu University Affiliated People’s Hospital, China
© 2024 Liu, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haoxi Liu NzE1MTQxMjc0QHFxLmNvbQ==
 Haoxi Liu*
Haoxi Liu* Liping Liu
Liping Liu