- 1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 2School of Public Health, Xi'an Jiaotong University Health Science Center, Xi'an, China
Aims: To explore the clinical characteristics of patients with symptomatic duodenal diverticula and to generalize how to make appropriate treatment choices for this group of patients.
Materials and methods: From January 2010 to September 2020, a total of 647 patients with duodenal diverticula (DD) were included in this study. 345 of them with relevant symptoms were divided into the symptomatic group and the other 302 patients were in the asymptomatic group.
Results: Among all patients, most DD were located in the periampullary area, <1 cm in size, and single in number. The distribution of DD localized in the 2nd portion/periampullary (P = 0.002/P < 0.001) and with a 1 cm size cut-off value (P = 0.003) was significantly different between the symptomatic and asymptomatic groups. Multivariate Logistics analysis further suggests that diverticular size (<1 cm, 1–3 cm) and combined biliary comorbidities (bile duct stones and gallstones, primary bile duct stones, cholangitis without bile duct stones) may be factors influencing the choice of treatment modality. Of all patients undergoing surgical treatment, a total of 7 cases developed various postoperative complications, and no one died.
Conclusions: Patients with DD ≥1 cm or located in the periampullary were more likely to be symptomatic. The specific size of the DD and the combination of specific biliary comorbidities may have an impact on the choice of treatment modality.
Introduction
Diverticulum is usually manifested as part of the intestinal wall structure herniated out due to the muscular layer defect, forming a pouch structure (1). Duodenal diverticula (DD) is one of the most common digestive tract diverticula (2), which was first discovered and reported by French pathologist Chomel. There are 70%–75% of DD that occurs in the radius of 2–3 cm around the ampullary of Vater (3), so it is also called periampullary duodenal diverticula (PAD). Detection rates for PAD under endoscopic retrograde cholangiopancreatography (ERCP) ranged from 5.1% to 32.8% (4–8), and from 23.0% to 32.0% at autopsy (9). PAD is more common among middle-aged and elderly people, and there is no obvious gender difference.
Generally, no special treatment is required for patients with asymptomatic DD. Only about 5% (10) of DD patients may show symptoms such as epigastric pain, nausea, and vomiting due to diverticulitis or diverticular perforation/hemorrhage. DD, especially PAD, may be associated with various biliopancreatic complications such as choledocholithiasis, pancreatitis, and common bile duct obstruction, and may accompany various relevant symptoms. Such as Lemmel's syndrome (11), which is defined as obstructive jaundice due to a PAD in the absence of choledocholithiasis or a neoplasm. At present, the studies on DD mostly focus on its association with biliopancreatic diseases or endoscopic therapy (12–14), while there are few reports on when and how to deal with symptomatic DD patients. Therefore, we designed this retrospective study aims to explore the clinical characteristics of symptomatic patients and to generalize how to make appropriate treatment choices for this group of patients.
Materials and methods
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University.
Patients with radiographic findings or clinical diagnosis of DD were searched through the PACS (picture archiving and communication system) and the medical record system for a decade (from January 2010 to September 2020), and a total of 831 patients were initially identified. Then we retrieved and recorded all patients' clinical data one by one with the hospital ID number identification, including basic characteristics like (1) name, sex, age, and other baseline characteristics; (2) the location, size, and number of DD; (3) clinical signs and symptoms, complications, and other positive imaging examinations; (4) treatment modalities and outcomes, etc. Patients who met one or more of the following exclusion criteria were excluded: (1) outpatients or those without complete data of hospitalization; (2) patients with clinically confirmed pseudo-DD (Ulcers, artifacts, etc.); (3) lack of reliable imaging data to confirm the existence of DD; (4) repeated patients. Figure 1 showed the Flow diagram of inclusion in the study.
All imaging data (Supplementary Figure S1) were retrieved from the picture archiving and communication system (PACS) of our hospital. The size of DD was evaluated on coronal reconstructed images at the PACS monitor using electronic calipers, and the size of the DD is based on the longest dimensions measured. To minimize the error caused by human factors as much as possible, two researchers independently conducted the data collection and collation stage, and finally summarized the data after comparison.
Statistical analysis was performed using SPSS 22.0 software (SPSS, Chicago, IL, USA). Measurement data with normal distribution were expressed as mean ± standard deviation while the non-normal distribution data are described by the median, and a t-test was used for comparison. The counting data were expressed as the number of cases and percentage, and the chi–square test was used for comparison. Univariate analysis and multivariate analyses (logistic regression) were used to judge the association between various parameters and specific outcome events. P value < 0.05 was considered statistically significant.
Results
After excluding 184 patients based on the exclusion criteria, a total of 647 patients with DD were enrolled in this study. And 345 patients with all kinds of relevant symptoms were divided into the symptomatic group, the remaining 302 patients were in the asymptomatic group. Patients in the symptomatic group were divided into different subgroups according to the treatment modality, and those who received surgical treatment were further differentiated by different procedures.
Of all 345 patients in the symptomatic group, the percentage of their clinical symptoms and the percentage of patients with a different number of comorbid symptoms were shown in Figure 2. The most common symptoms of them were epigastric pain (21.2%), next by nausea (13.8%), vomiting (11.8%), abdominal distension (11.0%), etc. Nearly half of the patients (172, 49.8%) had 3 or more symptoms at the same time, and the fewest patients (82, 23.8%) had a single symptom.
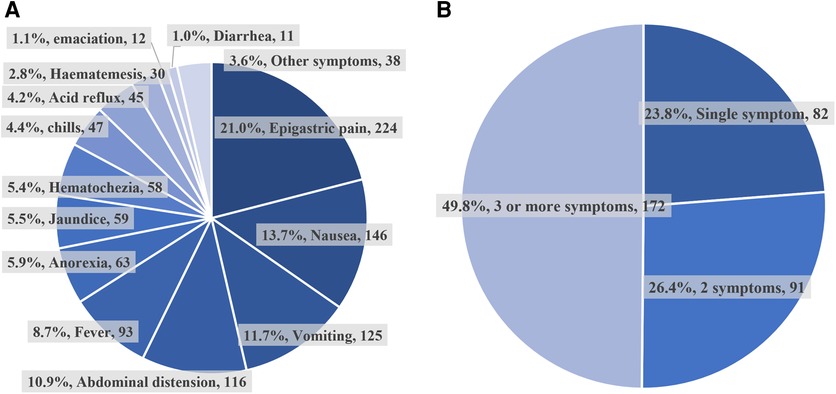
Figure 2. The ratio of different symptomatic types and numbers. (A) The percentage of different clinical symptoms in the symptomatic group. (B) The percentage of patients with a different number of symptoms in the symptomatic group.
Differences in baseline characteristics between patients with DD in the symptomatic and asymptomatic groups
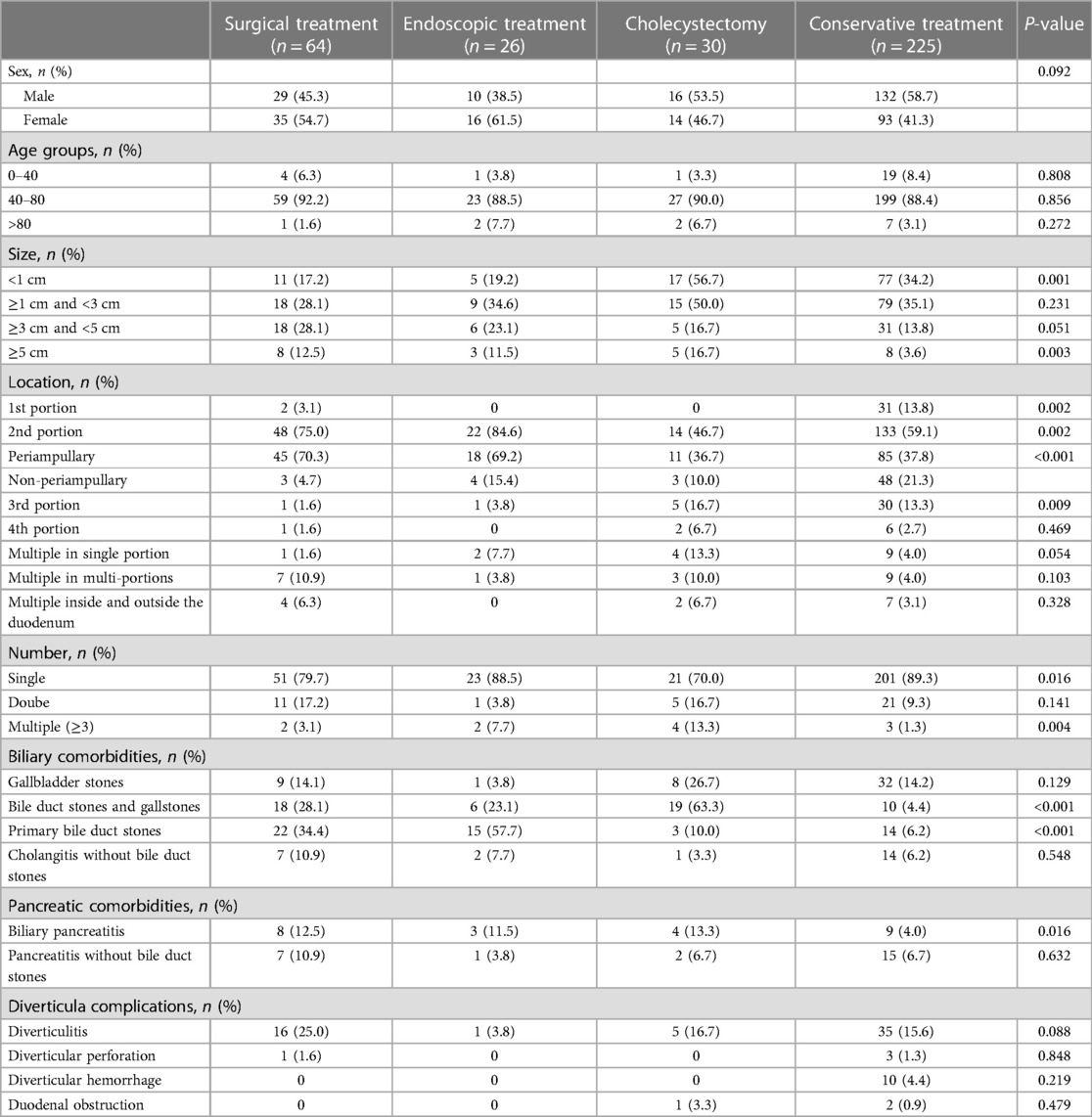
Among all patients 359 (55.5%) were male and 288 (44.5%) were female, with a male-to-female ratio of 1.25:1. The mean age of all symptomatic group patients was younger than that of the asymptomatic group (60.01 ± 13.79 vs. 61.12 ± 13.43 years, P = 0.303). And the majority of patients with DD were first diagnosed in the 40–80 age group, both in the symptomatic group and asymptomatic group (Table 1).
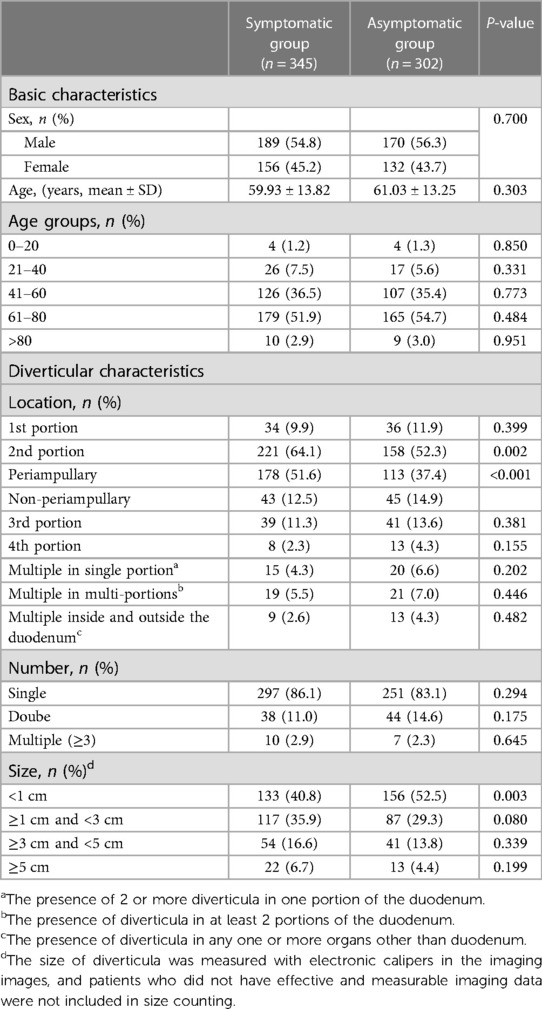
Table 1. Distribution of diverticular patient in baseline characteristics between the symptomatic and asymptomatic groups.
The most common location of DD was the 2nd portion, which showed a statistically significant difference between the two groups (P = 0.002). Also, the distribution of patients with PAD was statistically significant between the two groups (P < 0.001). We have also found that the majority of patients (84.7%) present with only single DD, with three or more DD occurring very rarely (2.6%). As for the size of DD, more patients (44.7%) had diverticula <1 cm in diameter. Further, the distribution of DD <1 cm and ≥1 cm was significantly different (P = 0.003) in the symptomatic and asymptomatic groups. Therefore, our results suggest that the location and size of a particular DD may be critical to the presence of symptoms.
Distribution of different biliopancreatic comorbidities and diverticular complications in symptomatic patients with 2nd portion DD
Given the significant differences in the distribution of DD located in the 2nd portion, periampullary, and with a cut-off value of 1 cm in size between the symptomatic and asymptomatic groups. To further corroborate this finding, we further explored the possible clinical sources of symptoms in patients with DD in specific sites and sizes, as the combination of various biliopancreatic comorbidities and diverticular complications.
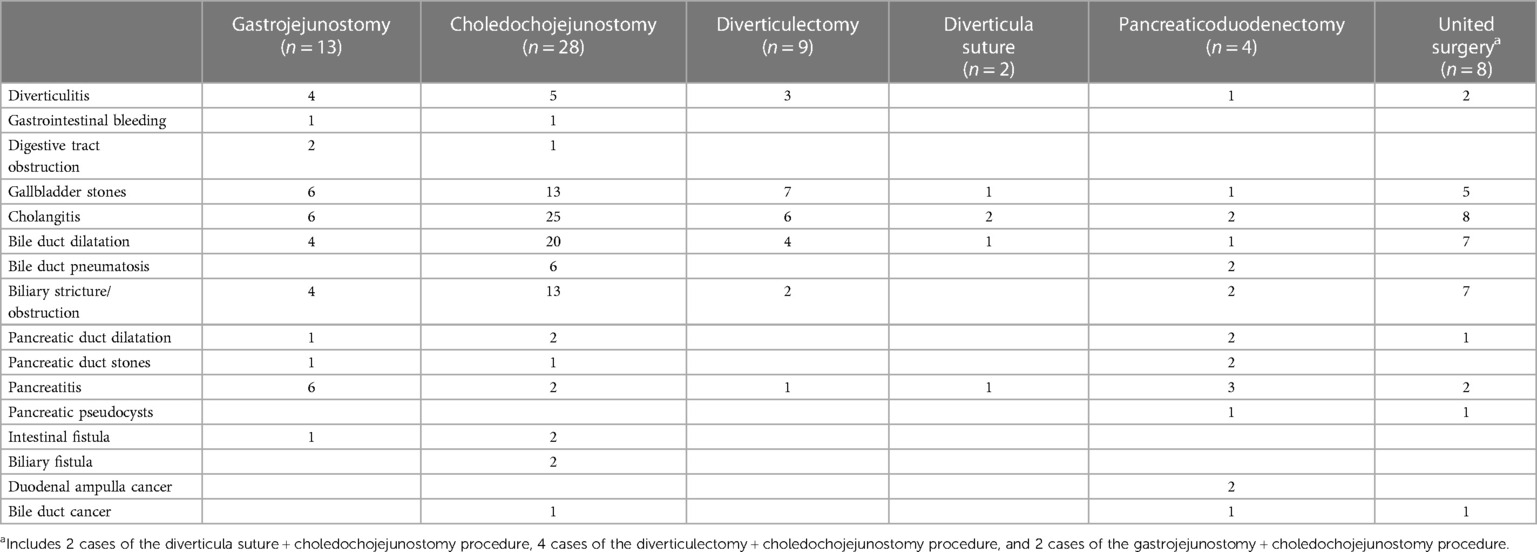
Our results show that various comorbidities and complications seem to be more frequent in the group of DD ≥1 cm, especially when the PAD and ≥1 cm (Table 2). Some biliopancreatic comorbidities (bile duct stones and gallstones, P = 0.034; primary bile duct stones, P = 0.045; pancreatitis without bile duct stones, P = 0.044) and some diverticular complications (diverticulitis, P = 0.008; diverticular hemorrhage, P = 0.026) had statistically significant differences in distribution between different groups of sites and sizes, which suggested that larger DD (≥1 cm) or/ (and) specific locations (periampullary) are more likely to have clinical manifestations that require management.
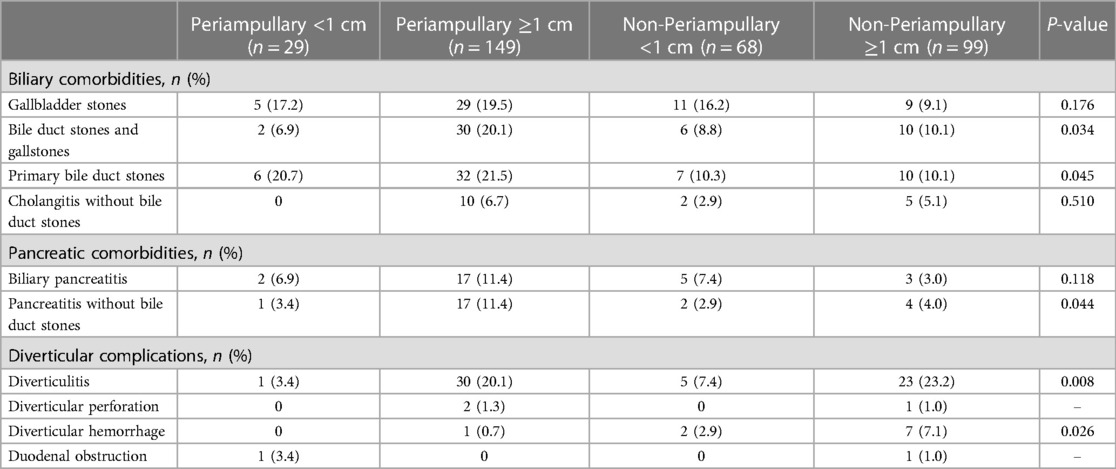
Table 2. Distribution of different comorbidities/complications in the symptomatic group of patients with 2nd portion diverticula.
Distribution of relevant clinical characteristics among different treatment modality groups of patients in the symptomatic group
After exploring the potential factors associated with symptomatic DD, we next turn to the management of this group of patients. We summarized the management of all 345 symptomatic patients, including direct surgical treatment of DD, indirect treatment of complications (endoscopic treatment or cholecystectomy), and conservative treatment. The most common treatment is conservative treatment (225, 65.2%), followed by surgical treatment (64, 18.6%) (Table 3). Univariate analysis showed a significant difference in the distribution of variables such as diverticular size (<1 cm, P = 0.001; ≥ 5 cm, P = 0.003), diverticular location (1st portion, P = 0.002; 2nd portion, P = 0.002; periampullary, P < 0.001; and 3rd portion, P = 0.009), number of diverticula (single, P = 0.016; multiple, P = 0.004), combined biliary comorbidities (bile duct stones and gallstones, P < 0.001; primary bile duct stones, P < 0.001) and pancreatic comorbidities (biliary pancreatitis, P = 0.016) between the different treatment groups.
To further confirm the findings, a multivariate analysis of variables was performed (Table 4). The results suggest that the combination of bile duct stones and gallstones, primary bile duct stones, and cholangitis without bile duct stones may be the reason for preferring surgical treatment as well as endoscopic treatment compared to conservative treatment, while DD sizes <1 cm or 1–3 cm are the possible reason for preferring conservative treatment. The above results suggest that the options of different treatments for patients with symptomatic DD may be related to the specific diverticular sizes and biliary comorbidities.
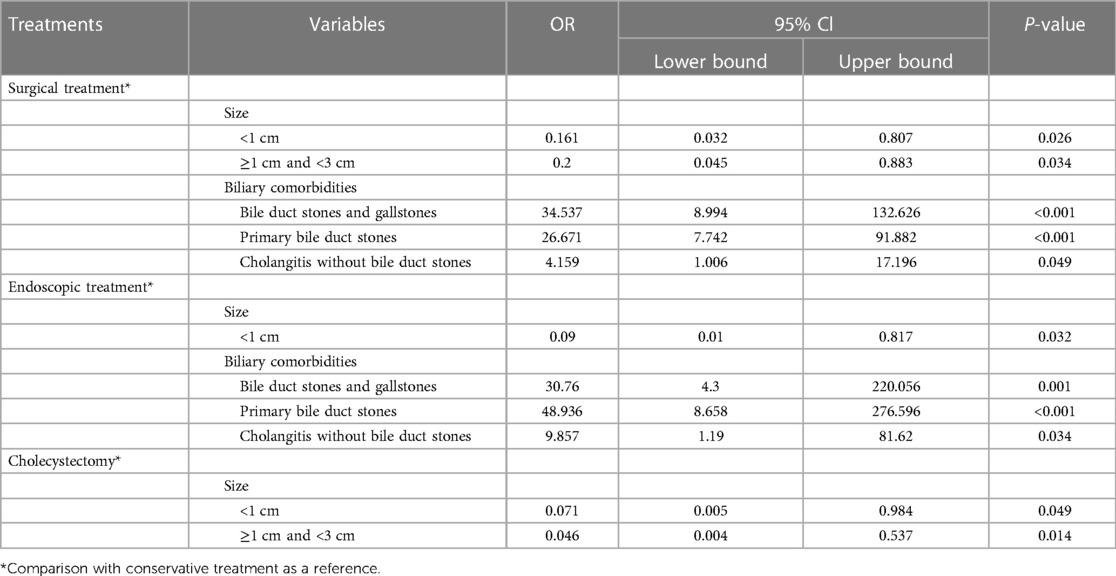
Table 4. Multivariate analysis of different treatment options for patients in the symptomatic group.
Analysis of surgical indications for patients with symptomatic DD and the corresponding complications
To make the appropriate surgical choice for patients with symptomatic DD, we reviewed the records of all 64 patients who underwent surgery and attempted to summarize the potential surgical indications for each procedure (Table 5). Our results showed that choledochojejunostomy (28, 43.8%) was the most common procedure, followed by gastrojejunostomy (13, 20.3%), diverticulectomy (9, 14.1%), etc. Patients undergoing gastrojejunostomy and diverticulectomy appear to be more commonly combined with gallbladder stones and cholangitis. As for choledochojejunostomy and united surgery, patients were more likely to have a combination of cholangitis and bile duct dilatation. There was no clear preference for the other two types of surgery, probably because of the low cases.
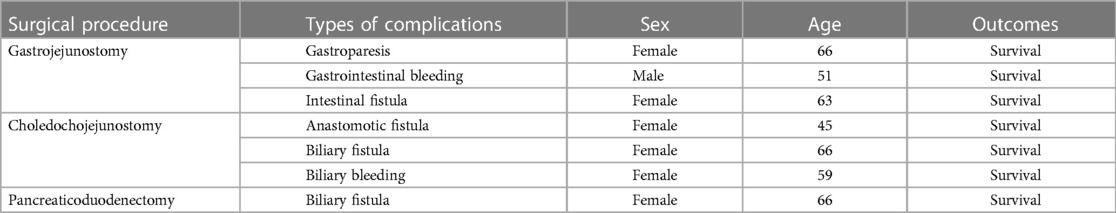
Encouragingly, the frequency of complications among patients who underwent surgical treatment was low (7/64, 10.9%). Complications are mainly from patients who have undergone gastrojejunostomy and choledochojejunostomy (Table 6), and most of them were middle-aged and elderly women. All complications were not fatal, as all patients were discharged in good condition after various conservative treatment measures, which suggests that surgical treatment of DD is safe and feasible.
Discussion
DD as a benign digestive tract disease is widely distributed in the population. Patients with DD are usually asymptomatic and therefore often receive insufficient attention (15), but in our study, more than half of the patients (53.3%) had a combination of various symptoms. Meanwhile, the most common clinical symptoms (such as epigastric pain, nausea, vomiting, and abdominal distension) of DD patients often lack specificity and are often combined with multiple symptoms at the same time, making them easy to misdiagnose in the clinic and preventing them from receiving appropriate treatment.
By comparing the differences in clinical characteristics between patients in the symptomatic and asymptomatic groups, we found that patients with DD located in periampullary and ≥1 cm seemed to be more likely to have clinical symptoms. It is well known that DD is more common in the 2nd portion and periampullary, and different hypotheses (16, 17) have also been proposed that DD in this area is more likely to be combined with various pathological states. Our results firstly provide the evidence of this association, meanwhile we suggesting a possible association between diverticular size and the presence of clinical symptoms.
Previous studies lacked a unified standard for the classification of DD size because it was difficult to accurately measure diverticular size even under endoscopy. Kim et al. (8) chose 1.5 cm and 3.0 cm as the judgment bounds, because the diameter of a commonly used endoscopic stone picking balloon was exactly 1.5 cm, and the size of PAD could be more accurately measured with this reference. Based on our precise measurements of all measurable imaging pictures, we chose 1 cm, 3 cm, and 5 cm as the measured value of diverticular size, then found that smaller DD with the size of <1 cm and 1–3 cm were the majority (78.0%). Interestingly, there were also lots of patients in our study who had no obvious clinical symptoms, so this phenomenon can be partially explained by the above conclusions, as we all know usually a small DD is less likely to cause symptoms because it has little effect on the primary physiological function.
Patients with DD usually have symptoms that do not arise directly from the diverticula, but rather from various types of diverticula-related complications and comorbidities. In addition to comorbidities such as diverticulitis, various complications related to the biliary system and the pancreas are also included. The association between DD and Biliopancreatic disorders has been confirmed by many previous studies. Karn et al. (18) recently completed a meta-analysis including 11 related studies and concluded that patients with PAD had a significantly increased risk of choledocholithiasis about 2.3 times that of normal people. Bruno et al. (19) conducted a 2,475 EUS examination on patients with PAD and showed that the prevalence of cholangitis, bile duct dilatation, and choledocholithiasis was significantly higher than those in the control group without DD.
In our study, patients with DD combined with different types of biliopancreatic comorbidities were significantly more common in PAD and DD >1 cm, again confirming our previous findings. As for the reasons for the association, there are several possible hypotheses in previous studies: (1) Mechanical pressure exerted by the diverticula on the distal portion of the common bile duct can impede bile excretion (20); (2) The diverticula may cause sphincter dysfunction of Oddi. It may be due to sphincter stenosis due to the accumulation of food or bezoar in the diverticula. Or chronic ampullary inflammation caused by diverticula can lead to chronic fibrosis of the nipple and subsequent stenosis (21); (3) Bile stasis and abnormal tension and contractile activity of Oddi sphincter may lead to the spread of overgrown bacteria in the diverticula to the biliary tract system more easily, and produce β-glucuronidase and debinding bile salts, thus forming stones (22, 23).
Given high proportion of patients in the symptomatic group in our study, the management of this group of patients deserves high attention. In our study, conservative treatment (65.2%) was predominant in the treatment of DD, which suggests that a high number of smaller-sized or single DD are not usually associated with a serious outcome. Depending on the type and severity of the complications, endoscopic treatment and cholecystectomy may be options for complications only, in addition to surgical treatments that directly target DD.
Our univariate analysis found that a greater number of clinical characteristics may be associated with the choice of treatment modality. Further, we performed a multivariate Logistics regression analysis and the results showed that patients with combined smaller-sized DD may prefer conservative treatment, while patients with combined biliary system stones and cholangitis without bile duct stones may prefer surgical treatment (direct or indirect). Previous studies on the treatment of patients with DD are scarce, and our findings will be very helpful in making decisions on the treatment for these patients.
The main surgical treatment modalities for patients with DD in previous studies (24) include diverticulectomy, duodenal resection, and diverticular inversion, and surgical treatment for DD is considered safe. In our study, DD patients were operated on more frequently with choledochojejunostomy (43.8%) and gastrojejunostomy (20.3%), next by diverticulectomy (14.1%). Based on a review of the indications for each procedure, we summarized the more frequent indications of each procedure for the reference of subsequent operators. As for postoperative complications, our results are consistent with those reported (24, 25), with a low probability of postoperative complications in diverticulosis patients (10.9%). Complications occur mostly in middle-aged and elderly female patients and are non-fatal, and all improved after conservative treatment.
As mentioned earlier, our study mainly focuses on two questions: What kind of DD patients deserve attention and management; and what kind of management is appropriate for this type of DD patient? Although it was a retrospective study, we suggest possible explanations by analyzing a large number of case data. Our study is also the only detailed and in-depth study done to date on the evaluation and management of DD.
Still, our study needs to be improved in the following aspects: groups based on DD size may be slightly biased from the true situation because we cannot ensure that the data measured by the electronic caliper is completely accurate, especially when it is influenced by the diverticular contents. More importantly, limited by the nature of retrospective studies, our answers to the two questions can only provide possible interpretations. As for the exact causal relationship, further confirmation is needed in subsequent multicenter, prospective studies.
In conclusion, DD is a common clinical pathology frequently occurring in the 2nd portion, mostly small in size and single in number. Patients with DD ≥1 cm or located in the periampullary are more likely to be combined with various types of comorbidities and complications, thus presenting as symptomatic. The size of the DD and the combination of specific biliary comorbidities may have an impact on the choice of treatment modality. Although most patients with symptomatic DD can be treated conservatively only, surgical treatment is also a safe and effective approach when the appropriate procedure is chosen. Our findings will provide important ideas for the clinical diagnosis and treatment of DD, but further prospective studies are needed to confirm that.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JR: Conceptualization, Writing – review & editing, Data curation, Methodology, Resources, Writing – original draft. JD: Conceptualization, Data curation, Methodology, Writing – review & editing, Software. TS: Formal Analysis, Writing – review & editing, Methodology. SW: Conceptualization, Formal Analysis, Methodology, Writing – review & editing. FC: Data curation, Investigation, Writing – review & editing. JL: Conceptualization, Formal Analysis, Writing – review & editing. ZW: Project administration, Supervision, Writing – review & editing. LH: Methodology, Writing – original draft, Writing – review & editing, Supervision. ZW: Conceptualization, Project administration, Validation, Writing – original draft, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (No.82203756) and Innovation Capacity Support Plan of Shaanxi Province (No. 2022PT-35) and Clinical research project of the First Affiliated Hospital of Xi'an Jiaotong University (No.XJTU1AF-CRF-2022-034).
Acknowledgments
The authors thank the clinicians of the Department of Radiology at the First Affiliated Hospital of Xi'an Jiaotong University for conducting the image assessment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1267436/full#supplementary-material
References
1. Cunningham SC, Gannon CJ, Napolitano LM. Small-bowel diverticulosis. Am J Surg. (2005) 190:37–8. doi: 10.1016/j.amjsurg.2005.03.024
2. Whitcomb JG. Duodenal diverticulum. A clinical evaluation. Arch Surg. (1964) 88:275–8. doi: 10.1001/archsurg.1964.01310200113023
3. Lobo DN, Balfour TW, Iftikhar SY, Rowlands BJ. Periampullary diverticula and pancreaticobiliary disease. Brit J Surg. (1999) 86:588–97. doi: 10.1046/j.1365-2168.1999.01121.x
4. Boix J, Lorenzo-Zúñiga V, Añaños F, Domènech E, Morillas RM, Gassull MA. Impact of periampullary duodenal diverticula at endoscopic retrograde cholangiopancreatography: a proposed classification of periampullary duodenal diverticula. Surg Laparosc Endosc Percutan Tech. (2006) 16:208–11. doi: 10.1097/00129689-200608000-00002
5. Chen L, Xia L, Lu Y, Bie L, Gong B. Influence of periampullary diverticulum on the occurrence of pancreaticobiliary diseases and outcomes of endoscopic retrograde cholangiopancreatography. Eur J Gastroenterol Hepatol. (2017) 29:105–11. doi: 10.1097/MEG.0000000000000744
6. Karaahmet F, Kekilli M. The presence of periampullary diverticulum increased the complications of endoscopic retrograde cholangiopancreatography. Eur J Gastroenterol Hepatol. (2018) 30:1009–12. doi: 10.1097/MEG.0000000000001172
7. Chen Q, Li Z, Li S, Ding X, Liu Z, Wu C, et al. Diagnosis and treatment of juxta-ampullary duodenal diverticulum. Clin Invest Med. (2010) 33:E298–303. doi: 10.25011/cim.v33i5.14355
8. Kim CW, Chang JH, Kim JH, Kim TH, Lee IS, Han SW. Size and type of periampullary duodenal diverticula are associated with bile duct diameter and recurrence of bile duct stones. J Gastroenterol Hepatol. (2013) 28:893–8. doi: 10.1111/jgh.12184
9. Jones TW, Merendino KA. The perplexing duodenal diverticulum. Surgery. (1960) 48:1068–84.13790597
10. Schnueriger B, Vorburger SA, Banz VM, Schoepfer AM, Candinas D. Diagnosis and management of the symptomatic duodenal diverticulum: a case series and a short review of the literature. J Gastrointest Surg. (2008) 12:1571–6. doi: 10.1007/s11605-008-0549-0
11. Bakula B, Romic M, Bakula M, Romic I. Duodenal diverticulum causing obstructive jaundice—lemmel's syndrome. Rev Esp Enferm Dig. (2021) 113:375–6. doi: 10.17235/reed.2020.7516/2020
12. Lee JJ, Brahm G, Bruni SG, Thipphavong S, Sreeharsha B. Biliary dilatation in the presence of a periampullary duodenal diverticulum. Br J Radiol. (2015) 88:20150149. doi: 10.1259/bjr.20150149
13. Namikawa T, Kawanishi Y, Fujisawa K, Munekage E, Munekage M, Maeda H, et al. Juxtapapillary duodenal diverticulum impacted with enterolith. J Gastrointest Surg. (2017) 21:920–2. doi: 10.1007/s11605-016-3271-3
14. Morosin T, De Robles MS, Still A. Duodenal diverticulum at the site of the major papilla may be a risk factor for biliary stent migration. J Surg Case Rep. (2021) 2021:rjab079. doi: 10.1093/jscr/rjab079
15. Tyagi P, Sharma P, Sharma BC, Puri AS. Periampullary diverticula and technical success of endoscopic retrograde cholangiopancreatography. Surg Endosc. (2009) 23:1342–5. doi: 10.1007/s00464-008-0167-7
16. Guerra F, Foghetti D, Patriti A. Laparoscopic duodenal diverticulectomy via the inframesocolic route. J Gastrointest Surg. (2021) 25:1360–62. doi: 10.1007/s11605-020-04905-y
17. Ishikawa-Kakiya Y, Maruyama H, Yamamoto K, Yamamura M, Tanoue K, Ominami M, et al. Ring-shaped thread counter traction-assisted endoscopic retrograde cholangiopancreatography of a huge periampullary diverticula. Am J Gastroenterol. (2021) 116:12. doi: 10.14309/ajg.0000000000000842
18. Wijarnpreecha K, Panjawatanan P, Manatsathit W, Cheungpasitporn W, Pungpapong S, Lukens FJ, et al. Association between juxtapapillary duodenal diverticula and risk of choledocholithiasis: a systematic review and meta-analysis. J Gastrointest Surg. (2018) 22:2167–76. doi: 10.1007/s11605-018-3865-z
19. Bruno M, Ribaldone DG, Fasulo R, Gaia S, Marietti M, Risso A, et al. Is there a link between periampullary diverticula and biliopancreatic disease? An EUS approach to answer the question. Dig Liver Dis. (2018) 50:925–30. doi: 10.1016/j.dld.2018.07.034
20. Kubota Y, Yamaguchi T, Tani K, Takaoka M, Fujimura K, Ogura M, et al. Anatomical variation of pancreatobiliary ducts in biliary stone diseases. Abdom Imaging. (1993) 18:145–9.8439754
21. Tomita R, Tanjoh K. Endoscopic manometry of the sphincter of oddi in patients with lemmel's syndrome. Surg Today. (1998) 28:258–61. doi: 10.1007/s005950050117
22. Cetta FM. Bile infection documented as initial event in the pathogenesis of brown pigment biliary stones. Hepatology. (1986) 6:482–9. doi: 10.1002/hep.1840060327
23. Skar V, Skar AG, Bratlie J, Osnes M. Beta-glucuronidase activity in the bile of gallstone patients both with and without duodenal diverticula. Scand J Gastroenterol. (1989) 24:205–12. doi: 10.3109/00365528909093038
24. Mathis KL, Farley DR. Operative management of symptomatic duodenal diverticula. Am J Surg. (2007) 193:308–9. doi: 10.1016/j.amjsurg.2006.09.024
Keywords: duodenal diverticula, symptomatic patients, diverticular size, biliary comorbidities, surgical treatment
Citation: Ren J, Ding J, Su T, Wu S, Chen F, Li J, Wang Z, Han L and Wu Z (2023) Evaluation and management of symptomatic duodenal diverticula: a single-center retrospective analysis of 647 patients. Front. Surg. 10:1267436. doi: 10.3389/fsurg.2023.1267436
Received: 26 July 2023; Accepted: 21 August 2023;
Published: 30 August 2023.
Edited by:
Ahmad Mahamid, Carmel Medical Center, IsraelReviewed by:
Ivan Romic, University Hospital Centre Zagreb, CroatiaRan Cui, Tongji University, China
© 2023 Ren, Ding, Su, Wu, Chen, Li, Wang, Han and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Wu d29vemhlbmdAeGp0dS5lZHUuY24=
Abbreviations DD, duodenal diverticula; PAD, periampullary duodenal diverticula.
 Jiaqiang Ren
Jiaqiang Ren Jiachun Ding1
Jiachun Ding1 Shuai Wu
Shuai Wu Jie Li
Jie Li Liang Han
Liang Han Zheng Wu
Zheng Wu