
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg., 11 August 2023
Sec. Visceral Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1258343
This article is part of the Research TopicColorectal Surgery and Proctology: Past, Present, and FutureView all 9 articles
 Michael K. Konstantinidis1,2*
Michael K. Konstantinidis1,2* Argyrios Ioannidis1
Argyrios Ioannidis1 Panteleimon Vassiliu2
Panteleimon Vassiliu2 Nikolaos Arkadopoulos2
Nikolaos Arkadopoulos2 Ioannis S. Papanikolaou3
Ioannis S. Papanikolaou3 Konstantinos Stavridis4
Konstantinos Stavridis4 Gaetano Gallo5
Gaetano Gallo5 Dimitrios Karagiannis6
Dimitrios Karagiannis6 Manish Chand7
Manish Chand7 Steven D. Wexner8
Steven D. Wexner8 Konstantinos Konstantinidis1
Konstantinos Konstantinidis1
Aim: To describe the currently available evidence regarding the efficacy and safety of preoperative tumor marking using indocyanine green (ICG) prior to laparoscopic or robotic colorectal resections.
Methods: A systematic search for relevant studies was conducted using the following databases: Embase (OVID), MEDLINE® (OVID), APA PsycInfo (OVID), Global Health (OVID) and HMIC Health Management Information Consortium (OVID) through June 2022 reported according to PRISMA 2020 guidelines. Primary outcome was the detection rate of the tumor sites preoperatively marked with ICG. Secondary outcomes were timing of ICG injection in days prior to the operation and technique-related complications.
Results: Eight single center studies, published between 2008 and 2022, were identified yielding a total of 1,061 patients, of whom 696 were preoperatively tattooed with ICG. Injection dosage of diluted ICG ranged from 0.1–1.5 ml. Four studies used the saline test injection method prior to ICG injection. When the marking was placed within one week, the visualization rate was 650/668 (97%), whereas when it was longer than one week, the detection rate was 8/56 (14%). No severe complications were reported.
Conclusion: Preoperative tumor marking using ICG prior to minimally invasive colorectal resections is safe and effective, allowing intraoperative tumor site location when performed up to a week prior to surgery without disturbing the surgical view in potential mild complications.
Indocyanine green (ICG) is a water-soluble fluorescent dye with binding affinity for plasma proteins, particularly lipoproteins and exhibits fluorescence within the near-infrared (NIR) spectrum (750–950 nm). Originally developed by Kodak Laboratories in 1955 for NIR photography, ICG obtained Food and Drug Administration (FDA) approval for human use in 1959 (1). Since then, it has found several applications as an imaging modality in various medical fields, including cardiac output determination as well as identification and assessment of ophthalmic angiography, hepatic function, liver and bowel blood flow, and cholangiography (2–4).
ICG administration routes include intravenous, interstitial (submucosa or subserosa) οr intra-ureteric. Following intravenous injection, the dye undergoes hepatic metabolism and is exclusively excreted into the bile, with its half-life dependent on liver function, typically ranging from 3 to 4 min (5). In cases where direct intravenous administration is not employed, ICG follows lymphatic drainage. The time required for ICG to reach the nearest lymph node is approximately 15 min (6).
The applications of ICG in colorectal surgery are extensive and continually evolving. ICG has been utilized for perfusion assessment, intraoperative ureter visualization, identification of sentinel nodes, and bowel visualization of lymphatic drainage in colorectal operations. Additionally, it has been described as a localization marker and intraoperative evaluation of peritoneal and hepatic metastases (7, 8).
Preoperative tumor marking with ICG has emerged as a promising tool in colorectal surgery. Studies evaluating preoperative tumor marking with ICG have demonstrated promising results, showcasing its potential to improve surgical outcomes. Enhanced visualization and precise tumor localization lead to increased resection accuracy, reduced positive margin rates, and decreased local recurrence rates. Additionally, ICG-guided lymph node mapping aids in determining lymph node status, allowing for more tailored treatment decisions. Ongoing research aims to refine techniques, optimize timing, dosage, and injection methods, and explore potential combination strategies with other imaging modalities. Furthermore, the integration of robotic surgery and fluorescence-guided techniques holds promise in further enhancing the accuracy and efficiency of tumor localization and resection. However, challenges such as limited depth penetration and potential adverse effects warrant caution. In this review, we critically assessed the existing body of literature regarding preoperative ICG tumor marking (tattoo) prior to colorectal operations, identified gaps in knowledge, and proposed future research directions to optimize its utilization in colorectal surgery.
A systematic literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (9). We performed an independent literature search for relevant studies from inception up to June 4, 2023, across five databases: Embase (OVID), MEDLINE® (OVID), APA PsycInfo (OVID), Global Health (OVID) and HMIC Health Management Information Consortium (OVID). The following search term was used in OVID: (colorectal surgery or colon surgery or colorectal tumor or colorectal lesions).mp AND (ICG or indocyanine green or preoperative colonoscopic marking or fluorescent guided surgery or tattooing or tattoo or endoscopic marking or tumor localization).mp. No geographical or age restrictions were applied.
Randomized controlled trials (RCTs), cohort studies (prospective or retrospective) and case-control studies reporting the use of preoperative colorectal tumor marking with ICG regarding the association between injection time and detection rate were included. Furthermore, restrictions included English language and human studies.
Studies reporting patient numbers below 10 are excluded. Reviews, case reports, editorials, viewpoints, letters to the editor, and conference abstracts are excluded.
After removing duplicates, publications were screened by title and abstract, then full texts were appraised to determine their eligibility by two independent authors (MK, KS). The full manuscripts of studies that met our inclusion criteria were obtained. In cases where reviewers disagreed on study eligibility, this was resolved by discussion with a third author (AI). Data from each article were extracted by one author (MK) and re-validated by another (KS). The following data were collected for each study: first author, publication year, country, study type, sample size (post-dropouts), operation type, primary endpoints (Table 1). Furthermore, procedure characteristics were collected for each study as following: ICG solution, N/S elevation, injection dosage, injection sites, injection time, and tumor location (Table 2).
The reported primary endpoint is the detection rate of the tumor sites preoperatively marked with ICG. The reported secondary outcomes were timing of ICG injection in days prior to the operation and technique-related complications.
The quality of the included studies was assessed by two independent reviewers (MK, KS) using the ROBINS-I tool for non-randomized studies (Figure 2) (10).
A database search identified 1,541 articles. After duplicates were removed, 1,299 articles remained. An analysis of titles and abstracts yielded 300 relevant articles for the full text review. Publications that met final inclusion criteria included 8 articles published between 2008 and 2022 (11–18). A total of 1,061 patients were included, of whom 696 were preoperatively tattooed with ICG. Figure 1 shows the study selection using the PRISMA flowchart. Four of the included studies were undertaken in Japan, three in South Korea, and one in Greece (Table 1). Overall, two studies were considered high risk of bias, one was moderate, whereas five were low risk, according to ROBINS-1 tool (Figure 2) (10). All of the studies were single center. These included two retrospective cohort studies, four prospective case-series studies, and two retrospective case-series. From the available reported data, a total of 201 patients (190 markings with ICG) underwent resection of the right colon (including transverse colon), 353 patients (294 markings with ICG) had an operation of the left colon, and 275 patients (257 markings with ICG) had a rectal tumor. Laparoscopic surgery was the most often used approach in a total of 937 patients, whereas an open operation was used in 75 patients and a robotic approach in 10 (Miyoshi et al. used both laparoscopic and open methods without the clarifying number of patients in each).
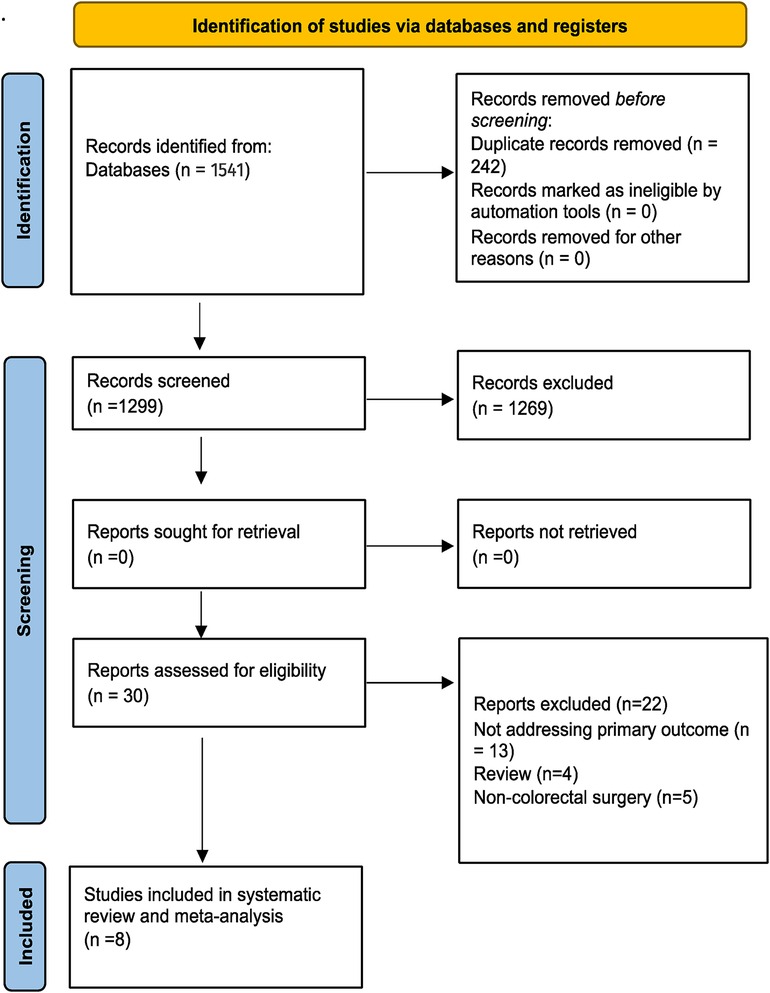
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart (9).
Indocyanine green (25 mg) was diluted with sterile water or normal saline and the final solution ranged from 2.5–12.5 mg/ml. Injection dosage ranged from 0.1–1.5 ml of solution at either 1 site distal to the tumor, 2 sites 180° apart, 3 sites 120° apart (circumferentially) or at all 4 quadrants. The saline test injection method was used in four studies prior to injecting ICG into the submucosal layer of the colon. Using either a 23-gauge or a 26-gauge injection needle, 0.2–2 ml of saline was injected into 1 or 2 sites of the submucosal layer to form proper submucosal elevation. Using a second syringe and needle, ICG solution was injected. After injecting ICG into elevated submucosal layers, both Sang Lee et al. and Miyoshi et al. replaced the syringe with the first one and injected 1–2 ml normal saline, respectively, to flush out the remaining ICG in the needle. Two studies did not use the saline test injection method and injected ICG directly into submucosal layer. Watanabe et al. as well as Nagata et al. did not specify whether they employed this technique (Table 2).
Patients were scheduled for preoperative colonoscopy and tumor marking on different days prior to surgery. In most of the included studies, the highest detection rates were achieved when the tattoo was performed within one week of surgery. More specifically, Satoyoshi et al. successfully detected all 141 (100%) tumors that were marked ≤6 days before surgery. Watanabe et al. presented similar results (75/76 tumors detected, 98.7%) in markings ≤7 days. Similarly, Miyoshi et al. described a 100% tattoo detection rate (28/28) within 8 days performed. When the preoperative colonoscopy was performed up to 3 days prior to the surgery, Kim et al. detected 85/90 (94.4%) of the tumor markings and Nagata et al. successfully found all 24 (100%) tumors intraoperatively. Sang Lee et al. reported 170/170 (100%) positive tumor detections after tattoo marking within 2 days, but only 2/5 (40%) after 2 days. Finally, when patients underwent colonoscopy one day prior to the operation, Park et al., Nagata et al. and Konstantinidis et al. detected all marked tumors [114/114 (100%), 24/24 (100%) and 10/10 (100%), respectively].
When the preoperative tattoo was performed more than one week prior to the operation, less tumors were detected. Satoyoshi et al. reported a 60% (6/10) successful rate in markings performed 7–9 days prior to the resection and 0% (0/4) when performed >10 days prior. Similarly, Watanabe et al. did not successfully detect any of the marked tumors in 4 patients (0/4, 0%) between 8 and 17 days prior to surgery. Finally, Miyoshi et al. observed positive staining in only 2/10 (20%) patients who underwent tumor marking ≥9 days prior to their operation day (Table 3). In total, when the marking was placed within one week, the visualization rate was 97% (650/668) whereas when it was longer than one week, the detection rate was 14% (8/5).
There were no severe complications related to tattooing in any of the included studies. Satoyoshi et al. described some cases (without defining the number) of spillage into serosa that obscured the separation boundary in a NIR view without disturbing the view in white-light. Kim et al. reported one patient with mild abdominal pain upon tattooing, but without any further preoperative findings and symptoms. Both Park et al. and Miyoshi et al. documented one patient each with peritoneal ICG spillage, but without inflammation. In addition, Park et al. observed one patient with mucosal edema (Table 3).
During minimally invasive surgery, identification of neoplasms has posed challenges due to the absence of tactile sensation. Consequently, surgeons must rely on visual assessment along with preoperative imaging to guide them in achieving a precise surgical resection. In complex cases, preoperative localization and marking of tumors by endoscopists hold vital importance as they provide surgeons with valuable anatomical guidance. Although extremely rare, positive longitudinal resection margins in colorectal surgery in cases where localization of the tumor cannot be verified intraoperatively, have negative implications in oncologic outcomes. The practice of endoscopic tattooing for detecting colorectal lesions has been documented as early as 1975 (19). Since then, it has been upgraded in many aspects and is one of the most popular approaches due to its accurate lesion localization and small tumor detection. Several substances have been used as markers including, methylene blue, indigo carmine, toluidine blue, and isosulfan blue, but only India ink and ICG were reportedly visible up to 48 h after injection (13, 20–22).
Lee et al. compared the duration of marking between ICG and India ink in a randomized animal study and concluded that India ink had better durability (23). However, India ink is a combination of several substances capable of inducing inflammatory reactions such as focal peritonitis, inflammatory pseudotumors, abscesses, and adhesions and for that reason, it is used under an autoclave and after dilution (15, 22). Depending on the technique, numerous complications have been reported using India ink (24–29). For instance, due to its permanent marking method, in the case of leakage, it interferes with the surgical view and detection of anatomical structures.
Conversely, ICG is transient rather than indelible (12) and has been safely used for more than 50 years (30–32). In 1993, Hammond et al. were the first to describe the use of ICG as a colorectal tumor marker instead of India ink (21). Since then, various techniques have been reported regarding preoperative colorectal tumor marking with ICG.
This systematic review included eight studies published between 2008 and 2022 that included a total of 1,061 patients. Included studies were both prospective and retrospective ones, providing a comprehensive evaluation of the utility of preoperative ICG tumor marking in colorectal surgery.
The utilization of ICG as a preoperative tumor marking agent in colorectal surgery offers numerous advantages in terms of enhanced visualization, precise tumor localization, and improved surgical outcomes.
Regarding the technique of preoperative ICG injection, the studies exhibited some variations in the dosage and administration method. The ICG solution, typically prepared by diluting 25 mg of ICG with sterile water or normal saline, ranged from 2.5 to 12.5 mg/ml. The injection dosage varied from 0.1 to 1.5 ml, depending on the number of sites and quadrants chosen for injection. Some studies employed the saline test injection method, which involved injecting saline into the submucosal layer before administering ICG. This technique aimed to achieve proper submucosal elevation for effective ICG delivery. However, not all studies clarified whether they utilized this method. The heterogeneity in injection techniques emphasizes the need for standardization and further investigation into the optimal dosage and injection methods.
The marking-detection rates demonstrated the effectiveness of preoperative ICG tumor marking in colorectal surgery. In general, the highest detection rates were achieved when the tattooing was performed within one week of surgery. Studies have consistently reported high detection rates, ranging from 94.4% to 100%, for tumor markings conducted within one week before the operation. However, when the preoperative colonoscopy and tumor marking were performed more than one week prior to surgery, the detection rates tended to decrease. The same findings were reported in 2009 by Watanabe et al., in 8 patients who underwent preoperative ICG tumor injection (33). Ahn et al. performed a prospective study that included 192 patients who underwent endoscopic submucosal ICG tattooing near the lesion 12–18 h before laparoscopic surgery for colorectal cancer. The aim of the study was to establish the optimal protocol for preoperative endoscopic submucosal ICG injection to perform fluorescence lymph node mapping, along with undisturbed fluorescent tumor localization and ICG angiography. The amounts of injected ICG were divided into 5 categories: >12, 1–12, 1–0.5, 0.5–0.3, and <0.3 mg. The authors concluded that the highest success rate was the dose of 0.5–1 mg (34). It is important to note that the optimal timing for tumor marking with ICG remains to be definitively determined. Further research is warranted to ascertain the ideal timing between marking and surgery to maximize detection rates and minimize the risk of false negatives.
Reported complications associated with preoperative ICG tumor marking were minimal. The reviewed studies reported no severe complications related to tattooing with ICG. Some cases of spillage into the serosa were observed, which occasionally obscured the separation boundary in the Near-Infrared Light (NIR) view but did not affect the view in white light. Additionally, mild abdominal pain and mucosal edema were reported in isolated cases, but these complications did not have significant clinical implications.
However, we should outline that body mass index (BMI) was not reported in the included studies, therefore any correlation between ICG-marked tumor identification and BMI cannot be made. We believe that BMI and intraperitoneal fat play important roles and can alter the efficacy of ICG. Moreover, another critical note is that tumor stage and detection rate were not correlated in any of the included studies.
Several other techniques have been employed for the detection of colorectal tumors, including computed tomography (CT) scans, CT colonography, preoperative barium enema, proctoscopy with stitch, as well as colonoscopy with metallic clipping (35–41). However, barium enemas have proven ineffective in visualizing small tumors (38, 39). The use of metallic clips for tumor localization presents challenges, as clips can be difficult to visualize and may migrate (35–37). Narihiro et al. compared technologies and reported on the safety and efficacy of NIR fluorescent clips, achieving a detection rate of 94.1% without encountering adverse effects associated with clip marking (40). Intraoperative colonoscopy can serve to identify gastrointestinal lesions, but it prolongs the overall operation time and can lead to intestinal distention, potentially limiting the surgical field (12, 35). An alternative approach utilizing autologous blood from the patient instead of a dye has also been described (41–43). However, additional blood collection is required and an accurate location may be difficult to find due to bleeding during surgery. Kim et al. employed 6–12 ml of autologous blood for endoscopic tattooing and reported a visualization rate of 92.2%, with three patients (5.9%) experiencing endoscopic adverse effects associated with the technique (41).
Our study has several limitations. Missing data may have led to an incomplete understanding of the subset(s) of patients may benefit from endoscopic tattooing. Moreover, the majority of the included studies did not report control groups of other tattooing agents or non-tattooed patients, which could have led to a more robust understanding of the value of this technique as well as its complications. Furthermore, the preoperative marking day varied between the studies and the exact day of injection was not clearly reported in every study, which could have led to more precise conclusions. Future large prospective cohorts or randomized studies are needed to better establish definitive conclusions regarding the effectiveness of ICG marking on tumor detection.
The available literature on preoperative tumor marking with ICG in colorectal surgery demonstrates its potential as a valuable tool for enhancing surgical precision and improving outcomes. The reviewed studies consistently highlight the benefits of enhanced visualization, precise tumor localization, and increased detection rates. ICG fluorescence marking with NIR light is a reliable method for tumor site marking if ICG is injected into the submucosal layer around the tumor within one week before laparoscopic or robotic colorectal surgery. Further research is also needed to investigate its cost-effectiveness compared to traditional permanent dyes as well as potential combination strategies with other imaging modalities. Despite the limitations and challenges associated with ICG, its utilization in preoperative tumor marking in colorectal surgery shows great promise and warrants continued investigation to refine its implementation and maximize its benefits for patients.
MK: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. AI: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. PV: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing. NA: Conceptualization, Formal Analysis, Methodology, Project administration, Validation, Visualization, Writing – review & editing. IP: Investigation, Software, Supervision, Validation, Writing – review & editing. KS: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft. DK: Investigation, Supervision, Validation, Writing – review & editing. MC: Investigation, Supervision, Validation, Writing – review & editing. SW: Investigation, Supervision, Validation, Writing – review & editing. KK: Conceptualization, Supervision, Validation, Writing – review & editing. GG: Conceptualization, Investigation, Project administration, Writing – review & editing.
SW reports receiving consulting fees from ARC/Corvus, Astellas, Baxter, Becton Dickinson, GI Supply, ICON Language Services, Intuitive Surgical, Leading BioSciences, Livsmed, Medtronic, Olympus Surgical, Stryker, Takeda and receiving royalties from Intuitive Surgical and Karl Storz Endoscopy America Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1258343/full#supplementary-material
1. Benson RC, Kues HA. Fluorescence properties of indocyanine green as related to angiography. Phys Med Biol. (1978) 23(1):159–63. doi: 10.1088/0031-9155/23/1/017
2. Weis F, Kilger E, Beiras-Fernandez A, Hinske CL, Nassau K, Adnan L, et al. Indocyanine green clearance as an outcome prediction tool in cardiac surgery: a prospective study. J Crit Care. (2014) 29:224–9. doi: 10.1016/j.jcrc.2013.10.023
3. Lee Z, Moore B, Giusto L, Eun DD. Use of indocyanine green during robot-assisted ureteral reconstructions. Eur Urol. (2015) 67:291–8. doi: 10.1016/j.eururo.2014.08.057
4. Yamamoto M, Orihashi K, Nishimori H, Wariishi S, Fukutomi T, Kondo N, et al. Indocyanine green angiography for intra-operative assessment in vascular surgery. Eur J Vasc Endovasc Surg. (2012) 43:426–32. doi: 10.1016/j.ejvs.2011.12.030
5. GAROUF. Available at: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/indocyanine-green-solution?redirect = true (Accessed 25 November, 2022).
6. Tajima Y, Murakami M, Yamazaki K, Masuda Y, Kato M, Sato A, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol. (2010) 17:1787–93. [CrossRef]. doi: 10.1245/s10434-010-0944-0
7. Ueda K, Ushijima H, Kawamura J. Lymphatic flow mapping during colon cancer surgery using indocyanine green fluorescence imaging. Minim Invasive Ther Allied Technol. (2023):1–7. doi: 10.1080/13645706.2022.2164468
8. Cassinotti E, Boni L, Baldari L. Application of indocyanine green (ICG)-guided surgery in clinical practice: lesson to learn from other organs-an overview on clinical applications and future perspectives. Updates Surg. (2023) 75(2):357–65. doi: 10.1007/s13304-022-01361-y
9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
10. Pe Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/bmj.i4919
11. Satoyoshi T, Okita K, Ishii M, Hamabe A, Usui A, Akizuki E, et al. Timing of indocyanine green injection prior to laparoscopic colorectal surgery for tumor localization: a prospective case series. Surg Endosc. (2021) 35(2):763–9. doi: 10.1007/s00464-020-07443-5
12. Kim YJ, Park JW, Lim HK, Kwon YH, Kim MJ, Choe EK, et al. Preoperative colonoscopic tattooing using a direct injection method with indocyanine green for localization of colorectal tumors: an efficacy and safety comparison study. J Minim Invasive Surg. (2020) 23(4):186–90. doi: 10.7602/jmis.2020.23.4.186
13. Lee SJ, Sohn DK, Han KS, Kim BC, Hong CW, Park SC, et al. Preoperative tattooing using indocyanine green in laparoscopic colorectal surgery. Ann Coloproctol. (2018) 34(4):206–11. doi: 10.3393/ac.2017.09.25
14. Watanabe M, Murakami M, Ozawa Y, Yoshizawa S, Matsui N, Aoki T. Intraoperative identification of colonic tumor sites using a near-infrared fluorescence endoscopic imaging system and indocyanine green. Dig Surg. (2017) 34(6):495–501. doi: 10.1159/000458450
15. Park JH, Moon HS, Kwon IS, Yun GY, Lee SH, Park DH, et al. Usefulness of colonic tattooing using indocyanine green in patients with colorectal tumors. World J Clin Cases. (2018) 6(13):632–40. doi: 10.12998/wjcc.v6.i13.632
16. Nagata J, Fukunaga Y, Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S, et al. Colonic marking with near-infrared, light-emitting, diode-activated indocyanine green for laparoscopic colorectal surgery. Dis Colon Rectum. (2016) 59(2):e14–8. doi: 10.1097/DCR.0000000000000542
17. Miyoshi N, Ohue M, Noura S, Yano M, Sasaki Y, Kishi K, et al. Surgical usefulness of indocyanine green as an alternative to India ink for endoscopic marking. Surg Endosc. (2009) 23(2):347–51. doi: 10.1007/s00464-008-9938-4
18. Konstantinidis MK, Ioannidis A, Vasiliou P, Arkadopoulos N, Papanikolaou IS, Chand M, et al. Preoperative tumor marking with indocyanine green prior of robotic colorectal resections. Front Surg. (2022) 9:1087889. doi: 10.3389/fsurg.2022.1087889
19. Ponsky JL, King JF. Endoscopic marking of colonic lesions. Gastrointest Endosc. (1975) 22(1):42–3. doi: 10.1016/S0016-5107(75)73687-8
20. Hammond DC, Lane FR, Welk RA, Madura MJ, Borreson DK, Passinault WJ. Endoscopic tattooing of the colon. An experimental study. Am Surg. (1989) 55:457–61.2472762
21. Hammond DC, Lane FR, Mackeigan JM, Passinault WJ. Endoscopic tattooing of the colon: clinical experience. Am Surg. (1993) 59:205–10.8476162
22. Technology Committee ASGE, Kethu SR, Banerjee S, Desilets D, Diehl DL, Farraye FA, et al. Endoscopic tattooing. Gastrointest Endosc. (2010) 72:681–5. doi: 10.1016/j.gie.2010.06.020
23. Lee JG, Low AH, Leung JW. Randomized comparative study of indocyanine green and India ink for colonic tattooing: an animal survival study. J Clin Gastroenterol. (2000) 31:233–6. doi: 10.1097/00004836-200010000-00010
24. Nizam R, Siddiqi N, Landas SK, Kaplan DS, Holtzapple PG. Colonic tattooing with India ink: benefits, risks, and alternatives. Am J Gastroenterol. (1996) 91:1804–8.8792702
25. Coman E, Brandt LJ, Brenner S, Frank M, Sablay B, Bennett B. Fat necrosis and inflammatory pseudotumor due to endoscopic tattooing of the colon with India ink. Gastrointest Endosc. (1991) 37:65–8. doi: 10.1016/S0016-5107(91)70626-3
26. Trakarnsanga A, Akaraviputh T. Endoscopic tattooing of colorectal lesions: is it a risk-free procedure? World J Gastrointest Endosc. (2011) 3:256–60. doi: 10.4253/wjge.v3.i12.256
27. Kim JH, Kim WH. Colonoscopic tattooing of colonic lesions. Korean J Gastroenterol. (2015) 66:190–3. doi: 10.4166/kjg.2015.66.4.190.26493503
28. Park JW, Sohn DK, Hong CW, Han KS, Choi DH, Chang HJ, et al. The usefulness of preoperative colonoscopic tattooing using a saline test injection method with prepackaged sterile India ink for localization in laparoscopic colorectal surgery. Surg Endosc. (2008) 22:501–5. doi: 10.1007/s00464-007-9495-2.17704874
29. Hwang MR, Sohn DK, Park JW, Kim BC, Hong CW, Han KS, et al. Small-dose India ink tattooing for preoperative localization of colorectal tumor. J Laparoendosc Adv Surg Tech A. (2010) 20:731–4. doi: 10.1089/lap.2010.0284.20879870
30. Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA, et al. Adverse reactions due to indocyanine green. Ophthalmology. (1994) 101:529–33. doi: 10.1016/S0161-6420(94)31303-0
31. Caesar J, Shaldon S, Chiandussi L, Guevara L, Sherlock S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. (1961) 21:43–57.13689739
32. Hiratsuka M, Miyashiro I, Ishikawa O, Furukawa H, Motomura K, Ohigashi H, et al. Application of sentinel node biopsy to gastric cancer surgery. Surgery. (2001) 129:335–40. doi: 10.1067/msy.2001.111699
33. Watanabe M, Tsunoda A, Narita K, Kusano M, Miwa M. Colonic tattooing using fluorescence imaging with light-emitting diode-activated indocyanine green: a feasibility study. Surg Today. (2009) 39:214–8. doi: 10.1007/s00595-008-3849-9.19280280
34. Ahn HM, Son GM, Lee IY, Shin DH, Kim TK, Park SB, et al. Optimal ICG dosage of preoperative colonoscopic tattooing for fluorescence-guided laparoscopic colorectal surgery. Surg Endosc. (2022) 36(2):1152–63. doi: 10.1007/s00464-021-08382-5
35. Kim SH, Milsom JW, Church JM, Ludwig KA, Garcia- Ruiz A, Okuda J, et al. Perioperative tumor localization for laparoscopic colorectal surgery. Surg Endosc. (1997) 11:1013–6. doi: 10.1007/s004649900514
36. Lee DW, Sohn DK, Han KS, Hong CW, Park HC, Oh JH. Promising novel technique for tumor localization in laparoscopic colorectal surgery using indocyanine green-coated endoscopic clips. Dis. Colon Rectum. (2021) 64:e9–13. doi: 10.1097/DCR.0000000000001876
37. Ryu S, Okamoto A, Nakashima K, Hara K, Ishida K, Ito R, et al. Usefulness of preoperative endoscopic fluorescent clip marking in laparoscopic gastrointestinal surgery. Anticancer Res. (2020) 40:6517–23. doi: 10.21873/anticanres.14675
38. Feingold DL, Addona T, Forde KA, Arnell TD, Carter JJ, Huang EH, et al. Safety and reliability of tattooing colorectal neoplasms prior to laparoscopic resection. J Gastrointest Surg. (2004) 8:543–6. doi: 10.1016/j.gassur.2003.12.016
39. Arteaga-Gonzalez I, Martın-Malagon A, Lopez-Fernandez EM, Arranz- Duran J, Parra-Blanco A, Nicolas-Perez D, et al. The use of preoperative endoscopic tattooing in laparoscopic colorectal cancer surgery for endoscopically advanced tumors: a prospective comparative clinical study. World J Surg. (2006) 30:605–11. doi: 10.1007/s00268-005-0473-3
40. Narihiro S, Yoshida M, Ohdaira H, Sato T, Suto D, Hoshimoto S, et al. Effectiveness and safety of tumor site marking with near-infrared fluorescent clips in colorectal laparoscopic surgery: a case series study. Int J Surg. (2020) 80:74–8. doi: 10.1016/j.ijsu.2020.06.014
41. Kim EJ, Chung JW, Kim SY, Kim JH, Kim YJ, Kim KO, et al. Autologous blood, a novel agent for preoperative colonic localization: a safety and efficacy comparison study. Surg Endosc. (2019) 33:1080–6. doi: 10.1007/s00464-018-6358-y
42. Lee SH, Kim DY, Oh SY, Lee KJ, Suh KW. Preoperative localization of early colorectal cancer or a malignant polyp by using the patient’s own blood. Ann Coloproctol. (2014) 30:115–7. doi: 10.3393/ac.2014.30.3.115
Keywords: fluorescence imaging, indocyanine green, colorectal surgery, colorectal cancer, colorectal tumor, preoperative tumor marking, preoperative tattoo
Citation: Konstantinidis MK, Ioannidis A, Vassiliu P, Arkadopoulos N, Papanikolaou IS, Stavridis K, Gallo G, Karagiannis D, Chand M, Wexner SD and Konstantinidis K (2023) Preoperative tumor marking with indocyanine green (ICG) prior to minimally invasive colorectal cancer: a systematic review of current literature. Front. Surg. 10:1258343. doi: 10.3389/fsurg.2023.1258343
Received: 13 July 2023; Accepted: 3 August 2023;
Published: 11 August 2023.
Edited by:
Roberta Tutino, Azienda Ospedaliero Universitaria Città della Salute e della Scienza di Torino, ItalyReviewed by:
Giulio Mari, Desio Hospital, Italy© 2023 Konstantinidis, Ioannidis, Vassiliu, Arkadopoulos, Papanikolaou, Stavridis, Gallo, Karagiannis, Chand, Wexner and Konstantinidis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael K. Konstantinidis bWlrZWtvbnN0YW50aW5pZGlzQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.