
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 30 August 2023
Sec. Neurosurgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1249366
Background: Glioblastoma is the most common and most aggressive primary brain tumor in adults. Despite multimodal treatment, the median survival time is 15–16 months and 5-year survival rate 5%–10%. The primary goal of this study was to identify prognostic factors for survival in an unselected population of patients operated for glioblastoma. The secondary goal was to explore changes in outcome and the clinical management of this patient group over time.
Methods: We identified 222 consecutive adults operated for glioblastoma between November 2012 and June 2016 at the Department of Neurosurgery, Sahlgrenska University Hospital in Gothenburg, serving a health care region in the western part of Sweden with 1.900.000 inhabitants. Clinical variables were identified and tested as predictors for prognosis in extended Poisson regression models. The results were compared with a previously published cohort from 2004 to 2008, before current standard of care based on molecular tumor diagnosis was fully implemented.
Results: Median overall survival was 1.07 years, which was significantly longer than in the 2004–2008 cohort (1.07 vs. 0.73 y, age- and sex adjusted HR = 1.89, p < 0.0001). Variables associated with longer survival in the multivariable model were MGMT promoter hypermethylation, non-central tumor location, complete resection of enhancing tumor, WHO performance status 0–1, unilateral tumor location, fewer lobes involved, younger age and no comorbidities.
Conclusion: The median survival for patients with glioblastoma treated according to current standard treatment has moderately but significantly increased, with MGMT promoter hypermethylation as the strongest predictor for survival.
Glioblastoma (GBM) is the most common and most malignant primary brain tumor (1), accounting for approximately 14% of all CNS tumors (2). The 2016 WHO Classification of CNS Tumors introduced molecular criteria for classification and divided GBMs into isocitrate dehydrogenase-wildtype (IDH-wt), previously classified as primary GBM (about 90%), and IDH-mutant GBM, previously secondary GBM (about 10%) (1). In the recent WHO classification, based on a multilayered approach and incorporating tumor morphology, molecular characteristics and DNA methylation profiles, all IDH-wt astrocytomas, irrespective of grade, are classified as GBM (3).
Several clinical factors have been shown to affect survival of GBM at group level. Among these, patient age (4–10), functional status (4, 6–10), tumor location (8, 10–12), extent of resection (6, 7, 9, 10, 12), multifocality (5, 6), bilaterality (5, 7, 10), type of oncological treatment (5–7, 9–12), and the methylation status of the MGMT (6O-methylguanine-DNA methyltransferase) gene promoter (13, 14) are the strongest predictors for survival. Often, these factors have been evaluated in isolation, while in the clinical situation a combination of patient-, tumor- and treatment-related factors is typically used.
The current standard treatment for newly diagnosed GBM is maximal safe resection followed by radiotherapy with concomitant and adjuvant temozolomide (TMZ) (13, 15). Addition of locoregional treatment by Tumor Treating Fields (TTFields, Optune®) to adjuvant TMZ has shown to prolong survival (16). Despite this multimodal treatment, patients with GBM still face poor prognosis (13, 17, 18).
Overall survival (OS) as reported in the above-mentioned randomized trials is around 15–20 months. In unselected cohorts with varying proportions of elderly patients or patients with poor performance status (PS), the median OS is 10.1–12.3 months (19–22). Thus, the survival benefit achieved in a controlled setting is only to a limited extent observed in a population-based setting. In our previously published GBM cohort from 2004 to 2008, the median OS was as low as 0.73 years (8.8 months) (5). Since patient recruitment was prior to full implementation of multimodal treatment, we found it of interest to perform a new and similarly designed study in patients treated by current standard of care. The primary goal was to report outcome and prognostic variables for survival after the introduction of multimodal treatment. The secondary goal was to compare these results with previous data, as to highlight the changes in management and outcome over time.
We performed a consecutive study with prospectively registered clinical data from patients referred to the Neurosurgical Department at Sahlgrenska University Hospital in Gothenburg. The department serves a health care region in the western part of Sweden with 1.900.000 inhabitants and all patients with radiologically suspected brain tumors are referred to multidisciplinary team conferences.
A total of 378 patients presented with a radiologically suspected GBM during the study period. Of these, 247 (65.3%) were considered suitable for surgery and thereby met the inclusion criteria for the present study. Inclusion criteria were adults (≥18 years) who underwent resection or biopsy for a supratentorial tumor and received a first-time histological diagnosis of GBM (1). The 131 (34.7%) patients not considered to benefit from surgery, who had radiological diagnosis of GBM without tissue diagnosis, have been presented in a separate study (23).
Patients were recruited from November 2012 through June 2016, included after informed consent, and followed until 30th of June 2018. Of the 247 operated patients, 25 patients had IDH-mutated tumors and were excluded from the analysis. The remaining 222 patients, 213 with confirmed IDH-wt GBM and 9 with missing IDH-status, were included in the present cohort (Figure 1). Demographics, preoperative symptoms, WHO PS as assessed by the surgeon, tumor location, presence of multifocality (defined as at least two separate contrast-enhancing tumors on MRI) and comorbidities were recorded.
One of the objectives of the present study was to investigate whether survival of GBM in our region had increased over the past decade. For comparison, we used our previous retrospective population-based study, including 229 consecutive adult patients operated at our hospital from January 2004 to December 2008, (and followed until 31st of December 2010) (5). In that study, we reported 430 patients discussed at multidisciplinary tumor conferences, of which 229 operated and included in the study. Patients with secondary GBMs met the earlier inclusion criteria. For objective comparative analysis of the two cohorts, the 25 patients with IDH-mutated tumors in the 2012–2016 cohort have therefore been included.
Analysis of IDH-gene mutations and MGMT promoter methylation were performed retrospectively. Mutation analysis of IDH1 (R132H) was performed by Sanger sequencing or by immunohistochemistry as indicated (24, 25). The methylation status of MGMT promoter was analyzed by pyrosequencing using the PyroMark PCR kit (Qiagen) as previously described (26), with cut-off value ≥9% for hypermethylated MGMT promoter.
Type of surgery, data on primary oncological treatment including treatment at recurrence, were recorded for all patients. Patients who underwent resection (but not biopsy) were evaluated with postoperative MRI within 72 h. The extent of surgical resection was defined as 1) complete resection of enhancing tumor (CRET), 2) incomplete resection or 3) biopsy (open or stereotactic).
For descriptive purposes, continuous variables were presented by mean, standard deviation, median, minimum and maximum, and categorical variables by numbers and percentages. For test between two groups Fisher's exact test was used for dichotomous variables, Mantel-Haenszel χ2 trend tests for ordered categorical variables, χ2 test for non-ordered categorical variables and Mann–Whitney U-test for continuous variables. Crude event rates were calculated as number of events divided by the sum of follow-up years for a specific group of patients and expressed per 10 patient years. The 95% confidence intervals were computed using exact Poisson limits. Extended Poisson regression was used as a method of survival analysis to study interaction between various variables and time in study (27). In the baseline hazard function, time was modelled including break points at follow-up of 1 and 3.5 years. The associated survival function showed to correspond well to the estimated Kaplan–Meier survival rates. For each main effect variable, the interaction term with time was tested. For significant interaction with time (p < 0.05) the hazard ratios (HR) for both the main effect variable and interaction with time were presented. Otherwise, the HR was only presented for the main effect variable. Oncological treatment was analyzed in two ways: (1) Assuming the group category to be assigned already at start, that leads to introduction of a statistical error called immortal time bias. These analyses were performed to be able to compare this study's results to other studies' results, where this error was ignored. One could interpret the results as the effect of following a certain pattern of treatment (if we already at baseline knew what pattern the patient would be able to follow), but not as the effect of the future treatment/intervention per se. (2) Handling the treatment/intervention as time-updated variable. In this analysis, a patient is categorized into no treatment/intervention until treatment is provided, which can be interpreted as the effect of treatment/intervention per se. Age- and sex-adjusted models were performed, including interaction with time if needed. A multivariable model was obtained using backward selection, keeping only statistically significant variables in the model. The HRs for finally selected variables were transformed into HRs per 1 SD increase, to be able to compare the impact between variables on survival. All tests were two-tailed and significance level of 0.05 was used. Survival duration was calculated from the day of the surgery to the day of death. Patients alive at the end of the study, 30th of June 2018, were censored All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
The baseline clinical characteristics of the 222 patients are shown in Table 1. As shown, there were 139 men (62.6%) and 83 women (37.4%) (male-to-female ratio of 1.67). The median age at surgery was 64 years (range 19; 82 years). At the time of diagnosis, 156 (70.3%) patients had WHO PS 0–1 and 66 (29.7%) had WHO PS 2–4. Most patients had multiple presenting symptoms (n = 153, 68.9%). The most common presenting symptoms, either as a single symptom or as one of several symptoms, were focal neurological deficits (n = 142, 64.0%), headache (n = 90, 40.5%), and seizures (n = 58, 26.1%). 132 (59.5%) patients had at least one comorbidity, e.g., hypertonia, diabetes, autoimmune disease. Most frequent tumor location was temporal (51.8%), and slightly more tumors in the right hemisphere compared to the left (49.5% vs. 42.8%). MGMT promoter methylation status was available for 210 cases, in 109 of all cases (49.1%) the MGMT promoter was hypermethylated.
The treatment characteristics are shown in Table 2. Of the 222 patients, 117 (52.7%) underwent CRET, 76 (34.2%) underwent incomplete resection, 29 (13.1%) had biopsy. 206 (92.8%) patients received postoperative oncological treatment. The median time from surgery to start of oncological treatment was 38 days. A total of 125 (56.3%) patients received both radiotherapy and chemotherapy as primary oncological treatment. Of these, 109 (49.1%) initiated radiotherapy with 60 Gy in 30 fractions with concomitant TMZ (75 mg/m2) followed by adjuvant TMZ (200 mg/m2 5 days every 4 week). In 40 (18.0%) patients, radiotherapy alone was provided, while in three cases radiotherapy was not completed. In those completing radiotherapy as monotherapy, the majority, 32 of 37 patients received 34 Gy. There were 41 (18.5%) patients that received chemotherapy alone, with TMZ being the chemotherapy of choice in 40 patients.
There were 30 (13.5%) patients undergoing reoperation at recurrence. Of these, 6 had a third surgery. The median time between first operation and reoperation was 424 days. Oncological treatment at recurrence was provided in 117 (52.7%) patients. The most frequent treatments at first recurrence were: Lomustine (49 patients; 22.1%) and TMZ (48 patients; 21.6%), other rare options were PCV, Bevacizumab, Irinotecan, Carboplatin-Etoposid and Nivolumab. A total of 7 (3.2%) patients received re-irradiation.
The median OS for the study population was 1.07 years. Survival rates after surgery were 82% at 6 months, 55% at 12 months and 19% at 24 months. At the end of the study, 18 patients (8.1%) were alive. For patients treated with postoperative chemoradiotherapy and adjuvant TMZ, the median OS was 19.5 months. Figure 2A shows the survival for all patients. Figure 2B shows the survival probability for the patients with the most and least favorable combination of treatment- and tumor-related factors, compared to all other patients.
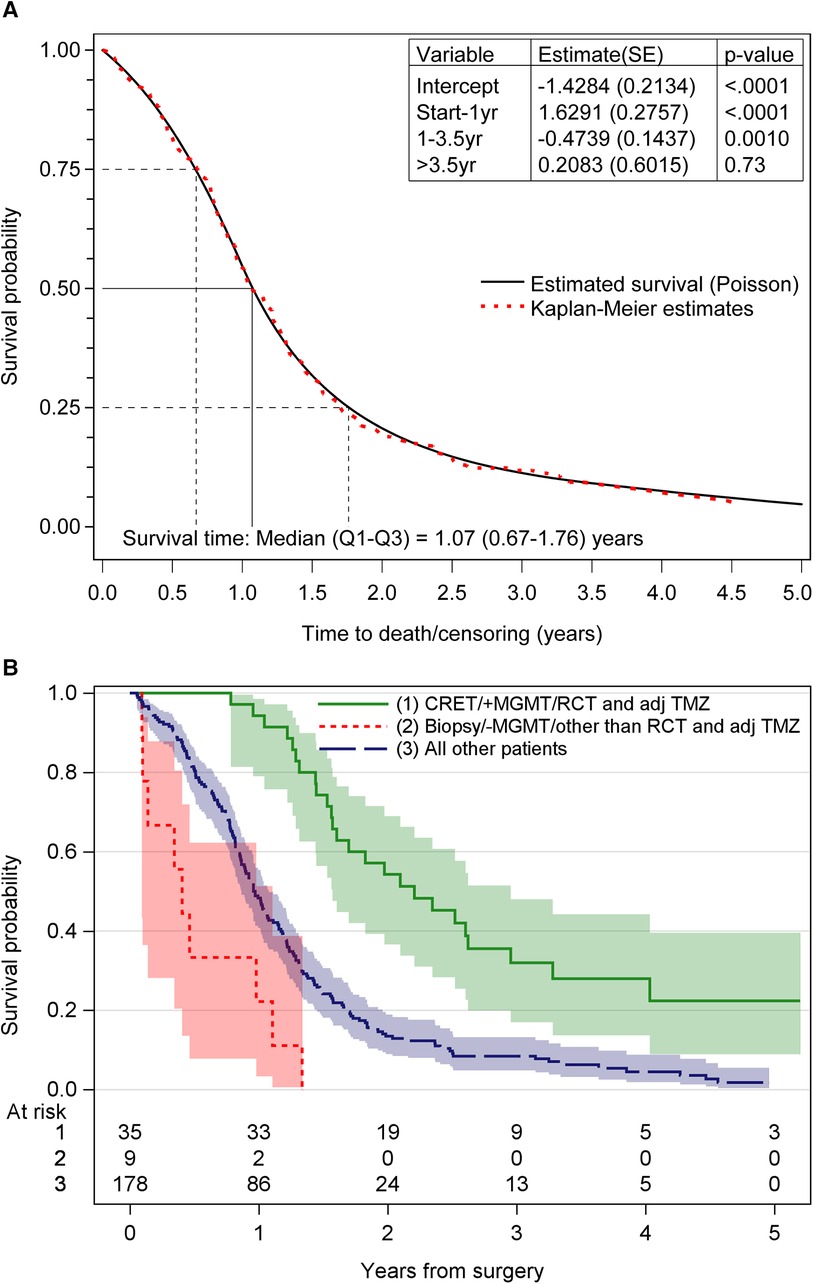
Figure 2. (A) The overall survival probability obtained by poisson regression for time-varying data and Kaplan–Meier technique. (B) Survival probability for the different groups of patients. CRET, complete resection of enhancing tumor; MGMT, 6O-methylguanine-DNA methyltransferase; RCT and adj TMZ, postoperative radiochemotherapy and adjuvant temozolomide; +MGMT, methylated MGMT promoter; –MGMT, unmethylated MGMT promoter.
Table 3 shows the age- and sex-adjusted survival estimates of the variables, including significant interactions with time. As shown, age was strongly associated to survival, each additional year in age represented a 3% increase in the risk of death. Patients with central tumor component, temporal tumor component, bilateral tumor location, right-sided tumor location, multilobular tumor also had an increased risk of death. In contrast, good performance status, no comorbidities, CRET and MGMT promoter methylation were associated with better prognosis. There were significant interactions with time for the variables central tumor location (HR: 0.52, meaning that the increased risk of the central tumors decreases with time) and oncological treatment (HR: 0.01, meaning that the decreased risk of the oncological treatment decreases further with time). In the sub-analysis of the different treatment modalities, we found significantly longer survival for the 109 patients receiving postoperative chemoradiotherapy and adjuvant TMZ compared to the other modalities. Of these, patients with hypermethylated MGMT promoter had significantly better prognosis, with a 63% lower risk of death.
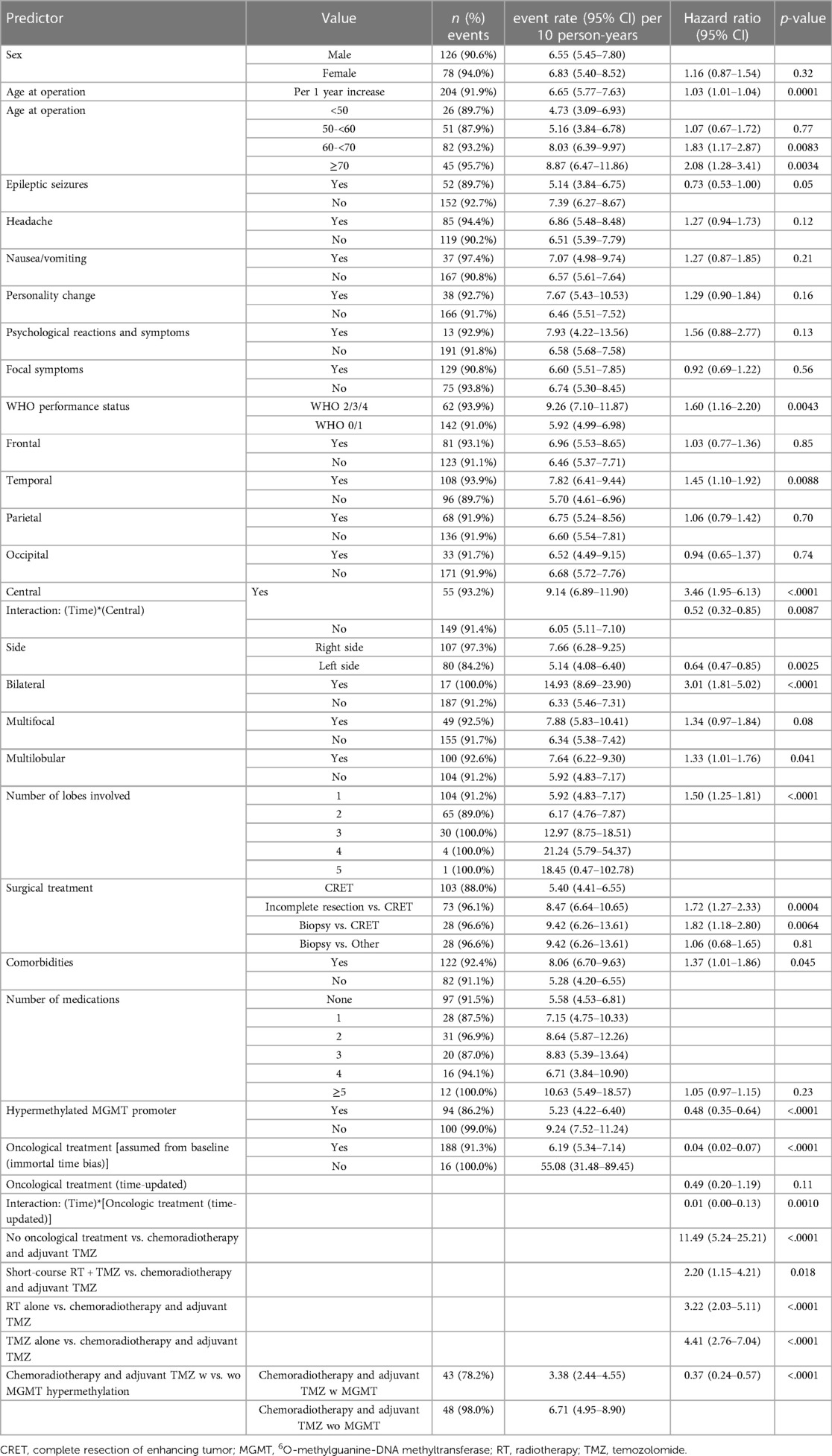
Table 3. Age- and sex-adjusted survival analysis using extended poisson regression including significant interactions with time.
The following variables, listed in order of importance (Table 4), were identified as independent predictors for longer survival: MGMT promoter hypermethylation (vs. unmethylated MGMT promoter), non-central tumor location including the interaction term with time, CRET (vs. no CRET), WHO PS 0–1 (vs. WHO PS 2–4), unilateral tumor location, one lobe involved, younger age and no comorbidities.
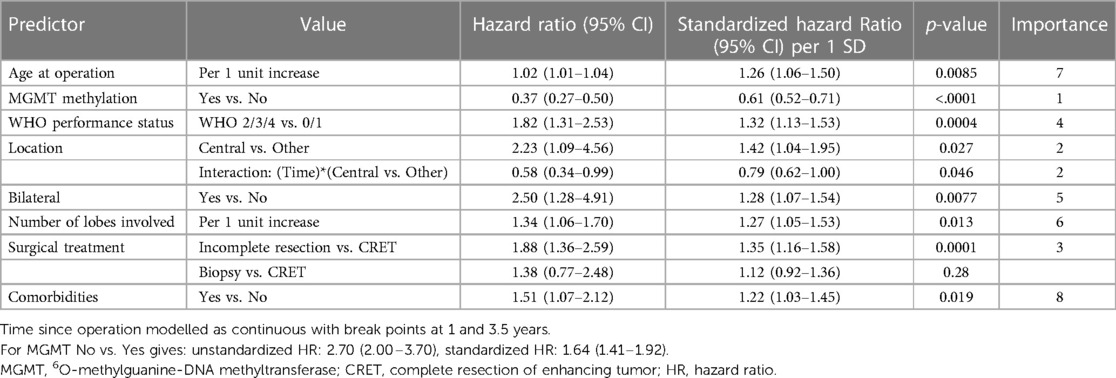
Table 4. Significant multivariable model including independent predictors using extended poisson regression models for time to death.
Table 5 shows the comparison of main characteristics between the two cohorts. The OS in the cohort from 2004 to 2008 was 0.73 years (8.8 months), compared to 1.07 years (12.8 months) in the cohort from 2012 to 2016, including the 25 patients with IDH-mutated tumors. Survival rates at 6, 12 and 24 months were respectively 65%, 36%, 11% in the previous cohort vs. 84%, 57%, 24% in the new cohort. Figure 3 shows the differences regarding median OS in relation to age, surgical treatment and post-operative oncological treatment between the two cohorts.
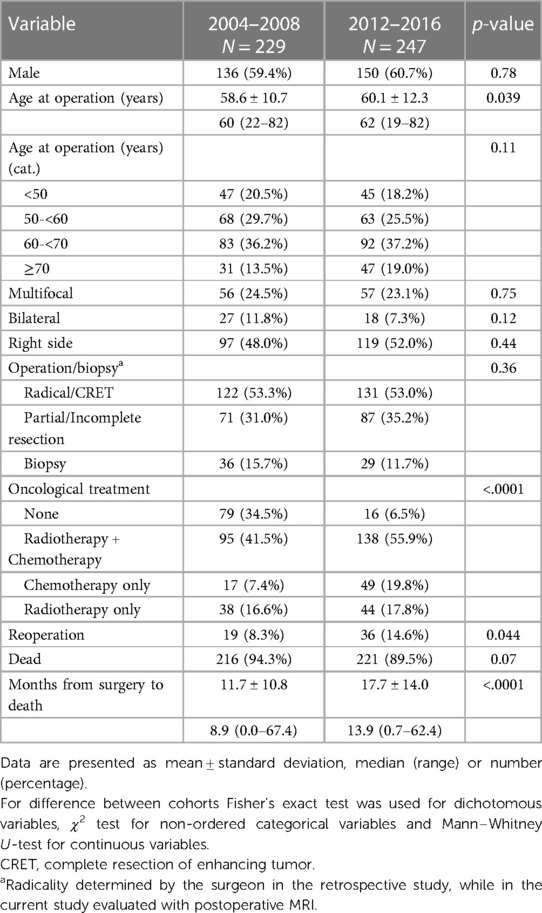
Table 5. Comparison of patient characteristics, tumor characteristics and treatment characteristics between our two cohorts.
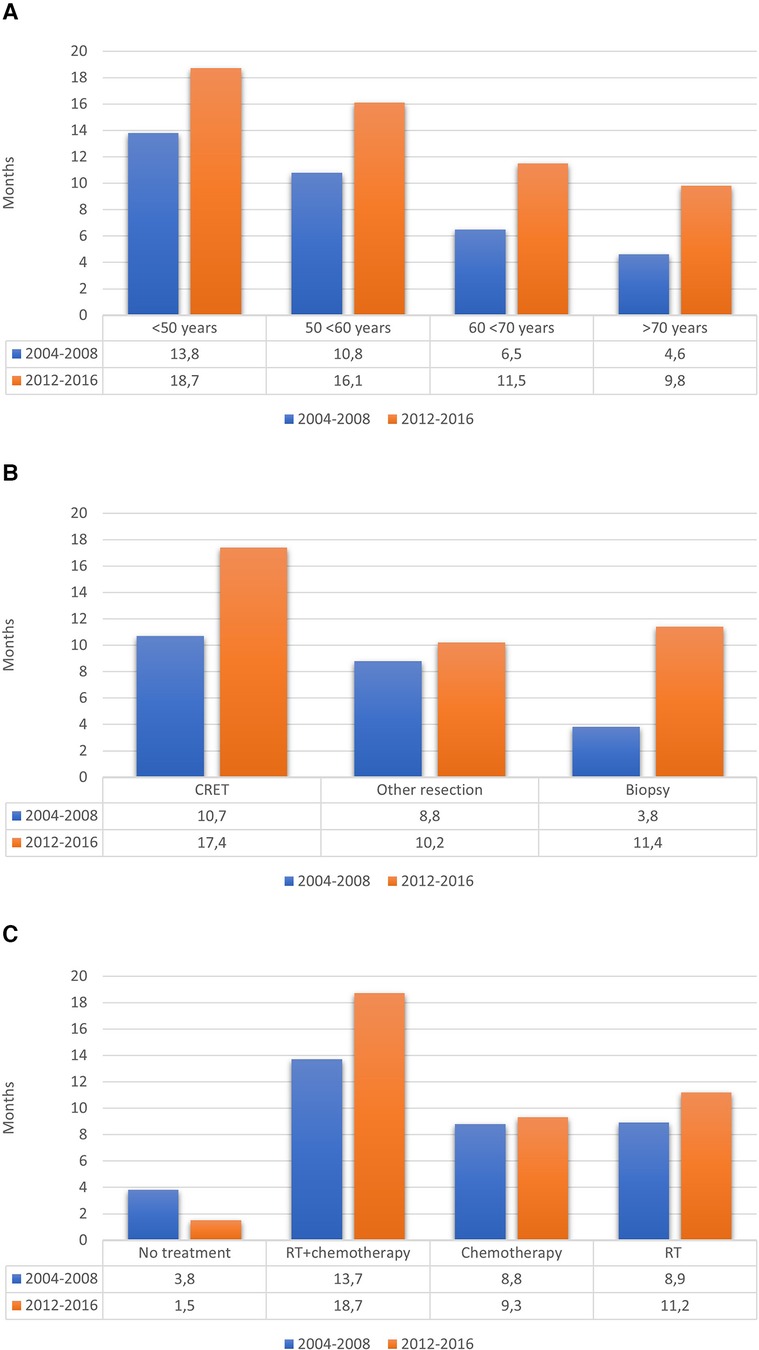
Figure 3. Median OS in relation to (A) age, (B) surgical treatment and (C) post-operative oncological treatment.
Healthcare in Sweden for patients with complicated or rare diseases such as GBM is centralized at university hospitals. This allows population-based studies, ensuring data from all patients presenting with the disease within a defined time period. Here, we present data on the clinical management and outcome in patients operated for GBM during 2012–2016. We found a significant change towards more active surgery and multimodal treatment over time with consequent increased survival, in comparison with a corresponding study from 2004 to 2008. Of the established prognostic markers, MGMT promoter hypermethylation was the strongest predictor for longer survival in our cohort. Other independent variables for prognosis were tumor location, extent of surgery, PS, age and comorbidities.
These findings are all consistent with earlier studies, including large, randomized trials that have provided accumulating evidence for the predictive value of MGMT promoter methylation in GBM (14, 28–31). It is important to note that there may have been synergism between various favourable treatment-related factors in our material. This is reflected by the long median OS of 2.21 years in the subgroup who had CRET, postoperative chemoradiotherapy and adjuvant TMZ, and hypermethylated MGMT promoter, in which more than 10% of patients survived 4 years or longer. It is obvious from our data and previously published studies that small subgroups of patients exist with GBM who will be long-term survivors. In contrast, central tumor location and bilateral tumor location were identified as independent predictors for poor survival. Similar findings have been shown in earlier studies (4, 7, 10, 32, 33). Fyllingen et al. for example reported reduced OS in patients with centrally located tumors, and suggested the actual distance from the center of third ventricle to the contrast-enhancing tumor as a possible prognostic factor for survival (34). A nuance in our study was that for patients with central tumor location, there was a significant interaction with time. This statistical interaction means that the value of HR changes over time. In other words, patients with a central tumor component have a significantly higher risk of death at the time of diagnosis than those with non-central tumor location, but this risk gradually decreases over time.
Regarding the role of surgery, MRI-defined CRET was a strong independent prognostic factor in our cohort, which is in line with previous studies, especially in post-TMZ era (6, 9, 22, 35). Of interest in this context, and corroborating earlier studies in GBM IDH-wt with data on MGMT methylation status, is the fact that the prognostic benefit of the incomplete resection was not different from biopsy (36, 37).The role of the extent of surgery and its effect on survival are controversial, with those proposing precise thresholds for the extent of resection to achieve a significant effect on survival, while others suggest a continuous relationship between the extent of surgery and survival (38).
Another expected finding was the favourable prognostic effect of good PS, confirming previous population-based studies (4, 21, 22, 29). We recorded PS at the time point before surgery, and found a similar distribution of the different WHO PS grades for these patients as reported by Hansen et al. (4).
Age was another strong prognostic factor, corroborating that younger age in patients with GBM is an independent prognostic factor for survival (4, 20, 39, 40).
Approximately 50% of the patients initiated the full multimodal treatment regime in our cohort with postoperative chemoradiotherapy and adjuvant TMZ, comparable with the population-based studies by Hansen et al. (50%) and Eriksson et al. (55%) (4, 22). The median OS for these patients was 19.5 months, i.e., somewhat higher than 16.2 months and 16.4 months reported in similar population-based settings (19, 29).
The secondary goal of this study was to investigate changes in outcome and clinical management over time. For this purpose, we compared the obtained data with those from a previously published cohort at our hospital. Although the inclusion criteria in both studies were similar, there were some differences in study designs, requiring carefulness in the interpretation of the results. As highlighted by Preusser et al., using historical controls strongly limits the comparability of data between studies with differences in inclusion criteria or study design (41). Another major difference is that in the current study, the extent of resection was evaluated by MRI, but evaluated by the operating surgeon in the historical cohort. Comparing data from several studies, Woehrer et al. describes a survival gain of about 1–2 months after the introduction of TMZ in the first line treatment (42). Postoperative oncological treatment with chemoradiotherapy and adjuvant TMZ was introduced in 2006 in our region, and successively implemented during the following years. Our hypothesis was that implementing this treatment contributed to longer survival in the population of GBM patients in our region. Compared to our previous study (2004–2008), there was indeed a significant increase in OS for patients with GBM treated according to current standard treatment (0.73 years = 8.8 months vs. 1.07 years = 12.8 months). These data are comparable to the result of a Swedish study by Bruhn et al. who reported a median OS of 8.6 months before vs. 12.3 months after the introduction of postoperative chemoradiotherapy and adjuvant TMZ (20). Other population-based studies from the post-TMZ era reported a slightly lower median OS; between 10.0 and 11.8 months (4, 19, 21, 22, 29, 40, 43).
It is, however, unlikely that the change in postsurgical treatment strategies is the only explanation for the increased OS for patients with GBM. Indeed, the proportion of patients with suspected GBM not selected for surgery has steadily decreased (46.7% vs. 34.8%) since our previous study, reflecting a more optimistic surgical attitude, while at the same time, the number of patients undergoing surgery has increased for each year. Thus, there was a clear trend in our region towards older patients being accepted for surgery compared to previous years. The proportion of operated patients over 70 years old was 19.0%, compared to 13.5% in the previous study. Interestingly, a numerically longer median OS was present for all age categories (Figure 3A), suggesting that the positive effect of a more active surgical approach was independent of biological age.
The proportion of patients that underwent complete resection was about the same in the previous retrospective study as in the recent study (53.3% and 53.0%, respectively). The high percentage of radically operated patients in the retrospective study may be explained by the fact that the extent of surgery was estimated by the surgeon, while in the recent study the assessment of radicality was based on postoperative MRI. By this approach, a larger proportion of patients underwent partial surgery/incomplete resection (35.2% vs. 31.0%) and a smaller proportion of patients underwent biopsy (15.7% vs. 11.7%).
To conclude, and as clearly illustrated in Figure 3B, the median OS for patients with GBM in recent years has increased in all groups, regardless of the extent of resection. There may be several factors explaining the increased OS: the surgical as well the imaging techniques have improved, and more patients have received postoperative oncological treatment. In the current study, considerably more patients initiated both radio- and chemotherapy, 55.9% vs. 41.5%. Furthermore, the proportion of patients who did not receive any postoperative oncological treatment has decreased significantly (34.5% vs. 6.5%) over time. Compared to the studies by Fabbro-Peray et al., Hansen et al. and Graus et al., this proportion was low (6.5% vs. 20%, 22% respectively 22.3%) (4, 21, 43). There was also a tendency to give radiotherapy or chemotherapy as single treatment to patients among those who have not been considered suitable for chemoradiotherapy.
The main strength of our study is that it is population-based, with high external validity. We were able to collect almost complete data for MGMT promoter methylation status. Although data were prospectively collected, the analysis was of retrospective character, which should be regarded as a limitation. Consequently, we were not always able to register if patients actually completed the prescribed oncological treatment.
MGMT promoter methylation status, followed by non-central tumor location and CRET, were the strongest prognostic factors for survival in this population-based study of patients with GBM. We found a significant change towards more active treatment strategies over time with consequent increased survival for patients with GBMs in our region, especially in the best prognostic group.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Regional Ethical Committee in the region of Västra Götaland. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
BF, KW and BR contributed to the study conception and design. Data collection was performed by BF and BR Interpretation of statistical analysis was performed by BF, KW, AS, AP, AJ, MT and KW The first draft of the manuscript was written by BF, and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
This study was financed by grants from the Health Medical Care Committee at Västra Götaland Regional FoU-support (BR: VGFOUREG-750851), AFA Research Foundation, the Gothenburg Foundation for Neurological Research, and the Sahlgrenska Academy at Gothenburg University.
The authors would like to acknowledge Gerd Ekstedt and Ünzüle Yildiz for support in collecting data, Thomas Olsson Bontell and Sandra Ferreyra Vega for help with the molecular analyses.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CRET, complete resection of enhancing tumor; GBM, glioblastoma; HR, hazard ratio; IDH, isocitrate dehydrogenase; MGMT, 6O-methylguanine-DNA methyltransferase; OS, overall survival; PS, performance status; RT, radiotherapy; wt, wildtype.
1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
2. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro-Oncol. (2021) 23(12 Suppl 2):iii1–105. doi: 10.1093/neuonc/noab200
3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncol. (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
4. Hansen S, Rasmussen BK, Laursen RJ, Kosteljanetz M, Schultz H, Norgard BM, et al. Treatment and survival of glioblastoma patients in Denmark: the Danish neuro-oncology registry 2009–2014. J Neuro-Oncol. (2018) 139(2):479–89. doi: 10.1007/s11060-018-2892-7
5. Fekete B, Werlenius K, Orndal C, Rydenhag B. Prognostic factors for glioblastoma patients–a clinical population-based study. Acta Neurol Scand. (2016) 133(6):434–41. doi: 10.1111/ane.12481
6. Stark AM, van de Bergh J, Hedderich J, Mehdorn HM, Nabavi A. Glioblastoma: clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg. (2012) 114(7):840–5. doi: 10.1016/j.clineuro.2012.01.026
7. Helseth R, Helseth E, Johannesen TB, Langberg CW, Lote K, Ronning P, et al. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand. (2010) 122(3):159–67. doi: 10.1111/j.1600-0404.2010.01350.x
8. Chaichana K, Parker S, Olivi A, Quinones-Hinojosa A. A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. J Neurosurg. (2010) 112(5):997–1004. doi: 10.3171/2009.9.JNS09805
9. Scoccianti S, Magrini SM, Ricardi U, Detti B, Buglione M, Sotti G, et al. Patterns of care and survival in a retrospective analysis of 1059 patients with glioblastoma multiforme treated between 2002 and 2007: a multicenter study by the central nervous system study group of airo (Italian association of radiation oncology). Neurosurgery. (2010) 67(2):446–58. doi: 10.1227/01.NEU.0000371990.86656.E8
10. Tait MJ, Petrik V, Loosemore A, Bell BA, Papadopoulos MC. Survival of patients with glioblastoma multiforme has not improved between 1993 and 2004: analysis of 625 cases. Br J Neurosurg. (2007) 21(5):496–500. doi: 10.1080/02688690701449251
11. Li SW, Qiu XG, Chen BS, Zhang W, Ren H, Wang ZC, et al. Prognostic factors influencing clinical outcomes of glioblastoma multiforme. Chin Med J. (2009) 122(11):1245–9. doi: 10.3760/cma.j.issn.0366-6999.2009.11.002
12. Mineo JF, Bordron A, Baroncini M, Ramirez C, Maurage CA, Blond S, et al. Prognosis factors of survival time in patients with glioblastoma multiforme: a multivariate analysis of 340 patients. Acta Neurochir. (2007) 149(3):245–52.; discussion 52-3. doi: 10.1007/s00701-006-1092-y
13. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330
14. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. (2005) 352(10):997–1003. doi: 10.1056/NEJMoa043331
15. Rodríguez-Camacho A, Flores-Vázquez JG, Moscardini-Martelli J, Torres-Ríos JA, Olmos-Guzmán A, Ortiz-Arce CS, et al. Glioblastoma treatment: state-of-the-art and future perspectives. Int J Mol Sci. (2022) 23(13). doi: 10.3390/ijms23137207
16. Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. (2017) 318(23):2306–16. doi: 10.1001/jama.2017.18718
17. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. (2009) 10(5):459–66. doi: 10.1016/S1470-2045(09)70025-7
18. Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. (2013) 31(32):4085–91. doi: 10.1200/JCO.2013.49.6968
19. Ronning PA, Helseth E, Meling TR, Johannesen TB. A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro Oncol. (2012) 14(9):1178–84. doi: 10.1093/neuonc/nos153
20. Bruhn H, Strandeus M, Milos P, Hallbeck M, Vrethem M, Lind J. Improved survival of Swedish glioblastoma patients treated according to stupp. Acta Neurol Scand. (2018) 138(4):332–7. doi: 10.1111/ane.12966
21. Fabbro-Peray P, Zouaoui S, Darlix A, Fabbro M, Pallud J, Rigau V, et al. Association of patterns of care, prognostic factors, and use of radiotherapy-temozolomide therapy with survival in patients with newly diagnosed glioblastoma: a French national population-based study. J Neuro-Oncol. (2019) 142(1):91–101. doi: 10.1007/s11060-018-03065-z
22. Eriksson M, Kahari J, Vestman A, Hallmans M, Johansson M, Bergenheim AT, et al. Improved treatment of glioblastoma - changes in survival over two decades at a single regional centre. Acta Oncol. (2019) 58(3):334–41. doi: 10.1080/0284186X.2019.1571278
23. Werlenius K, Fekete B, Blomstrand M, Carén H, Jakola AS, Rydenhag B, et al. Patterns of care and clinical outcome in assumed glioblastoma without tissue diagnosis: a population-based study of 131 consecutive patients. PLoS One. (2020) 15(2):e0228480. doi: 10.1371/journal.pone.0228480
24. Ferreyra Vega S, Wenger A, Kling T, Olsson Bontell T, Jakola AS, Carén H. Spatial heterogeneity in DNA methylation and chromosomal alterations in diffuse gliomas and meningiomas. Mod Pathol. (2022) 35(11):1551–61. doi: 10.1038/s41379-022-01113-8
25. Ferreyra Vega S, Olsson Bontell T, Corell A, Smits A, Jakola AS, Carén H. DNA methylation profiling for molecular classification of adult diffuse lower-grade gliomas. Clin Epigenetics. (2021) 13(1):102. doi: 10.1186/s13148-021-01085-7
26. Wenger A, Ferreyra Vega S, Kling T, Bontell TO, Jakola AS, Carén H. Intratumor DNA methylation heterogeneity in glioblastoma: implications for DNA methylation-based classification. Neuro-Oncol. (2019) 21(5):616–27. doi: 10.1093/neuonc/noz011
27. Holford TR. The analysis of rates and of survivorship using log-linear models. Biometrics. (1980) 36(2):299–305. doi: 10.2307/2529982
28. Sonoda Y, Yokosawa M, Saito R, Kanamori M, Yamashita Y, Kumabe T, et al. O(6)-methylguanine DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression is correlated with progression-free survival in patients with glioblastoma. Int J Clin Oncol. (2010) 15(4):352–8. doi: 10.1007/s10147-010-0065-6
29. Brandes AA, Franceschi E, Ermani M, Tosoni A, Albani F, Depenni R, et al. Pattern of care and effectiveness of treatment for glioblastoma patients in the real world: results from a prospective population-based registry. Could survival differ in a high-volume center? Neurooncol Pract. (2014) 1(4):166–71. doi: 10.1093/nop/npu021
30. Urhie O, Turner R, Lucke-Wold B, Radwan W, Ahn J, Gyure K, et al. Glioblastoma survival outcomes at a tertiary hospital in appalachia: factors impacting the survival of patients following implementation of the stupp protocol. World Neurosurg. (2018) 115:e59–66. doi: 10.1016/j.wneu.2018.03.163
31. Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. (2000) 343(19):1350–4. doi: 10.1056/NEJM200011093431901
32. Lutterbach J, Sauerbrei W, Guttenberger R. Multivariate analysis of prognostic factors in patients with glioblastoma. Strahlenther Onkol. (2003) 179(1):8–15. doi: 10.1007/s00066-003-1004-5
33. Kumar N, Kumar P, Angurana SL, Khosla D, Mukherjee KK, Aggarwal R, et al. Evaluation of outcome and prognostic factors in patients of glioblastoma multiforme: a single institution experience. J Neurosci Rural Pract. (2013) 4(Suppl 1):S46–55. doi: 10.4103/0976-3147.116455
34. Fyllingen EH, Bø LE, Reinertsen I, Jakola AS, Sagberg LM, Berntsen EM, et al. Survival of glioblastoma in relation to tumor location: a statistical tumor atlas of a population-based cohort. Acta Neurochir. (2021) 163(7):1895–905. doi: 10.1007/s00701-021-04802-6
35. Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. (2008) 62(3):564–76; discussion -76. doi: 10.1227/01.neu.0000317304.31579.17
36. Gessler F, Bernstock JD, Braczynski A, Lescher S, Baumgarten P, Harter PN, et al. Surgery for glioblastoma in light of molecular markers: impact of resection and MGMT promoter methylation in newly diagnosed IDH-1 wild-type glioblastomas. Neurosurgery. (2019) 84(1):190–7. doi: 10.1093/neuros/nyy049
37. Kreth FW, Thon N, Simon M, Westphal M, Schackert G, Nikkhah G, et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. (2013) 24(12):3117–23. doi: 10.1093/annonc/mdt388
38. Skardelly M, Kaltenstadler M, Behling F, Mäurer I, Schittenhelm J, Bender B, et al. A continuous correlation between residual tumor volume and survival recommends maximal safe resection in glioblastoma patients: a nomogram for clinical decision making and reference for non-randomized trials. Front Oncol. (2021) 11:748691. doi: 10.3389/fonc.2021.748691
39. Teo M, Martin S, Owusu-Agyemang K, Nowicki S, Clark B, Mackinnon M, et al. A survival analysis of GBM patients in the west of Scotland pre- and post-introduction of the stupp regime. Br J Neurosurg. (2013) 28(3): 351–5. doi: 10.3109/02688697.2013.847170
40. Efremov L, Abera SF, Bedir A, Vordermark D, Medenwald D. Patterns of glioblastoma treatment and survival over a 16-years period: pooled data from the German cancer registries. J Cancer Res Clin Oncol. (2021) 147(11):3381–90. doi: 10.1007/s00432-021-03596-5
41. Preusser M, van den Bent MJ. Autologous tumor lysate-loaded dendritic cell vaccination (DCVax-L) in glioblastoma: breakthrough or fata morgana? Neuro-Oncol. (2022) 25(4):631–4. doi: 10.1093/neuonc/noac281
42. Woehrer A, Bauchet L, Barnholtz-Sloan JS. Glioblastoma survival: has it improved? Evidence from population-based studies. Curr Opin Neurol. (2014) 27(6):666–74. doi: 10.1097/WCO.0000000000000144
Keywords: glioblastoma, population-based, survival, prognostic factors, treatment
Citation: Fekete B, Werlenius K, Tisell M, Pivodic A, Smits A, Jakola AS and Rydenhag B (2023) What predicts survival in glioblastoma? A population-based study of changes in clinical management and outcome. Front. Surg. 10:1249366. doi: 10.3389/fsurg.2023.1249366
Received: 28 June 2023; Accepted: 17 August 2023;
Published: 30 August 2023.
Edited by:
Eberval Figueiredo, University of São Paulo, BrazilReviewed by:
Maixmilian Scheer, University Hospital in Halle, Germany© 2023 Fekete, Werlenius, Tisell, Pivodic, Smits, Jakola and Rydenhag. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: B. Fekete Ym9nbGFya2EuZmVrZXRlQHZncmVnaW9uLnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.