- 1Department of General Surgery, Koç University School of Medicine, Istanbul, Turkey
- 2Koc University School of Medicine, Istanbul, Turkey
- 3Department of General Surgery, VKV American Hospital, Istanbul, Turkey
Background: Anastomotic leakage is a major complication in colorectal surgery, resulting in significant morbidity and mortality rates. Despite substantial progress in surgical technique, anastomotic leakage rates remain stable. An early diagnosis of anastomotic leaks was proven to reduce adverse outcomes and improve survival.
Objective: This study aims to find a novel scoring system for detecting anastomotic leaks using inflammatory and nutritional indicators after colorectal surgery. Our purpose was to analyze the diagnostic accuracy of leak scores in predicting postoperative complications.
Design: The study included colorectal cancer patients who underwent curative surgery at Koc University Hospital between 2014 and 2018. Patients were categorized into two groups depending on the presence of anastomotic leaks and compared in terms of preoperative albumin levels, CRP levels in postoperative days 1 and 3, anastomotic leakage rates, length of hospital stay, and CRP quotient, which was calculated by dividing POD 3 CRP level to POD 1 CRP level. The bedside leak score is calculated by dividing the CRP quotient by the preoperative albumin level. The predictive value of bedside leak score, CRP quotient, and preoperative albumin levels in estimating anastomotic leakage was analyzed, and a cutoff value for the leak score was calculated.
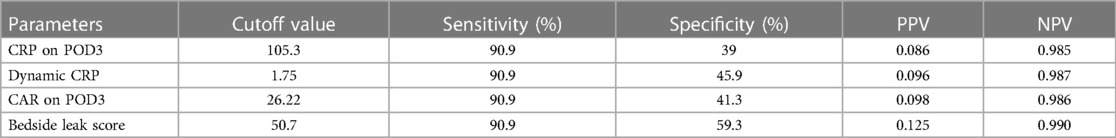
Results: A total of 184 patients were included in the study. The leak score, CRP POD 3–1 ratio, and preoperative albumin levels were found to successfully detect anastomotic leakage. The area under the curve for the leak score was calculated as 0.78. The optimal cutoff value was found to be 50.3 for the bedside leak score, which shows 90.9% sensitivity and 59.3% specificity.
Conclusion: The leak score may represent a valuable diagnostic tool for detecting patients at risk for anastomotic leakage after colorectal surgery and planning a better strategy to reduce morbidity and mortality rates and associated costs. However, further multicenter studies with large cohorts are necessary to confirm these results.
Introduction
Anastomotic leakage (AL) is considered the “Achilles heel” of gastrointestinal tract surgery and is a devastating complication of colorectal surgery. Even in very experienced colorectal surgery centers, the incidence rate of AL is approximately 7% (1, 2). Anastomotic leakage can cause significant morbidity and mortality rates, resulting in decreased disease-free and overall survival along with higher healthcare costs (3–5). Despite recent advances in surgical techniques and perioperative care, the incidence rate of AL remains unchanged (6). Nonetheless, an early diagnosis of AL can prevent adverse short- and long-term outcomes by reducing the rate of permanent stomas and cancer recurrence, which leads to prolonged long-term survival (7).
Once an AL becomes clinically overt, it is usually too late to prevent complications and adverse clinical outcomes. Therefore, risk assessment and prediction scores for AL are crucial in colorectal surgery (8–14). C-reactive protein (CRP), a commonly used inflammatory marker in patient care after colorectal surgery, has clinical significance for detecting infectious complications. However, the CRP level alone does not reflect a patient's nutritional status, including hypoalbuminemia, which is a well-known risk factor in detecting AL (15–17). The CRP/albumin ratio (CAR) was found to have higher predictivity and accuracy. However, the CAR lacks clarity in the trajectory of CRP, which was shown to help rule out AL (11). C-reactive protein reaches its peak value 3 days after surgery, but increased values are not always associated with complications. As a result, a new score that incorporates the change in the inflammatory state and the nutritional status which can be practically calculated at the bedside is needed (11, 13, 18).
This study aims to find a novel scoring system for detecting colorectal AL using inflammatory and nutritional indicators. The CRP quotient on postoperative days 1 and 3 (POD1 and POD3) is divided into preoperative albumin levels and multiplied by 100 to obtain a new score: the bedside leak score. This study's purpose is to establish the success of the bedside leak score in detecting AL in advance.
Materials and methods
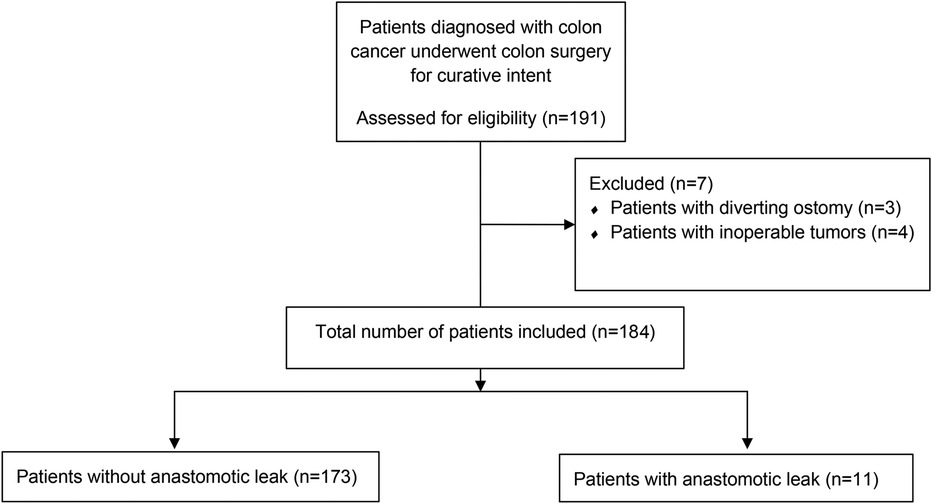
This study included patients with a histopathological diagnosis of colon cancer who underwent curative surgery between 2014 and 2018 in a tertiary care colorectal surgery clinic. Patient records were prospectively collected and retrospectively analyzed. Data, including patient demographics, tumor location, type of tumor pathology, Clavien–Dindo score, mortality within 30 days, surgical procedure, type of surgery, preoperative albumin levels, CRP levels on POD 1 and 3, CAR on POD 1 and 3, time of operation, day of diagnosis of AL, presence of AL, and length of hospital stay, were obtained (Figure 1). The study excluded patients who have inoperable tumors, underwent emergent or urgent surgery, received neoadjuvant treatment, and have diverting ostomies. We did not routinely create a diverting ostomy unless the patient had a tumor that caused an obstruction. A diagnosis of AL was based on clinical suspicion confirmed by extraluminal contrast extravasation, gas-containing collections around anastomosis shown on computed tomography scans with oral contrast, or anastomotic disruption seen on endoscopy.
The CRP quotient on POD 1 and 3 was divided into preoperative albumin levels and multiplied by 100 to obtain the bedside leak score for all patients (Figure 2).
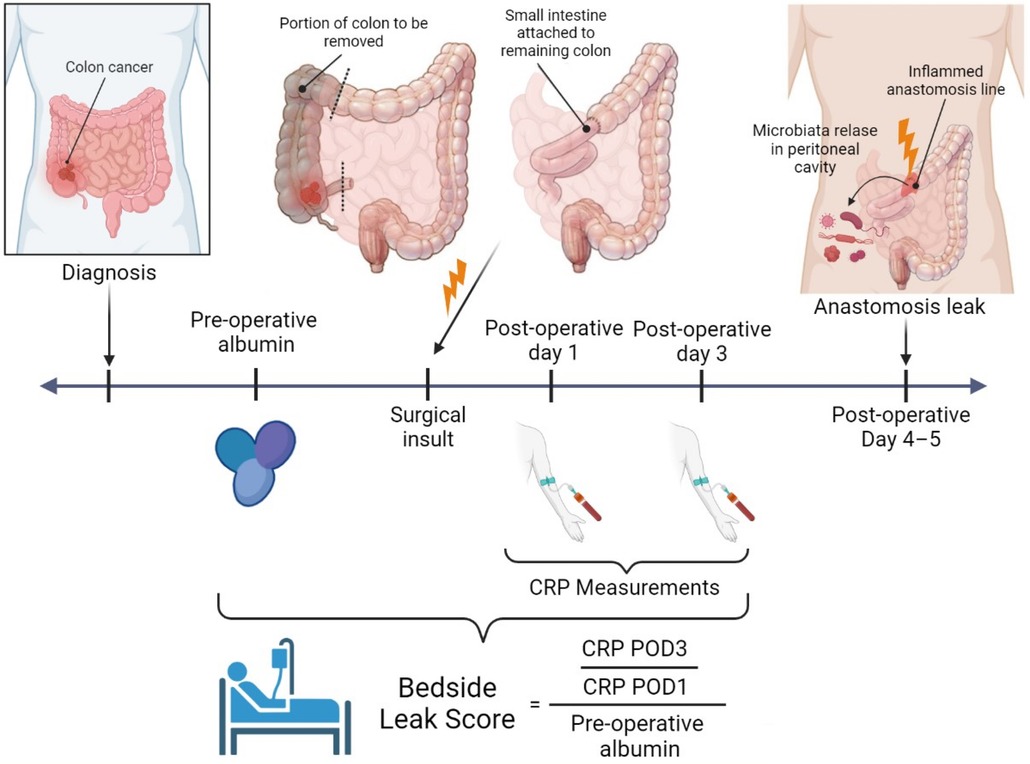
Figure 2. Bedside leak score—created with BioRender.com.
This study was approved by the Koc University Ethics Committee with Approval No. 2021. 314.IRB1.140, and all patients filled out a written informed consent form for participation and consent for publication. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Statistical analysis was performed using SPSS version 26 software (IBM, USA). The bedside leak score, POD 1 and POD3/POD1 (dynamic) CRP levels, CAR on POD1 and POD3, and albumin levels in patients who experienced AL were compared with those of the patients who did not have AL using the independent samples Kruskal–Wallis test. The patient's age, body mass index (BMI), and CRP levels on POD3 were tested using an independent sample T-test. The Mann–Whitney U-test was used to compare the length of hospital stay and operative time in patients with and without AL. Receiver operating characteristic (ROC) curves were obtained for the dynamic CRP levels, CRP on POD1 and POD3, CAR on POD1 and POD3, and bedside leak score to estimate AL. The area under the ROC curve values was divided into four groups: >0.90 = excellent, 0.80–0.90 = good, 0.70–0.80 = fair, and <0.60 = poor. A statistically significant value was accepted at p ≤ 0.050.
Results
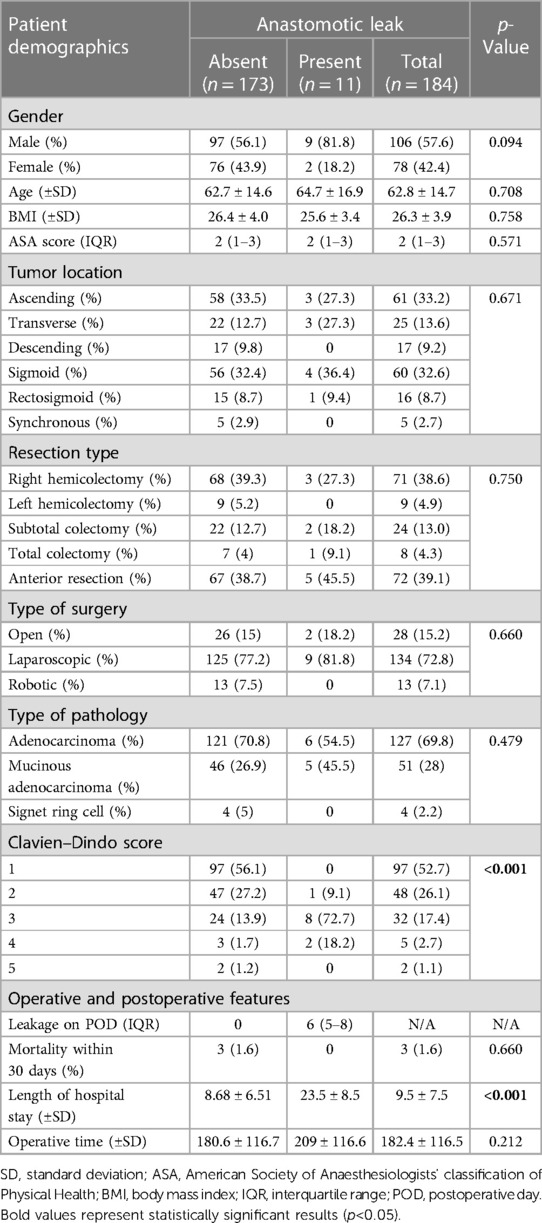
After excluding patients with inoperable tumors (n = 4) and diverting ostomies (n = 3), this study included 183 patients. Table 1 shows the clinical data of the patients. The two groups were comparable in terms of patient demographics (gender, age, BMI, and ASA scores). The Clavien–Dindo scores were found to be higher in the AL group. No mortality was observed in the AL group. The length of hospital stay was significantly longer in patients with AL (23.5 vs. 8.7 days, p < 0.010). The operative time was found to be longer in the AL group (209 vs. 180 min), despite failing to reach statistical significance.
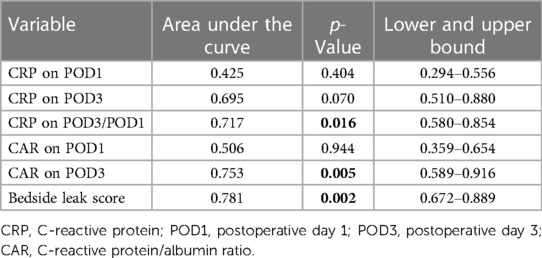
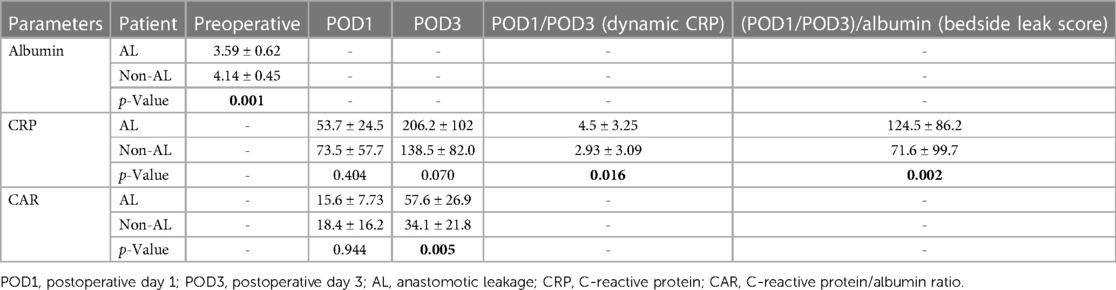
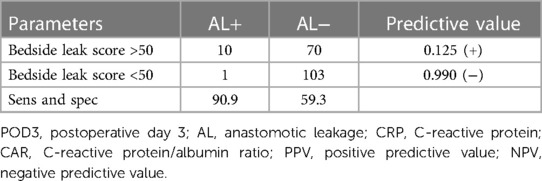
The association between AL and preoperative albumin levels, bedside leak score, CAR on POD3, and dynamic CRP (CRP POD3/POD1) was statistically significant with p-values of 0.001, 0.002, 0.005, and 0.016, respectively (Table 3). Furthermore, to compare diagnostic values among bedside leak score, dynamic CRP levels, CRP on POD1 and POD3, and CAR on POD1 and POD3, ROC curves were generated, and the areas under the curve (AUCs) for each diagnostic parameter were calculated (Tables 2, 3). The AUCs for the bedside leak score, CRP POD 3–1 ratio, CRP POD3, and CAR POD3 were 0.781, 0.717, 0.695, and 0.753, respectively (Figure 3 and Table 2). The bedside leak score was found to have a sensitivity of 90.9, with a cutoff value of 50.7, which shows the lowest false positive ratio among them (Tables 4, 5).
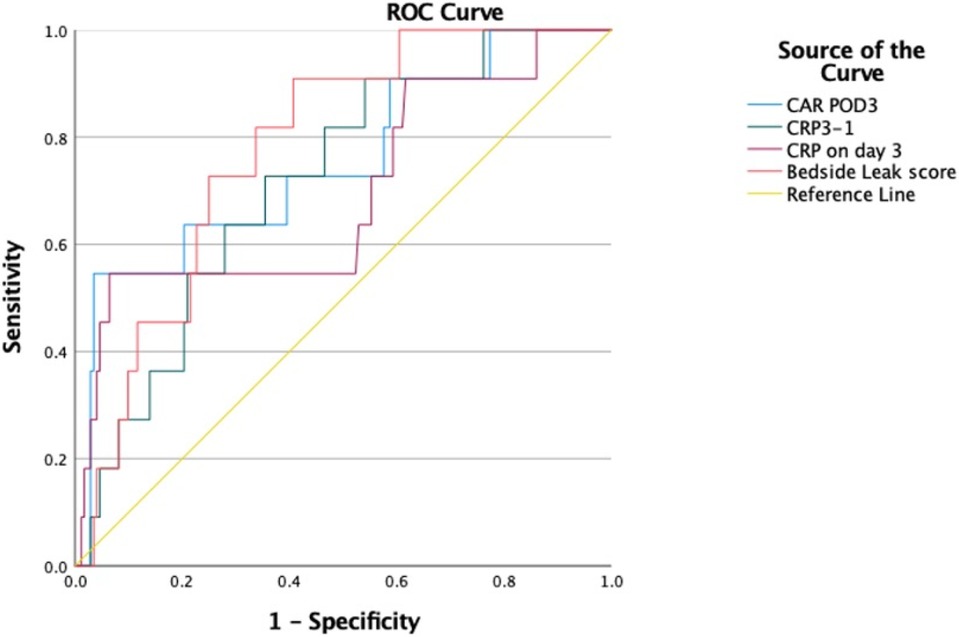
Figure 3. ROC curves for bedside leak score, CRP 3–1 ratio, CRP on day 3, and CRP to albumin ratio in postoperative day 3.
Discussion
Compared with other traditional diagnostic measures, including preoperative albumin levels and CRP levels on postoperative days 1 and 3, the bedside leak score provides statistically valuable diagnostic predictive power. With a cutoff value of 50.7, the sensitivity of the bedside leak score is 90.9, which is a significant value for a screening score.
Anastomotic leakage is one of the most devastating complications of colorectal surgery because of its high morbidity and mortality rates. Early AL prediction and diagnosis AL improve short-term morbidity and mortality rates and long-term survival (7). Therefore, substantial efforts are made to predict and prevent this complication, especially after colon cancer surgery (19, 20).
To detect AL early and prevent morbidity, several blood markers and extravascular fluid biomarkers, including CRP levels, are used in many studies. In addition, the clinical use of the spot and trajectory values of CRP (11, 13), CAR (18, 21), and novel systemic inflammatory biomarkers (10) is still under investigation in several surgical practices.
This study examined the accuracy of dynamic CRP changes (POD3 to POD1), spot CRP values (POD1 and POD3), CAR on POD 1 and 3, and the bedside leak score (dynamic CRP/albumin) in predicting AL. The results indicated that the bedside leak score was the most accurate predictive test.
Looking at the predictive value of CRP, one of the key differences between this study and previous ones is that this study assessed dynamic CRP rather than spot values (13). The inflammatory response due to AL increases gradually. Therefore, systemic inflammatory markers such as CRP increase progressively in response to AL. A postoperative increase in CRP is expected, but the level depends on the degree of surgical damage. Using daily spot values of CRP to assess evolving conditions is less likely to be accurate. A test to evaluate dynamic changes is more advantageous than an isolated spot value because AL gradually develops (11). This study also reveals how the CRP levels of patients with AL exponentially increased compared with those of the non-AL group, highlighting the importance of the trajectory model.
This study evaluated the prediction of AL with a dynamic CRP to preoperative albumin ratio (the bedside leak score), which is the trajectory model. The CAR is the arbitrary CRP to albumin ratio (18, 21). In this study, albumin is preferred because it is a good predictor of malnutrition and has been proven to be an early detection marker for postoperative complications. Low preoperative albumin levels increase the risk of postoperative complications such as AL (15, 22). Previous studies used the CAR to determine the prognosis and overall survival of cancer patients (23–25). On the other hand, only a few articles use CAR to predict AL following colorectal cancer surgery (18, 21), and only one article used the trajectory model of CRP to predict AL (11). However, no studies in the literature specifically anticipated AL by considering the ratio of dynamic CRP changes to preoperative albumin levels.
Few articles investigated the predictive value of inflammatory biomarkers [interleukin (IL)-1β, IL-6, IL-10, TNF-α, and MMP-2 and -9] in the peritoneal fluid of cancer patients who underwent surgery and experienced AL in different locations (11, 26, 27). Even though peritoneal fluid biomarkers have a higher potential of detecting AL than systemic markers, Shi et al. (26) stated that investigating such inflammatory markers in the peritoneal fluid cannot be applied in every institution due to their low cost-effectivity and limited availability of kits. The components of the bedside leak score (i.e., the preoperative albumin and the CRP on POD1 and POD3) are commonly used blood parameters in patient care after colon cancer surgeries. Therefore, the bedside leak score prevents additional costs and provides a tremendous economic advantage by avoiding complications due to AL.
The high sensitivity score and AUC of the bedside leak score make this test highly valuable for AL screening. Smith et al. (28) showed high sensitivity of the trajectory model of CRP values. However, this model has low specificity compared with the bedside leak score. This finding emphasizes that the bedside leak score is more accurate in predicting AL, with lower false positive results, than the trajectory model. Therefore, using the bedside leak score may prevent unnecessary tests or interventions aimed at confirming the diagnosis of AL. Paliogiannis et al. (18) revealed that the CAR had great potential in predicting AL with very low false positive rates. However, compared with the bedside leak score, its sensitivity is lower as a screening test for AL. The high sensitivity and relatively high specificity of the bedside leak score are crucial for anticipating AL and preventing unnecessary, costly diagnostic tests.
The bedside leak score also adds objectivity to clinical suspicion. In clinical practice, evaluating patients for potential AL includes a physical examination, drain inspection, and close monitoring of postoperative vital signs. Fever, abdominal pain, nausea and vomiting, low blood pressure, and tachycardia are some clinical symptoms of AL. Hence, suspicion of an AL is up to the surgeon's clinical experience. The subjectivity of the assessment may cause delays in an interventional attempt to prevent complications from AL, especially in centers with low experience. However, the bedside leak score can predict AL before any symptoms appear. In a study that was validated prospectively, an online objective anastomotic risk calculator was found to be a remarkably reliable tool in predicting AL. Differences in risk perception, personal risk tolerance, or the pressure of external biases could affect surgeons’ decisions before taking action (29). Surgeons’ personal experience can also affect their assessment. For these reasons, it is vital to have a scoring system based on patient data, allowing the surgeon to evaluate the situation objectively. A subjective scoring system such as the bedside leak score allows other healthcare providers to verify the condition with the same impartiality.
Fluorescent-guided surgery, particularly the use of indocyanine green, has emerged as a valuable tool for the prediction of anastomotic leakage in colorectal surgery. However, indocyanine green was not routinely used in this cohort of patients as the study enrollment period ended in 2018. However, in recent years, with promising reports on the use of intraoperative fluorescence imaging in evaluating anastomosis perfusion, we have started to incorporate this technique into our routine clinical practice, especially during minimally invasive surgeries (30, 31). The addition of ICG to the bedside leak score might be valuable and can increase its predictive value. Future studies might consider utilizing ICG in scoring systems.
There were several limitations to this study. First, this investigation was performed in a single center, and the sample size of this retrospective study was not big enough to validate the bedside leak score internally and externally. As surgical technique and perioperative management are crucial in postoperative complications, multicentric and prospective studies are necessary to confirm the results of this study. In addition, one of the major limitations of this study is that it is a retrospective study and relies on previously collected data. The study's retrospective nature makes it susceptible to selection and information bias.
Conclusion
This retrospective study compared the novel bedside leak score with commonly used serum biomarkers and newly used formulations in predicting AL following colon cancer surgeries. Among them, the bedside leak score, with a cutoff value of 50, was the most accurate in terms of providing preoperative risk evaluation and predicting AL based on the trajectory of CRP levels. Its high sensitivity indicates that the bedside leak score may represent a promising screening tool for predicting AL and assist surgeons in planning a better strategy to reduce morbidity and mortality rates and associated healthcare costs. However, due to the inherent limitations of the study design, further multicenter studies with large cohorts are necessary to confirm the real value of this score in clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Koc University Ethics Committee with the Approval No. 2021. 314.IRB1.140.
Author contributions
IO: study conception and design, drafting the article, critical revision for intellectual content, and final approval. EB: study conception and design and final approval. SS: data analysis and interpretation and final approval. SK: data analysis and interpretation and final approval. FCa: data acquisition and final approval. FCe: data acquisition and final approval. EO: data analysis and interpretation and final approval. OA: critical revision for intellectual content and final approval. EB: critical revision for intellectual content and final approval. DB: critical revision for intellectual content and final approval. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alves A, Panis Y, Trancart D, Regimbeau JM, Pocard M, Valleur P. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg. (2002) 26(4):499–502. doi: 10.1007/s00268-001-0256-4
2. Phitayakorn R, Delaney CP, Reynolds HL, Champagne BJ, Heriot AG, Neary P, et al. Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J Surg. (2008) 32(6):1147–56. doi: 10.1007/s00268-008-9468-1
3. Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK. Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum. (2016) 59(3):236–44. doi: 10.1097/DCR.0000000000000554
4. Takahashi H, Haraguchi N, Nishimura J, Hata T, Yamamoto H, Matsuda C, et al. The severity of anastomotic leakage may negatively impact the long-term prognosis of colorectal cancer. Anticancer Res. (2018) 38(1):533–9. doi: 10.21873/anticanres.12255
5. Turrentine FE, Denlinger CE, Simpson VB, Garwood RA, Guerlain S, Agrawal A, et al. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg. (2015) 220(2):195–206. doi: 10.1016/j.jamcollsurg.2014.11.002
6. Vallance A, Wexner S, Berho M, Cahill R, Coleman M, Haboubi N, et al. A collaborative review of the current concepts and challenges of anastomotic leaks in colorectal surgery. Colorectal Dis. (2017) 19(1):O1–o12. doi: 10.1111/codi.13534
7. Branagan G, Finnis D. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum. (2005) 48(5):1021–6. doi: 10.1007/s10350-004-0869-4
8. Coakley KM, Sarasani S, Prasad T, Steele SR, Paquette I, Heniford BT, et al. MELD-Na score as a predictor of anastomotic leak in elective colorectal surgery. J Surg Res. (2018) 232:43–8. doi: 10.1016/j.jss.2018.04.012
9. Mik M, Dziki L, Berut M, Trzcinski R, Dziki A. Neutrophil to lymphocyte ratio and C-reactive protein as two predictive tools of anastomotic leak in colorectal cancer open surgery. Dig Surg. (2018) 35(1):77–84. doi: 10.1159/000456081
10. Shi J, Wu Z, Wu X, Shan F, Zhang Y, Ying X, et al. Early diagnosis of anastomotic leakage after colorectal cancer surgery using an inflammatory factors-based score system. BJS Open. (2022) 6(3):1–6. doi: 10.1093/bjsopen/zrac069
11. Stephensen BD, Reid F, Shaikh S, Carroll R, Smith SR, Pockney P. C-reactive protein trajectory to predict colorectal anastomotic leak: PREDICT study. Br J Surg. (2020) 107(13):1832–7. doi: 10.1002/bjs.11812
12. Wada H, Tominaga T, Nonaka T, To K, Hamada K, Araki M, et al. Charlson comorbidity index predicts anastomotic leakage in patients with resected right-sided colon cancer. Surg Today. (2022) 52(5):804–11. doi: 10.1007/s00595-022-02472-0
13. Yeung DE, Peterknecht E, Hajibandeh S, Hajibandeh S, Torrance AW. C-reactive protein can predict anastomotic leak in colorectal surgery: a systematic review and meta-analysis. Int J Colorectal Dis. (2021) 36(6):1147–62. doi: 10.1007/s00384-021-03854-5
14. Zhong B, Lin ZY, Ma DD, Shang ZH, Shen YB, Zhang T, et al. A preoperative prediction model based on lymphocyte-C-reactive protein ratio predicts postoperative anastomotic leakage in patients with colorectal carcinoma: a retrospective study. BMC Surg. (2022) 22(1):283. doi: 10.1186/s12893-022-01734-5
15. Lai CC, You JF, Yeh CY, Chen JS, Tang R, Wang JY, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis. (2011) 26(4):473–81. doi: 10.1007/s00384-010-1113-4
16. McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. (2015) 102(5):462–79. doi: 10.1002/bjs.9697
17. Telem DA, Chin EH, Nguyen SQ, Divino CM. Risk factors for anastomotic leak following colorectal surgery: a case-control study. Arch Surg. (2010) 145(4):371–6; discussion 376. doi: 10.1001/archsurg.2010.40
18. Paliogiannis P, Deidda S, Maslyankov S, Paycheva T, Farag A, Mashhour A, et al. C reactive protein to albumin ratio (CAR) as predictor of anastomotic leakage in colorectal surgery. Surg Oncol. (2021) 38:101621. doi: 10.1016/j.suronc.2021.101621
19. Hüser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J, et al. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg. (2008) 248(1):52–60. doi: 10.1097/SLA.0b013e318176bf65
20. Phan K, Oh L, Ctercteko G, Pathma-Nathan N, El Khoury T, Azam H, et al. Does a stoma reduce the risk of anastomotic leak and need for re-operation following low anterior resection for rectal cancer: systematic review and meta-analysis of randomized controlled trials. J Gastrointest Oncol. (2019) 10(2):179–87. doi: 10.21037/jgo.2018.11.07
21. Yu Y, Wu Z, Shen Z, Cao Y. Preoperative C-reactive protein-to-albumin ratio predicts anastomotic leak in elderly patients after curative colorectal surgery. Cancer Biomark. (2020) 27(3):295–302. doi: 10.3233/CBM-190470
22. Moghadamyeghaneh Z, Hwang G, Hanna MH, Phelan MJ, Carmichael JC, Mills SD, et al. Even modest hypoalbuminemia affects outcomes of colorectal surgery patients. Am J Surg. (2015) 210(2):276–84. doi: 10.1016/j.amjsurg.2014.12.038
23. Haruki K, Shiba H, Shirai Y, Horiuchi T, Iwase R, Fujiwara Y, et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg. (2016) 40(9):2254–60. doi: 10.1007/s00268-016-3491-4
24. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. (2016) 23(3):900–7. doi: 10.1245/s10434-015-4948-7
25. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. (2015) 22(3):803–10. doi: 10.1245/s10434-014-4048-0
26. Shi J, Wu Z, Wu X, Shan F, Zhang Y, Ying X, et al. Early diagnosis of anastomotic leakage after gastric cancer surgery via analysis of inflammatory factors in abdominal drainage. Ann Surg Oncol. (2022) 29(2):1230–41. doi: 10.1245/s10434-021-10763-y
27. Sparreboom CL, Komen N, Rizopoulos D, Verhaar AP, Dik WA, Wu Z, et al. A multicentre cohort study of serum and peritoneal biomarkers to predict anastomotic leakage after rectal cancer resection. Colorectal Dis. (2020) 22(1):36–45. doi: 10.1111/codi.14789
28. Smith SR, Pockney P, Holmes R, Doig F, Attia J, Holliday E, et al. Biomarkers and anastomotic leakage in colorectal surgery: C-reactive protein trajectory is the gold standard. ANZ J Surg. (2018) 88(5):440–4. doi: 10.1111/ans.13937
29. Sammour T, Lewis M, Thomas ML, Lawrence MJ, Hunter A, Moore JW. A simple web-based risk calculator (www.anastomoticleak.com) is superior to the surgeon’s estimate of anastomotic leak after colon cancer resection. Tech Coloproctol. (2017) 21(1):35–41. doi: 10.1007/s10151-016-1567-7
30. Iguchi K, Watanabe J, Suwa Y, Chida K, Atsumi Y, Numata M, et al. The usefulness of indocyanine green fluorescence imaging for intestinal perfusion assessment of intracorporeal anastomosis in laparoscopic colon cancer surgery. Int J Colorectal Dis. (2023) 38(1):7. doi: 10.1007/s00384-023-04307-x
31. Losurdo P, Mis TC, Cosola D, Bonadio L, Giudici F, Casagranda B, et al. Anastomosis leak: is there still a place for indocyanine green fluorescence imaging in colon-rectal surgery? A retrospective, propensity score-matched cohort study. Surg Innov. (2022) 29(4):511–8. doi: 10.1177/1553350620975258
Keywords: colorectal cancer, colon cancer, colorectal surgery, anastomosis, anastomotic leakage, scoring systems
Citation: Ozata IH, Bozkurt E, Sucu S, Karahan SN, Camci F, Cetin F, Ozoran E, Agcaoglu O, Balik E and Bugra D (2023) A novel scoring system for the early detection of anastomotic leakage: bedside leak score—a pilot study. Front. Surg. 10:1204785. doi: 10.3389/fsurg.2023.1204785
Received: 12 April 2023; Accepted: 12 July 2023;
Published: 28 July 2023.
Edited by:
Francesco Pata, Nicola Giannettasio Hospital, ItalyReviewed by:
Audrius Dulskas, National Cancer Institute, LithuaniaAndrea Picchetto, Sapienza University of Rome, Italy
© 2023 Ozata, Bozkurt, Sucu, Karahan, Camci, Cetin, Ozoran, Agcaoglu, Balik and Bugra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibrahim H. Ozata aW96YXRhQGt1aC5rdS5lZHUudHI=
†ORCID Ibrahim H. Ozata orcid.org/0000-0001-6749-8518 Emre Bozkurt orcid.org/0000-0001-7971-3772 Serkan Sucu orcid.org/0000-0001-7040-6696 Salih N. Karahan orcid.org/0000-0002-3610-7390 Furkan Camci orcid.org/0000-0002-9227-9015 Feyza Cetin orcid.org/0000-0001-92387692 Emre Ozoran orcid.org/0000-0002-9371-6811 Orhan Agcaoglu orcid.org/0000-0003-1617-3953 Emre Balik orcid.org/0000-0001-5751-1133 Dursun Bugra orcid.org/0000-0003-0316-6818
 Ibrahim H. Ozata
Ibrahim H. Ozata Emre Bozkurt1,†
Emre Bozkurt1,† Serkan Sucu
Serkan Sucu Salih N. Karahan
Salih N. Karahan Furkan Camci
Furkan Camci Orhan Agcaoglu
Orhan Agcaoglu Emre Balik
Emre Balik