- 1Department of Cardiovascular Surgery, First Affiliated Hospital of Gannan Medical University, Ganzhou, China
- 2The First Clinical Medical College, Gannan Medical University, Ganzhou, China
- 3Department of Breast Disease Comprehensive Center, First Affiliated Hospital of Gannan Medical University, Ganzhou, China
Superior vena cava (SVC) stenosis is rarely caused by iatrogenic trauma. Herein, the case of a 5-year-old boy who underwent radiofrequency ablation for paroxysmal supraventricular tachycardia but developed SVC stenosis and related syndromes is reported. Notably, the child exhibited an enlarged left atrial appendage that had partially breached the pericardium. Subsequent interventions involved successful removal of the stenosis, artificial vascular reconstruction, and comprehensive radiofrequency ablation of the entire right atrium, along with ligation of the left atrial appendage under direct vision. As a result, the child experienced relief from symptoms.
Background
Superior vena cava (SVC) stenosis is a critical condition characterised by SVC compression by intrathoracic masses and/or endovascular devices (1). While a recent study suggests that intraluminal tumour metastasis can also cause SVC stenosis (2), it is rarely observed as a result of radiofrequency ablation. Surgical interventions are typically required to address SVC stenosis and SVC syndrome by eliminating the obstruction. In cases where the obstruction is caused by intraluminal tumour metastasis, radiotherapy and chemotherapy are also feasible. Without intervention, oedema might compress the brain, midbrain, and medulla oblongata, resulting in acute syndromes, like hernia, loss of consciousness, and respiratory arrest (3). An enlarged left atrial appendage, known as left atrial appendage aneurysm (LAAA), is a rare condition that can be classified as intrapericardial or extrapericardial LAAA based on pericardial integrity or as congenital and secondary LAAA based on the underlying causes (4–6). Secondary left auricular enlargement can result from severe mitral stenosis or reflux, which increases left atrial pressure. Congenital LAAA might arise from congenital defects in the atrial comb muscle, particularly when the atrium expands due to blood flow and intracardiac pressure (6). Tumours with an enlarged left atrial appendage can compress the ventricles and cause cardiac insufficiency. Additionally, the presence of thrombi in the left atrial appendage could result in systemic circulation embolism and malignant arrhythmias (7). Therefore, early treatment is crucial once these conditions are diagnosed. Herein, a case of artificial vascular replacement of SVC stenosis in a 5-year-old boy with LAAA is reported and a concise literature review on SVC stenosis and LAAA is provided (Table 1) (8–19). Common surgical interventions for SVC stenosis include SVC angioplasty, percutaneous transluminal angioplasty, and SVC stenting. However, there have been no previous reports of vascular replacement in a 5-year-old child.
Case presentation
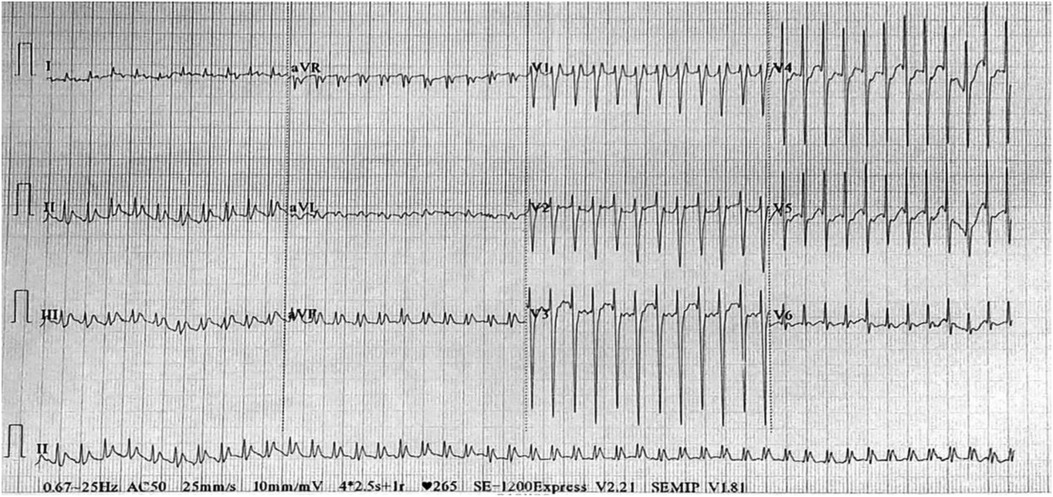
A 5-year-old boy was hospitalised due to facial and upper limb oedema. He was diagnosed with paroxysmal supraventricular tachycardia at another hospital a month ago. Prior to admission, prehospital electrophysiological (EP) testing and electroanatomical mapping had confirmed the ectopic stimulation point in the SVC. Subsequently, the patient underwent catheter ablation using a temperature-controlled mode, with the target temperature set at 50°C and energy at 30 Watts. However, a month after the procedure, the child developed facial and upper limb oedema, complicated by headache and mental disorder. Furthermore, episodes of supraventricular tachycardia persisted. Upon admission to our hospital, computed tomography (CT) angiography (CTA) revealed SVC stenosis (Figures 1A,B). Contrast-enhanced CT revealed an enlarged left atrial appendage (Figures 1C,D). Electrocardiogram findings indicated paroxysmal supraventricular tachycardia (Figure 2). Esmolol was administered to control the heart rate.
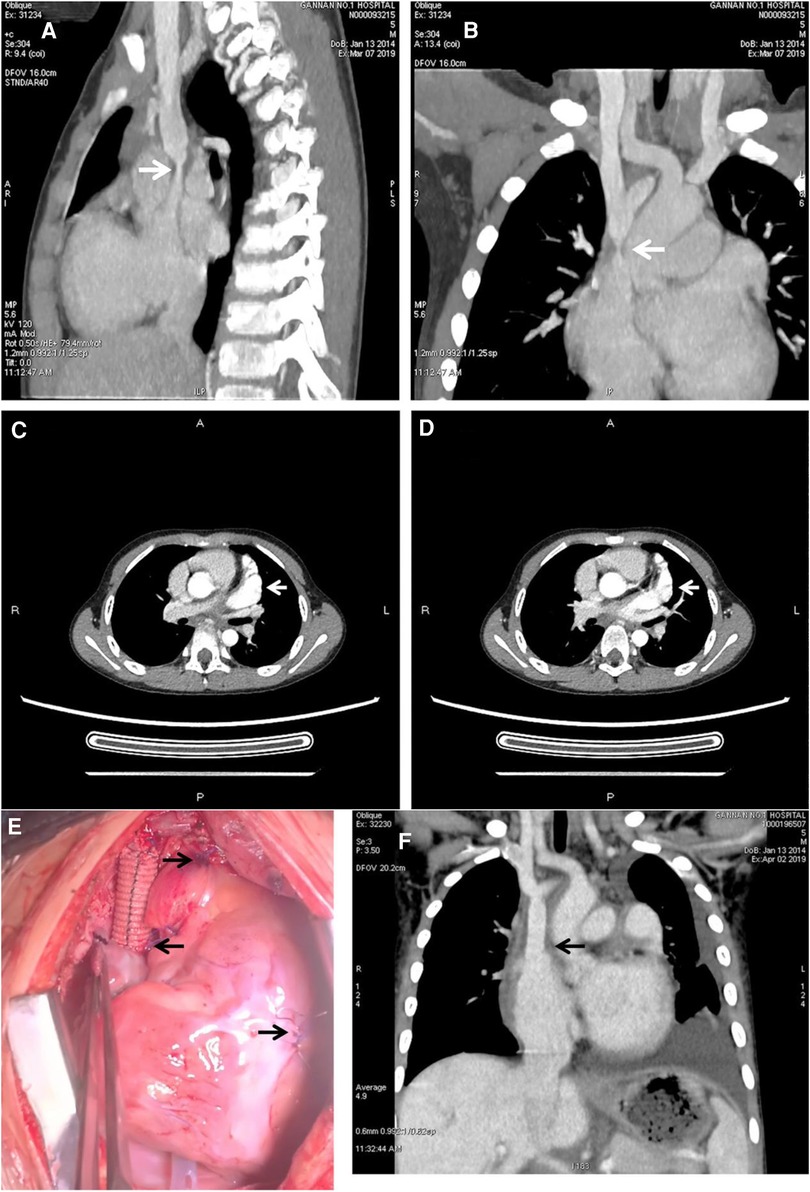
Figure 1. Preoperative CT and CTA showed the stenosis of the superior vena cava and left atrial appendage aneurysm. Intraoperative and postoperative CT showed the reconstruction of the superior vena cava with artificial blood vessels. (A,B) Showed severe stenosis at the junction of superior vena cava and right atrium (arrowhead). (C,D) Showed that the enhancement of the left atrial appendage was consistent with that of the ascending aortic cavity, and the arrowhead referred to the enlarged left atrial appendage (arrowhead). (E) Showed the details of the operation; the left arrow showed the site of anastomosis between the artificial vessel and the right atrium; the first arrow on the right showed the site of ligation and suture at the root of the left atrial appendage; and the second arrow on the right showed the site of the temporary pacemaker. (F) Showed that the blood flow between the artificial vessel and the right atrium was unobstructed, which relieved the stenosis of the superior vena cava.
After obtaining informed consent, the patient underwent surgery. Ascending aortic cannulation was performed, and an SVC drainage tube was inserted at the confluence of the innominate vein and the SVC. Additionally, an inferior vena cava drainage tube was inserted through the right atrium. Cold blood cardioplegia solution was infused to protect the myocardium. Intermittent anterograde delivery of cold blood cardioplegia solution was performed via the aortic root during normothermic cardiopulmonary bypass. The initial plan was to use a patch to expand the narrowed segment of the SVC. However, upon opening the SVC, severe vascular wall damage and fragile scar tissue that could not support the patch were observed. Therefore, a 10 mm artificial vessel was used to replace the SVC. The stenosis was excised, and the SVC was reconstructed. Special care was taken to safeguard the sinoatrial node during suturing. Subsequently, the patient underwent a maze procedure using surgical ablation. The Medtronic bipolar radiofrequency ablation system (Medtronic, Minneapolis, MN, USA) was employed to perform radiofrequency ablation of the entire right atrium (including the right atrial appendage, right atrial wall, atrial septum, and tricuspid isthmus) to isolate ectopic rhythms or re-entrant activations originating from the right atrium. Furthermore, ligation of the left atrial appendage was performed to prevent the formation of thrombi or thromboembolism, such as cerebral embolism and arterial embolism, resulting from LAAA. Upon restoration of heart function, radiofrequency ablation demonstrated satisfactory efficacy.
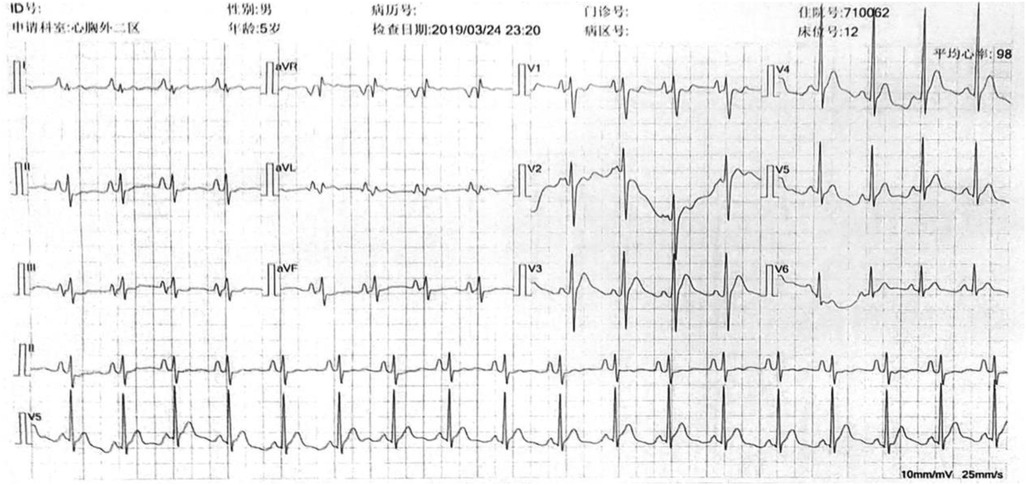
Following the surgery, the patient's facial and upper extremity oedema subsided. CTA revealed that the SVC regained vascular patency (Figure 1F), and the electrocardiogram showed a recovered sinus rhythm (Figure 3). Subsequently, the patient was discharged and prescribed aspirin for 3 months. Telephonic follow-up assessments were performed at 3 months and 6 months after discharge, during which the patient remained asymptomatic without any adverse events.
Discussion
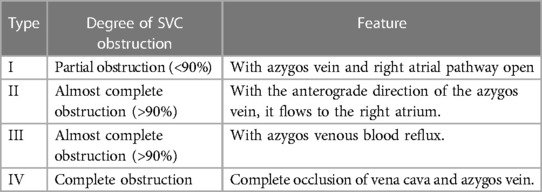
SVC syndrome is a complex condition primarily characterised by severe stenosis or obstruction of the SVC. The degree of obstruction can be categorised into four types (Table 2) (31). In 1997, Lumsden et al. (32) proposed diagnostic criteria for severe SVC stenosis, defining it as a stenosis with a diameter reduction of >50% in the SVC, with or without upstream vein stenosis. These criteria have been widely adopted in the field of veno-occlusive diseases. Clinical symptoms associated with severe SVC stenosis include facial, neck, and/or upper chest oedema, which can extend to the proximal end of the upper limbs. Venography could be used to assess the presence and severity of SVC stenosis. Besides the congenital causes, SVC stenosis is primarily attributed to internal or external factors, including long-term indwelling central venous catheter (CVC), transvenous pacemakers, surgeries, and radiotherapy. Prolonged exposure to these factors stimulates vascular endothelial cells, leading to intimal inflammation, intimal hyperplasia, and eventually SVC stenosis (30). The most common cause is long-term CVC retention in patients with end-stage renal disease undergoing haemodialysis (27, 33). External factors contributing to SVC stenosis often involve compression by mediastinal masses, malignant tumours, or fibrous mediastinitis. The SVC, located in the middle mediastinum, is surrounded by mediastinal fat, lymph nodes, the right lung and pleura, and the left trachea and ascending aorta. Enlargement of these structures might compress the SVC. Malignant tumours, including small cell and non-small cell lung carcinoma, lymphoma, metastatic lymphadenopathy from intrathoracic and extrathoracic malignant tumours, and tracheal malignant tumours, are the most common external factors causing SVC stenosis (8, 20). Chest CT is the most common imaging technique, while magnetic resonance (MR) angiography can provide better visualisation of cancer cell infiltration in blood vessels and pericardium. The main approach for addressing stenosis caused by internal factors is removing the stimulating factor, such as removing CVC or pacemaker leads. If infection and thrombosis occur, symptomatic support treatments such as antibiotics and anticoagulation might be necessary. In cases of stenosis due to external factors, relieving the compression becomes essential. If the compression cannot be relieved, venous angioplasty and stenting could be considered to improve patient symptoms.
In this case, the patient exhibited progressive facial oedema and developed headaches one week after undergoing radiofrequency ablation. CTA confirmed the presence of severe local SVC stenosis, which was believed to be caused by SVC injury after radiofrequency ablation. Urgent removal of the SVC obstruction was necessary to prevent complications such as cerebral oedema, cerebral compression, cerebral herniation, and acute compression syndromes affecting the midbrain and medulla, resulting in loss of consciousness, respiratory arrest, and other complications (3). The width of the artificial vessel was chosen to suit the age of the child, considering that the patient was too young and that an artificial vessel that was too thick might interfere with cranial blood flow. Therefore, in this patient, a 10 mm artificial vessel was used for SVC replacement. Following the surgery, the patient's facial and upper extremity oedema subsided. And it is essential to monitor the biological adaptability of the artificial vessel and assess whether further surgical intervention will be required. Cardiac radiofrequency ablation is the most effective method for treating paroxysmal tachycardia. By delivering radiofrequency current through the electrode, it induces myocardial coagulative necrosis, thereby blocking the abnormal conduction bundle and subsequent tachyarrhythmia. Precisely identifying the location of the sinoatrial node is crucial for cardiac radiofrequency ablation as it determines the ablation approach and significantly affects patient prognosis. The distribution of the sinoatrial node is primarily based on two parameters: height distribution and plane distribution. Height distribution involves three locations: the SVC, right atrium, and SVC-right atrial junction. The sinoatrial node is considered to be in the right atrium when its height is two-thirds lower than the SVC-right atrial junction, and in the SVC when its height is two-thirds higher than the SVC-right atrial junction (21). Plane distribution is described by the head and foot position, 1–3 o’clock position corresponds to the postero-septal position, 3–5 o’clock to the antero-septal position, 5–7 to the anterior position, 7–9 o’clock to the anterolateral position, 9–11 o’clock to the posterolateral position, and 11–1 o’clock to the posterior position (Figure 4). In cases of paroxysmal supraventricular tachycardia originating from the SVC, ablation can be performed on the septal side of the SVC-right atrium without cardioversion. However, if the sinoatrial node is located within the superior vena cava or the ablation target in the SVC overlaps with the functional sinoatrial node, the ablation level on the side of the sinoatrial node should be at least 5 mm higher than that of the sinoatrial node. This approach ensures the maximum protection of the superior vena cava from injury, which is particularly crucial in paediatric patients whose SVC walls are not fully developed.
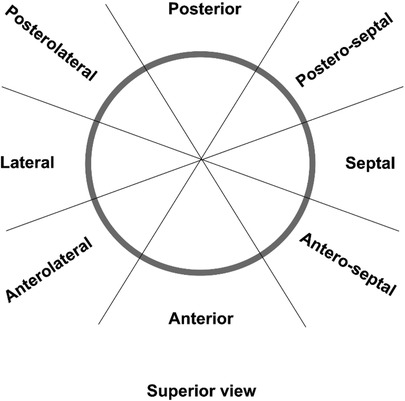
Figure 4. Distribution of the ablation points at the superior vena cava-right atrium (SVC-RA) junction. Superior view of the SVC-RA junction is shown.
Typically, radiofrequency currents can ablate an area within a radius of 1–3 mm. Supraventricular tachycardia often presents with sudden attacks, palpitation, shortness of breath, chest tightness, dizziness, and lethal syncope or shock (22). For patients experiencing recurrent episodes, endocardial EP examination and radiofrequency ablation are recommended. Transcatheter ablation is safe and effective for children; however, those under 4 years of age and weighing <15 kg have a higher likelihood of experiencing complications (23). The most frequently observed complications of radiofrequency catheter ablation are sinoatrial node injury, phrenic nerve paralysis, and SVC stenosis (24, 25). Given that the cardiovascular system of children is still maturing, radiofrequency ablation might result in vascular damage and pericardial tamponade.
LAAA is a rare abnormality with potentially fatal complications (26). The size of LAAA can range from 4 cm × 3 cm to 22 cm × 15 cm, with an average size of 11 ± 5 × 7 ± 3 cm. This first reported case of LAA was documented by Parmley in 1962 (28). While LAAA can occur in individuals of all age groups, it is most commonly observed in patients aged approximately 30 years old, with an average age of 30 ± 20 years (16). The incidence of LAAA tends to increase with age. As the aneurysm grows larger, patients might experience symptoms such as supraventricular arrhythmia, cardiac insufficiency, atypical chest pain, and an elevated risk of intra-atrial thrombosis and systemic embolism (29). Several reports have linked LAAA to atrial or supraventricular arrhythmias (7, 34). Among the patients, 27 (26.7%) patients exhibited atrial fibrillation/flutter, 10 (9.9%) had supraventricular tachycardia, and 60 (59.4%) maintained sinus rhythm. The elevated tension exerted by LAAA on the conduction system or congenital defects in the conduction tissues might contribute to the occurrence of supraventricular arrhythmias (16). Therefore, in young patients presenting with atrial fibrillation without other associated cardiac abnormalities, it is crucial to consider the possibility of LAAA. The diagnosis of LAA can be confirmed using transthoracic echocardiography or transoesophageal echocardiography with colour Doppler, which can reveal blood flow between the left atrium and LAAA (35). Other imaging modalities, such as CT, MR imaging, CTA, and angiocardiography, are valuable in diagnosing LAAA and ruling out other conditions, such as cardiac or mediastinal tumours, pericardial cysts, acquired left atrial enlargement secondary to mitral valve disease, and left atrial hernia with pericardial defects (19, 36, 37). Once diagnosed, surgery is the recommended treatment option, even in asymptomatic patients, as it can prevent potential thromboembolism and address associated arrhythmias. The two most commonly used techniques are cardiopulmonary bypass-assisted median sternotomy and left lateral sternotomy. The former is employed in patients with left atrial thrombus, as cardiopulmonary bypass blocking the aorta could prevent systemic embolism during aneurysm resection. On the other hand, left lateral sternotomy offers better visualisation and causes less patient trauma compared with median sternotomy.
In this case report, median sternotomy aided with cardiopulmonary bypass was employed due to the requirement for angioplasty. During the surgery, a pericardial defect surrounding the left atrial appendage was incidentally discovered, necessitating an extrapericardial repair. An enlarged left atrial appendage can compress the ventricles, resulting in cardiac insufficiency, while sluggish blood flow within the left atrial appendage could contribute to thrombus formation and subsequent systemic circulation embolism. Therefore, early intervention is necessary. In this case, prehospital EP testing and electroanatomical mapping confirmed the ectopic stimulation point was located at the SVC. Subsequently, radiofrequency ablation of the right SVC was performed immediately after diagnosis. Although the arrhythmia symptoms subsided within a month, they reappeared later with increased frequency. This suggests the possibility of multiple tachycardia origin points or incomplete success of the previous interventional radiofrequency ablation. Therefore, radiofrequency ablation was performed on the entire right atrium to avoid the recurrence of arrhythmia. Additionally, considering the patient's young age (5 years), ligation of the left atrial appendage was performed to prevent thrombus formation or thromboembolism, such as cerebral embolism and arterial embolism, resulting from LAAA.
Conclusions
Continuous documentation of these rare cases is important for guiding future practices. This case stands apart from previous cases in that the SVC syndrome resulted from radiofrequency ablation, and the patient is a 5-year-old boy with congenital LAAA. Therefore, for children with arrhythmia and LAAA, surgical intervention might offer a more favourable option than radiofrequency ablation. Simultaneous to the surgical intervention, surgical ablation can be performed to achieve complete resolution of LAAA and arrhythmia. Additionally, for children with irreparable superior vena cava stenosis, artificial vascular replacement serves as a potential alternative. Nonetheless, it is important to note that the long-term prognosis of children in such cases warrants ongoing observation and follow-up.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med. (2007) 356(18):1862–9. doi: 10.1056/NEJMcp067190
2. Nicholson AA, Ettles DF, Arnold A, Greenstone M, Dyet JF. Treatment of malignant superior vena cava obstruction: metal stents or radiation therapy. J Vasc Interv Radiol. (1997) 8(5):781–8. doi: 10.1016/S1051-0443(97)70660-2
3. Ganeshan A, Hon LQ, Warakaulle DR, Morgan R, Uberoi R. Superior vena caval stenting for SVC obstruction: current status. Eur J Radiol. (2009) 71(2):343–9. doi: 10.1016/j.ejrad.2008.04.014
4. Saul JP, Kanter RJ, Abrams D, Asirvatham S, Bar-Cohen Y, Blaufox AD, et al. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease: developed in partnership with the pediatric and congenital electrophysiology society (PACES) and the heart rhythm society (HRS). endorsed by the governing bodies of PACES, HRS, the American academy of pediatrics (AAP), the American heart association (AHA), and the association for European pediatric and congenital cardiology (AEPC). Heart Rhythm. (2016) 13(6):e251–89. doi: 10.1016/j.hrthm.2016.02.009
5. Sigfusson G, Park SC, Ettedgui JA, Newman B, Siewers RD, Neches WH. Intrapericardial left atrial aneurysm: noninvasive diagnosis. Pediatr Cardiol. (1997) 18(3):240–3. doi: 10.1007/s002469900164
6. Soleimani A, Sattarzadeh R. Left atrial appendage aneurysm: a rare cause of paroxysmal supraventricular tachycardia. Heart Lung Circ. (2008) 17(3):246–7. doi: 10.1016/j.hlc.2007.04.004
7. Foale RA, Gibson TC, Guyer DE, Gillam L, King ME, Weyman AE. Congenital aneurysms of the left atrium: recognition by cross-sectional echocardiography. Circulation. (1982) 66(5):1065–9. doi: 10.1161/01.CIR.66.5.1065
8. Rosch J, Uchida BT, Hall LD, Antonovic R, Petersen BD, Ivancev K, et al. Gianturco-Rosch expandable Z-stents in the treatment of superior vena cava syndrome. Cardiovasc Intervent Radiol. (1992) 15(5):319–27. doi: 10.1007/BF02733957
9. Labriola L, Seront B, Crott R, Borceux P, Hammer F, Jadoul M. Superior vena cava stenosis in haemodialysis patients with a tunnelled cuffed catheter: prevalence and risk factors. Nephrol Dial Transplant. (2018) 33(12):2227–33. doi: 10.1093/ndt/gfy150
10. Mahajan S, Khanna S, Rohit M, Chakraborty NS, Singhal A. Congenital superior vena cava (SVC) stenosis and obstructed supracardiac total anomalous pulmonary venous connection (TAPVC)-A surgical perspective. J Card Surg. (2020) 35(9):2399–402. doi: 10.1111/jocs.14792
11. Yokose M, Sato H, Akutsu H, Misawa Y. Superior vena cava stenosis due to lipomatosis of the right atrium. J Card Surg. (2018) 33(3):135–6. doi: 10.1111/jocs.13548
12. Economopoulos GC, Dimitrakakis GK, Brountzos E, Kelekis DA. Superior vena cava stenosis: a delayed BioGlue complication. J Thorac Cardiovasc Surg. (2004) 127(6):1819–21. doi: 10.1016/j.jtcvs.2003.12.041
13. Kuhne M, Schaer B, Osswald S, Sticherling C. Superior vena cava stenosis after radiofrequency catheter ablation for electrical isolation of the superior vena cava. Pacing Clin Electrophysiol. (2010) 33(4):e36–8. doi: 10.1111/j.1540-8159.2009.02588.x
14. Zhang X, Li P, Cao Y, Li X, Duan X, Bai S, et al. Left atrial appendage aneurysm in pediatrics. Echocardiography. (2020) 37(6):917–21. doi: 10.1111/echo.14677
15. Yeung DF, Miu W, Turaga M, Tsang MYC, Tsang TSM, Jue J, et al. Incidentally discovered left atrial appendage aneurysm managed conservatively. Heart Lung Circ. (2020) 29(5):e53–5. doi: 10.1016/j.hlc.2019.10.015
16. Wang B, Li H, Zhang L, He L, Zhang J, Liu C, et al. Congenital left atrial appendage aneurysm: a rare case report and literature review. Medicine (Baltimore). (2018) 97(2):e9344. doi: 10.1097/MD.0000000000009344
17. Chowdhury UK, Seth S, Govindappa R, Jagia P, Malhotra P. Congenital left atrial appendage aneurysm: a case report and brief review of literature. Heart Lung Circ. (2009) 18(6):412–6. doi: 10.1016/j.hlc.2008.10.015
18. Yakut K, Varan B, Erdogan I. Asymptomatic giant congenital left atrial aneurysm. Turk J Pediatr. (2019) 61(1):117–9. doi: 10.24953/turkjped.2019.01.019
19. Brazier A, Hasan R, Jenkins P, Hoschtitzky A. Urgent resection of a giant left atrial appendage aneurysm and mitral valve replacement in a complex case of hurler-scheie syndrome. BMJ Case Rep. (2015) 2015. doi: 10.1136/bcr-2015-211551
20. McDevitt JL, Goldman DT, Bundy JJ, Hage AN, Jairath NK, Gemmete JJ, et al. Gianturco Z-stent placement for the treatment of chronic central venous occlusive disease: implantation of 208 stents in 137 symptomatic patients. Diagn Interv Radiol. (2021) 27(1):72–8. doi: 10.5152/dir.2020.19282
21. Nishiyama N, Hashimoto K, Yamashita T, Miyama H, Fujisawa T, Katsumata Y, et al. Visualization of the electrophysiologically defined junction between the superior vena cava and right atrium. J Cardiovasc Electrophysiol. (2020) 31(8):1964–9. doi: 10.1111/jce.14613
22. Gaita F, Haissaguerre M, Scaglione M, Jais P, Riccardi R, Lamberti F, et al. Catheter ablation in a patient with a congenital giant right atrial diverticulum presented as wolff-Parkinson-white syndrome. Pacing Clin Electrophysiol. (1999) 22(2):382–5. doi: 10.1111/j.1540-8159.1999.tb00457.x
23. Thomas PE, Macicek SL. Catheter ablation to treat supraventricular arrhythmia in children and adults with congenital heart disease: what we know and where we are going. Ochsner J. (2016) 16(3):290–6.27660579
24. Jin MN, Lim B, Yu HT, Kim TH, Uhm JS, Joung B, et al. Long-term outcome of additional superior vena Cava to septal linear ablation in catheter ablation of atrial fibrillation. J Am Heart Assoc. (2019) 8(22):e013985.31726961
25. Zhang T, Wang Y, Liang Z, Zhao H, Han Z, Wang Y, et al. Effect of combined pulmonary vein and superior vena Cava isolation on the outcome of second catheter ablation for paroxysmal atrial fibrillation. Am J Cardiol. (2020) 125(12):1845–50. doi: 10.1016/j.amjcard.2020.03.030
26. Jiang B, Wang X, Liu F, Song L. Left atrial appendage aneurysm. Interact Cardiovasc Thorac Surg. (2020) 30(3):495–6. doi: 10.1093/icvts/ivz283
27. Stanford W, Jolles H, Ell S, Chiu LC. Superior vena cava obstruction: a venographic classification. AJR Am J Roentgenol. (1987) 148(2):259–62. doi: 10.2214/ajr.148.2.259
28. Parmley LF Jr. Congenital atriomegaly. Circulation. (1962) 25:553–8. doi: 10.1161/01.CIR.25.3.553
29. Aryal MR, Hakim FA, Ghimire S, Giri S, Pandit A, et al. Left atrial appendage aneurysm: a systematic review of 82 cases. Echocardiography. (2014) 31(10):1312–8. doi: 10.1111/echo.12667
30. Quaretti P, Galli F, Moramarco LP, Corti R, Leati G, Fiorina I, et al. Dialysis catheter-related superior vena cava syndrome with patent vena cava: long term efficacy of unilateral viatorr stent-graft avoiding catheter manipulation. Korean J Radiol. (2014) 15(3):364–9. doi: 10.3348/kjr.2014.15.3.364
31. Stanford W, Doty DB. The role of venography and surgery in the management of patients with superior vena cava obstruction. Ann Thorac Surg. (1986) 41(2):158–63. doi: 10.1016/S0003-4975(10)62659-8
32. Lumsden AB, MacDonald MJ, Isiklar H, Martin LG, Kikeri D, Harker LA, et al. Central venous stenosis in the hemodialysis patient: incidence and efficacy of endovascular treatment. Cardiovasc Surg. (1997) 5(5):504–9. doi: 10.1016/S0967-2109(97)00043-4
33. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. (1986) 146(2):259–61. doi: 10.1001/archinte.146.2.259
34. Fountain-Dommer RR, Wiles HB, Shuler CO, Bradley SM, Shirali GS. Recognition of left atrial aneurysm by fetal echocardiography. Circulation. (2000) 102(18):2282–3. doi: 10.1161/01.CIR.102.18.2282
35. Clark JB, Ting JG, Polinsky RJ Jr, Wolfe LT. Resection of a giant left atrial appendage aneurysm via limited thoracotomy. World J Pediatr Congenit Heart Surg. (2014) 5(3):475–7. doi: 10.1177/2150135114524602
36. LaBarre TR, Stamato NJ, Hwang MH, Jacobs WR, Stephanides L, Scanlon PJ. Left atrial appendage aneurysm with associated anomalous pulmonary venous drainage. Am Heart J. (1987) 114(5):1243–5. doi: 10.1016/0002-8703(87)90206-7
Keywords: superior vena cava, left atrial appendage aneurysm, radiofrequency ablation, arrhythmias, complication
Citation: Xiong J, Wenbo Y, Gao J, Li M and Yu D (2023) Radiofrequency ablation-induced superior vena cava stenosis in a 5-year-old boy with congenital left atrial appendage deformity: a case report and literature review. Front. Surg. 10:1199335. doi: 10.3389/fsurg.2023.1199335
Received: 3 April 2023; Accepted: 21 June 2023;
Published: 10 July 2023.
Edited by:
Professor Xiaofeng Yang, Temple University, United StatesReviewed by:
Maruti Haranal, U N Mehta Institute of Cardiology and Research, IndiaMichael Hofmann, University of Zurich, Switzerland
© 2023 Xiong, Wenbo, Gao, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongmin Yu MTgzNzA5NTU1NjhAMTYzLmNvbQ==
 Jianxian Xiong
Jianxian Xiong Yu Wenbo
Yu Wenbo Jianfeng Gao
Jianfeng Gao Meifang Li3
Meifang Li3 Dongmin Yu
Dongmin Yu