
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 02 June 2023
Sec. Neurosurgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1188517
 Xiaofeng Jiang1,2,†
Xiaofeng Jiang1,2,† Lili Gu3,2,†
Lili Gu3,2,† Gang Xu3,2
Gang Xu3,2 Xuezhong Cao3,2
Xuezhong Cao3,2 Jian Jiang1,2
Jian Jiang1,2 Daying Zhang3,2
Daying Zhang3,2 Mu Xu3,2*
Mu Xu3,2* Yi Yan3,2*
Yi Yan3,2*
Objective: To investigate and integrate multiple independent risk factors to establish a nomogram for predicting the unfavourable outcomes of percutaneous endoscopic transforaminal discectomy (PETD) for lumbar disc herniation (LDH).
Methods: From January 2018 to December 2019, a total of 425 patients with LDH undergoing PETD were included in this retrospective study. All patients were divided into the development and validation cohort at a ratio of 4:1. Univariate and multivariate logistic regression analyses were used to investigate the independent risk factors associated with the clinical outcomes of PETD for LDH in the development cohort, and a prediction model (nomogram) was established to predict the unfavourable outcomes of PETD for LDH. In the validation cohort, the nomogram was validated by the concordance index (C-index), calibration curve, and decision curve analysis (DCA).
Results: 29 of 340 patients showed unfavourable outcomes in the development cohort, and 7 of 85 patients showed unfavourable outcomes in the validation cohort. Body mass index (BMI), course of disease (COD), protrusion calcification (PC), and preoperative lumbar epidural steroid injection (LI) were independent risk factors associated with the unfavourable outcomes of PETD for LDH and were identified as predictors for the nomogram. The nomogram was validated by the validation cohort and showed high consistency (C-index = 0.674), good calibration and high clinical value.
Conclusions: The nomogram based on patients' preoperative clinical characteristics, including BMI, COD, LI and PC, can be used to accurately predict the unfavourable outcomes of PETD for LDH.
Low back pain is a leading cause of disability and absenteeism in the developed and developing countries (1). It not only reduces the quality of life for patients, but also causes an enormous economic burden to both health-care and social support systems (2, 3). Previous studies have shown that approximately 70% of people have low back pain in their lifetime, and with an annual prevalence of 15%–45% (4, 5). Up to half of the low back pain is caused by lumbar disc herniation (LDH), and the incidence of LDH has risen steeply and gradually affected more younger individuals in the past two decades (6).
At present, percutaneous endoscopic transforaminal discectomy (PETD) has been widely used in LDH due to the merits of normal paraspinal structures preservation, less soft tissue injury, fewer complications and shorter operation time (7). Most patients can relieve pain and return to normal life after PETD (8), but some patients have unfavourable outcomes postoperatively, and even need reoperation (9, 10). To improve clinical decision making and patient satisfaction, it is important to figure out the risk factors and predict the unfavourable outcomes of PETD for LDH.
Previous studies have identified several clinical factors as the significant risk factors associated with the clinical outcomes of PETD for LDH (11, 12), but they did not perform comprehensive analysis of these risk factors, which provided little help in improving clinical decision making. The development of nomograms make up for this deficiency, as nomograms can integrate a variety of significant risk factors and generate a single numerical estimation of event probability, which can be applied to predict the clinical outcomes of surgery (13). To our knowledge, only one study has developed a nomogram for pain and functional outcomes after lumbar spine fusion surgery (14), and this predictive model is rarely used in predicting the unfavourable outcomes of PETD for LDH.
Therefore, we performed a retrospective study to develop and validate a nomogram to predict the unfavourable outcomes of PETD for LDH.
From January 2018 to December 2019, LDH patients treated with PETD at the Department of Pain Medicine of The First Affiliated Hospital of Nanchang University were collected. The PETD was performed by two senior and experienced surgeons and the detailed surgical procedure was same as that described in our previous study (15) and was shown in Figure 1. The inclusion criteria were leg pain or leg pain + low back pain, diagnosis as single-segment LDH by computed tomography (CT) and/or magnetic resonance imaging (MRI), failure of conservative treatment for more than 2 months. The exclusion criteria were unclear diagnosis, multi-segment LDH, recurrent LDH, prior spine surgery, other lumbar diseases (tuberculosis, infection, tumour, etc), non-transforaminal approach or failure of the transforaminal approach and loss to follow-up. All patients were followed up for 12 months by outpatient or telephone and clinical outcomes were evaluated by the Numeric Rating Scale (NRS) and the modified MacNab criteria (16). The modified MacNab criteria evaluated as excellent or good and NRS < 3 were defined as favourable outcomes, and those evaluated as moderate or poor and NRS ≥ 3 were defined as unfavourable outcomes. A total of 447 patients with LDH who were treated with PETD were collected. Finally, 425 patients completed 12 months of follow-up and were included in this retrospective study. All patients were randomly divided in a ratio of 4:1 into the development and validation cohort by computer-generated random order (https://www.randomizer.org).
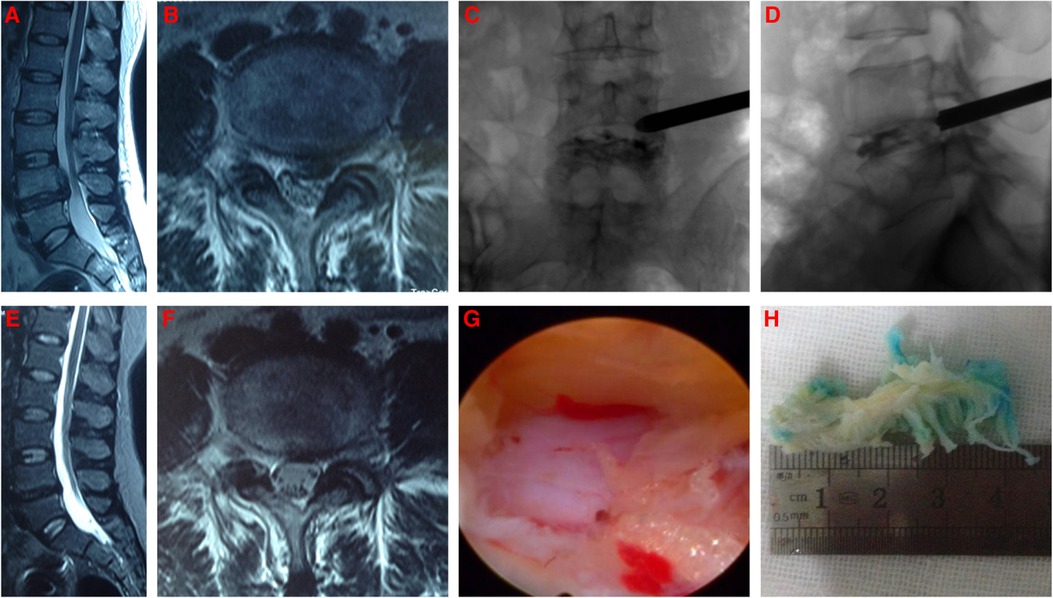
Figure 1. The typical PETD procedure. (A,B) Preoperative MRI of L4/5 LDH; (C,D) The working catheter of PETD; (E,F) 10 months postoperative MRI of L4/5 showing good decompression of nerve root; (G) Decompression of nerve root under endoscope; (H) Intraoperative removal of the nucleus pulposus.
Clinical characteristics including age, gender, body mass index (BMI), history of lumbar trauma (LT), preoperative lumbar epidural steroid injection (LI), course of disease (COD), symptoms, segments, disc degeneration (DD), Modic change (MC) and protrusion calcification (PC) were collected from all patients. Age, gender, BMI, LT, COD, LI, symptoms and other clinical data were obtained from medical records or radiological examinations. Age was divided into ≥50 years and <50 years (17). BMI was classified as overweight (BMI ≥ 25 kg/m2) and normal (BMI < 25 kg/m2) according to World Health Organization standards; COD was divided into ≥6 months and <6 months (18). Symptoms were divided into leg pain and leg pain + low back pain. MC was assessed by MRI (19). DD was classified as mild (Pfirrmann grade Ⅰ-Ⅲ) or severe (Pfirrmann grade Ⅳ-Ⅴ) (20). PC was assessed by CT.
All the variables were categorical. Univariate and multivariate logistic regression analyses were used to investigate the independent risk factors associated with the clinical outcomes of PETD for LDH. The odds ratio (OR) and 95% confidence interval (CI) were analyzed by SPSS 22.0 software (SPSS Inc, Chicago, IL, USA). Then we put the data into R software (http://www.R-project.org) to establish the nomogram. The discrimination and calibration of the nomogram were validated by the calibration curve, concordance index (C-index) and decision curve analysis (DCA). The calibration curve, C-index and DCA curve were calculated and drawn by R software. P < 0.05 was considered statistically significant.
A total of 447 patients with LDH who were treated with PETD were collected. Finally, 425 patients completed 12 months of follow-up, and all patients were randomly divided in a ratio of 4:1 into the development cohort (n = 340) and the validation cohort (n = 85). Unfavourable outcomes of PETD for LDH were found in 29 of 340 patients in the development cohort, and 7 of 85 patients in the validation cohort (Figure 2). The clinic characteristics of the development and validation cohort were summarized in Table 1.
The univariate logistic analysis showed significant differences in age, BMI, COD, DD, MC, PC and LI (Table 2). Multivariate logistic regression analysis demonstrated that BMI, COD, LI and PC were independent risk factors associated with the unfavourable outcomes of PETD for LDH (Table 3).
Univariate and multivariate logistic regression analyses showed that BMI, COD, LI and PC were independent risk factors associated with the clinical outcomes of PETD for LDH. Subsequently, these four independent risk factors were used to establish a nomogram. Each independent risk factor in the nomogram is scored based on its corresponding points scale. The scores for the four independent risk factors are added to obtain the total score. Finally, a vertical line is drawn down from the total score row to generate a single numerical estimation of the event probability, which can predict the unfavourable outcomes of PETD for LDH (Figure 3).
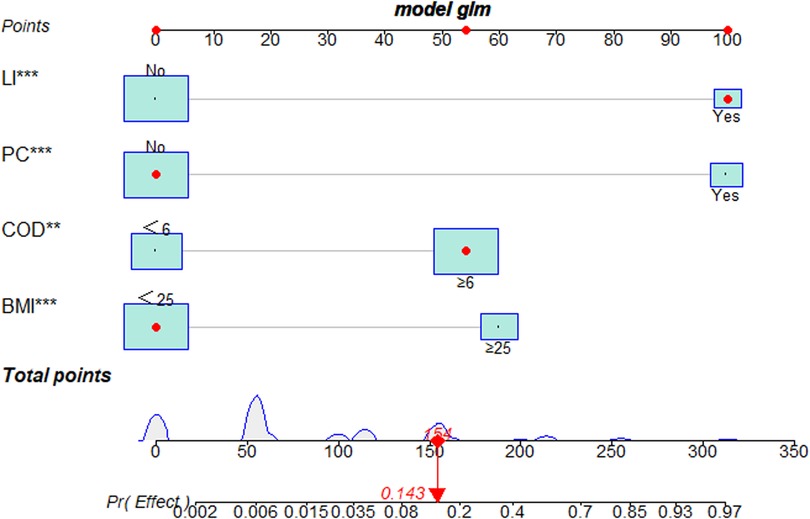
Figure 3. The nomogram for predicting the clinical outcomes of PETD for LDH. A representative of the LDH patient to show how to excute the nomogram. Each independent risk factor of the nomogram was scored on its corresponding points scale. The scores for four independent risk factors were then added to obtain the total score. A vertical line corresponded to the total score and generated a single numerical estimation of event probability, which can predict the unfavourable outcomes of PETD for LDH. The patient's total score was 154, which corresponds to a probability of 0.143 for having unfavorable outcomes of PETD for LDH (**: P < 0.01, ***:P < 0.001).
In the validation cohort, the nomogram showed good discrimination (C-index = 0.837) in distinguishing the clinical outcomes of PETD for LDH. Moreover, we calculated the calibration curve, which showed that the regression fitting curve was close to the standard curve (P = 0.674) (Figure 4), meaning that the actual outcomes of PETD for LDH were consistent with the outcomes predicted by the nomogram. In addition, the DCA curve was conducted to calculate the clinical value of the nomogram by quantifying the net benefits at different threshold probability. The DCA curve showed that the clinical value of the nomogram presented more net benefits at the threshold probability of 0%–32% and 58%–85%, indicating that the nomogram had good clinical efficacy (Figure 5).

Figure 4. Calibration and discrimination of the nomogram. In the validation cohort, the prediction model showed good discrimination (C-index = 0.674) in differentiating the clinical outcomes of PETD for LDH, and the actual probability of the clinical outcomes of PETD for LDH was consistent with the probability predicted by the nomogram.
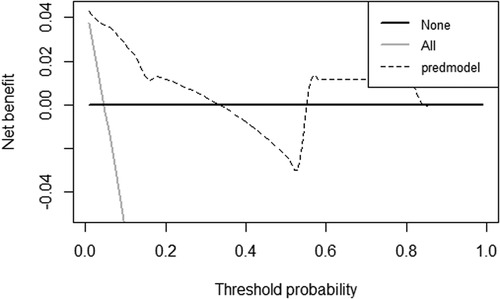
Figure 5. The decision curve analysis (DCA) curve for the clinical values of the nomogram. The DCA curve showed that the clinical value of the nomogram presented more net benefits at the threshold probability of 0%–32% and 58%–85%, indicating that the nomogram had good clinical efficacy.
The clinical outcomes of PETD for LDH can be influenced by multiple factors. Nonetheless, previous studies mainly focused on single risk factors that may influence the outcomes of PETD for LDH (11, 12), which did not contribute much to improve clinical decision making or patient satisfaction. Clinically, since few surgeons will act on the reason of a single risk factor, recent studies have focused on integrating multiple risk factors into a tool that can help guide clinical decision making (21). In this study, a prediction model (nomogram) was established for the first time to predict the unfavourable outcomes of PETD for LDH by integrating multiple independent risk factors to provide reliable evidence for clinical decision making.
Similar to previous studies, the present study revealed that COD (18) and BMI (22) were independent risk factors associated with the clinical outcomes of PETD for LDH. In the early stage of LDH, the nucleus pulposus does not adhere with the intraspinal tissues, which can be completely removed by forceps (as shown in Figure 1H). However, a long course of disease might lead to epidural venous congestion or epidural adhesions and even protrusion calcification, which increase the surgical difficulty and result in nucleus pulposus residue and even surgical failure. Jeffrey et al. (18) showed that LDH patients with a course of disease longer than 6 months had a longer operative time and increased intraoperative bleeding. Moreover, our previous study found that chronic low back pain can affect brain structure and function, leading to neuropathic pain (23). Heuch et al. (24) suggested that low back pain was associated with increased levels of BMI. Böstman (25) revealed that the BMI of LDH patients undergoing surgery was 25.1–27.3 kg/m2, while 22.3–23.1 kg/m2 in the general population, indicating that obese patients are more likely to have LDH. In addition, obese patients who undergo surgery for LDH have a longer operative time, more intraoperative bleeding and longer hospital stays (22). Consistent with previous correlational study (26), the results of this study suggested that BMI was an independent risk factor associated with the clinical outcomes of PETD for LDH, and LDH patients with BMI ≥ 25 kg/m2 were more likely to have unfavourable outcomes after PETD. High BMI may influence the biomechanical characteristics of discs, especially in degenerated and postoperative discs (26). Therefore, obese patients should prolong the time of using the lumbar belt and gradually reduce their weight after PETD. If obese patients can not reduce the load of discs, there is still a risk of recurrent LDH or LDH in other lumbar segments (26). For this reason, Meredith et al. (27) suggested that weight loss counseling should be considered in the preoperative conversation.
LI is one of the most commonly non-surgical treatments of LDH between drugs and surgery (28). The North American Spine Society has recommended that LI for LDH as a grade A choice. LI in the treatment of LDH has been shown to be effective, where with a wide variation in reported efficacy (29). For the varational efficacy, we think that LI can eliminate inflammation, relieve neuroedema, and has a good effect on early mild LDH (30), which can successfully prevent surgical intervention (31). However, for severe chronic LDH, LI could not remove the nucleus pulposus and may result in poor outcomes. Consistent with our results, Koltsov et al. (32) revealed that patients with preoperative LI did experience higher rates of reoperation than those with no preoperative LI. Additionally, Bhattacharjee et al. (33) believed that steroid may impede annulus fibrosus healing and thus predispose poor outcomes after surgery. PC is more common in patients with a longer course of disease (34). Previous study has reported that PC is associated with chronic inflammation and immunity (35). Due to the limitation of the endoscopic visual field, it is difficult to completely remove PC, and with high risk of nerve root injury to expose the PC fragment (36). Further, partial removal of PC may affect the stability of the posterior tissues of the intervertebral disc, and form new fissures and tears postoperatively, which leads to recurrent LDH. Moreover, the removal of PC requires a dynamic bur, which greatly damages the normal structure of the spinal canal and may cause epidural adhesion and fibrosis, leading to postoperative pain (37). We believe that the key to PETD for LDH is the decompression of the nerve root. If PC is directly related to nerve root compression, it should be removed as much as possible; if not, it should be retained to enhance the stability of the posterior tissues of the intervertebral disc and prevent recurrent LDH.
Pathologically, the four independent risk factors associated with the clinical outcomes of PETD for LDH are closely related. With the developed nomogram, we can perform a comprehensive analysis of these independent risk factors. Nomograms are visual format of the predictive model that allow improved predictive accuracy for outcomes by calculating the cumulative effect of each independent risk factor compared with the previous studies. Thus, surgeons can preoperatively predict the expected clinical outcomes of PETD for LDH based on the patients' preoperative clinical characteristics. In the validation cohort, the prediction model showed good discrimination (C-index = 0.674) in differentiating the clinical outcomes of PETD for LDH, and the actual probability of the clinical outcomes of PETD for LDH was consistent with the probability predicted by the nomogram. In terms of clinical efficacy, the prediction model showed good clinical efficacy, compared with the extreme curves in the threshold probability of 0%-32% and 58%-85%, indicating that the prediction model had high clinical efficacy and safety.
There are some limitations in this retrospective study. Firstly, this study established a prediction model for the clinical outcomes of PETD for LDH based on patients’ preoperative clinical characteristics but did not include related intraoperative and postoperative risk factors, such as surgeon experience (38), lifestyle and inappropriate physical workload (39). However, based on the results of this study, surgeons can improve clinical decision making, predict the expected postoperative clinical outcomes and guide postoperative rehabilitation. Secondly, we used a validation cohort independent of the development cohort to validate the prediction model and avoid overfitting. However, if the nomogram is validated with data from other hospitals, the results would be more convincing. Finally, this study is a single-centre, retrospective study, may have potential biases in patient collection. A multi-centre study with a larger sample size and more related risk factors may further optimise and validate the model and confirm its value in clinical practice.
The prediction model (nomogram) based on patients' preoperative clinical characteristics, including BMI, COD, LI and PC, can be used to accurately predict the unfavourable outcomes of PETD for LDH. As a reliable, simplified and well-understood scoring system, the nomogram can be implemented in clinical practice to aid surgeons in clinical decision making, and it can allow for a more informed and well-understood discussion with patients regarding the expected clinical outcomes when considering PETD for LDH.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was a retrospective study and was approved by the Ethics Committee of the In review First Affiliated Hospital of Nanchang University. According with the declaration of Helsinki, all patients provided written informed consent, and all clinical data was kept confidential.
XJ and LG designed this study and collected the clinical data. XJ, GX, XC, and JJ analyzed the data. XJ and YY drafted the manuscript. DZ, MX, and YY supervised this study and revised the article.All authors made a significant contribution to this study. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China [81860216 to DZ] and the Natural Science Foundation of Jiangxi Province [20224BAB216046 to YY].
We appreciate the effort of the individuals to executing this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hartvigsen J, Hancock M, Kongsted A, Louw Q, Ferreira M, Genevay S, et al. What low back pain is and why we need to pay attention. Lancet. (2018) 391(10137):2356–67. doi: 10.1016/s0140-6736(18)30480-x
2. Schizas C, Kulik G, Kosmopoulos V. Disc degeneration: current surgical options. Eur Cell Mater. (2010) 20:306–15. doi: 10.22203/ecm.v020a25
3. Buchmann N, Preuß A, Gempt J, Ryang Y, Vazan M, Stoffel M, et al. Outcome after surgical treatment for late recurrent lumbar disc herniations in standard open microsurgery. World Neurosurg. (2016) 89:382–6. doi: 10.1016/j.wneu.2016.02.028
4. Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Pract Res Clin Rheumatol. (2010) 24(6):769–81. doi: 10.1016/j.berh.2010.10.002
5. Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. (2009) 12(4):E35–70. doi: 10.36076/ppj.2009/12/35
6. Tang S, Qian X, Zhang Y, Liu Y. Treating low back pain resulted from lumbar degenerative instability using Chinese Tuina combined with core stability exercises: a randomized controlled trial. Complement Ther Med. (2016) 25:45–50. doi: 10.1016/j.ctim.2016.01.001
7. Gibson JN, Cowie JG, Iprenburg M. Transforaminal endoscopic spinal surgery: the future “gold standard” for discectomy?—a review. Surgeon. (2012) 10(5):290–6. doi: 10.1016/j.surge.2012.05.001
8. Ruetten S, Komp M, Merk H, Godolias G. Recurrent lumbar disc herniation after conventional discectomy: a prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J Spinal Disord Tech. (2009) 22(2):122–9. doi: 10.1097/BSD.0b013e318175ddb4
9. Shin S, Hwang B, Keum H, Lee S, Park S, Lee S Epidural steroids after a percutaneous endoscopic lumbar discectomy. Spine. (2015) 40(15):E859–865. doi: 10.1097/brs.0000000000000990
10. Cheng J, Wang H, Zheng W, Li C, Wang J, Zhang Z, et al. Reoperation after lumbar disc surgery in two hundred and seven patients. Int Orthop. (2013) 37(8):1511–7. doi: 10.1007/s00264-013-1925-2
11. Kim HS, You JD, Ju CI. Predictive scoring and risk factors of early recurrence after percutaneous endoscopic lumbar discectomy. Biomed Res Int. (2019) 2019:6492675. doi: 10.1155/2019/6492675
12. Park C, Park E, Lee S, Lee K, Kwon Y, Kang M, et al. Risk factors for early recurrence after transforaminal endoscopic lumbar disc decompression. Pain Physician. (2019) 22(2):E133–138. doi: 10.36076/ppj/2019.22.E133
13. Lin Y, Wang M, Jia J, Wan W, Wang T, Yang W, et al. Development and validation of a prognostic nomogram to predict recurrence in high-risk gastrointestinal stromal tumour: a retrospective analysis of two independent cohorts. EBioMedicine. (2020) 60:103016. doi: 10.1016/j.ebiom.2020.103016
14. Janssen E, Punt I, van Kuijk S, Hoebink E, van Meeteren N, Willems P. Development and validation of a prediction tool for pain reduction in adult patients undergoing elective lumbar spinal fusion: a multicentre cohort study. Eur Spine J. (2020) 29(8):1909–16. doi: 10.1007/s00586-020-06473-w
15. Yan Y, Zhu M, Cao X, Zhang Y, Zhang X, Xu M, et al. Different approaches to percutaneous endoscopic lumbar discectomy for L5/S1 lumbar disc herniation: a retrospective study. Br J Neurosurg. (2020):1–7. doi: 10.1080/02688697.2020.1861218
16. Lee JH, Lee SH. Clinical efficacy of percutaneous endoscopic lumbar annuloplasty and nucleoplasty for treatment of patients with discogenic low back pain. Pain Med. (2016) 17(4):650–7. doi: 10.1093/pm/pnv120
17. Yao Y, Liu H, Zhang H, Wang H, Zhang C, Zhang Z, et al. Risk factors for recurrent herniation after percutaneous endoscopic lumbar discectomy. World Neurosurg. (2017) 100:1–6. doi: 10.1016/j.wneu.2016.12.089
18. Rihn J, Hilibrand A, Radcliff K, Kurd M, Lurie J, Blood E. Duration of symptoms resulting from lumbar disc herniation: effect on treatment outcomes: analysis of the spine patient outcomes research trial (SPORT). J Bone Joint Surg Am. (2011) 93(20):1906–14. doi: 10.2106/jbjs.J.00878
19. Mok F, Samartzis D, Karppinen J, Fong D, Luk K, Cheung K Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J. 2016;16(1):32–41. doi: 10.1016/j.spinee.2015.09.060
20. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. (2001) 26(17):1873–8. doi: 10.1097/00007632-200109010-00011
21. Khor S, Lavallee D, Cizik A, Bellabarba C, Chapman J, Howe C, et al. Development and validation of a prediction model for pain and functional outcomes after lumbar spine surgery. JAMA Surg. (2018) 153(7):634–42. doi: 10.1001/jamasurg.2018.0072
22. Rihn J, Kurd M, Hilibrand A, Lurie J, Zhao W, Albert T, et al. The influence of obesity on the outcome of treatment of lumbar disc herniation: analysis of the spine patient outcomes research trial (SPORT). J Bone Joint Surg Am. (2013) 95(1):1–8. doi: 10.2106/jbjs.K.01558
23. Zhang Y, Zhu Y, Pei Y, Zhao Y, Zhou F, Huang M, et al. Disrupted interhemispheric functional coordination in patients with chronic low back-related leg pain: a multiscale frequency-related homotopic connectivity study. J Pain Res. (2019) 12:2615–26. doi: 10.2147/jpr.S213526
24. Heuch I, Heuch I, Hagen K, Zwart JA. Body mass index as a risk factor for developing chronic low back pain: a follow-up in the Nord-Trøndelag Health Study. Spine. (2013) 38(2):133–9. doi: 10.1097/BRS.0b013e3182647af2
25. Böstman OM. Body mass index and height in patients requiring surgery for lumbar intervertebral disc herniation. Spine. (1993) 18(7):851–4. doi: 10.1097/00007632-199306000-00007
26. Kong M, Xu D, Gao C, Zhu K, Han S, Zhang H, et al. Risk factors for recurrent L4-5 disc herniation after percutaneous endoscopic transforaminal discectomy: a retrospective analysis of 654 cases. Risk Manag Healthc Policy. (2020) 13:3051–65. doi: 10.2147/rmhp.S287976
27. Meredith DS, Huang RC, Nguyen J, Lyman S. Obesity increases the risk of recurrent herniated nucleus pulposus after lumbar microdiscectomy. Spine J. (2010) 10(7):575–80. doi: 10.1016/j.spinee.2010.02.021
28. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. (2007) 32(16):1754–60. doi: 10.1097/BRS.0b013e3180b9f96e
29. Labaran L, Puvanesarajah V, Rao S, Chen D, Shen F, Jain A, et al. Recent preoperative lumbar epidural steroid injection is an independent risk factor for incidental durotomy during lumbar discectomy. Global Spine J. (2019) 9(8):807–12. doi: 10.1177/2192568219833656
30. Cohen SP, Bicket MC, Jamison D, Wilkinson I, Rathmell JP. Epidural steroids: a comprehensive, evidence-based review. Reg Anesth Pain Med. (2013) 38(3):175–200. doi: 10.1097/AAP.0b013e31828ea086
31. Bhatti AB, Kim S. Role of epidural injections to prevent surgical intervention in patients with chronic sciatica: a systematic review and meta-analysis. Cureus. (2016) 8(8):e723. doi: 10.7759/cureus.723
32. Koltsov J, Smuck M, Alamin T, Wood K, Cheng I, Hu S. Preoperative epidural steroid injections are not associated with increased rates of infection and dural tear in lumbar spine surgery. Eur Spine J. (2021) 30(4):870–7. doi: 10.1007/s00586-020-06566-6
33. Bhattacharjee S, Pirkle S, Shi LL, Lee MJ. Preoperative lumbar epidural steroid injections administered within 6 weeks of microdiscectomy are associated with increased rates of reoperation. Eur Spine J. (2020) 29(7):1686–92. doi: 10.1007/s00586-020-06410-x
34. Xu D, Chen Z, Zhao Y, Ni H, Chen K, Liu Y, et al. The clinical results of percutaneous endoscopic interlaminar discectomy (PEID) in the treatment of calcified lumbar disc herniation: a case-control study. Pain Physician. (2016) 19(2):69–76.26815251
35. Shao J, Yu M, Jiang L, Wei F, Wu F, Liu Z, et al. Differences in calcification and osteogenic potential of herniated discs according to the severity of degeneration based on pfirrmann grade: a cross-sectional study. BMC Musculoskelet Disord. (2016) 17:191. doi: 10.1186/s12891-016-1015-x
36. Chen Y, Wang J, Sun B, Cao P, Tian Y, Shen X, et al. Percutaneous endoscopic lumbar discectomy in treating calcified lumbar intervertebral disc herniation. World Neurosurg. (2019) 122:e1449–1456. doi: 10.1016/j.wneu.2018.11.083
37. Zhao Z, Guo L, Zhu Y, Luo W, Ou Y, Quan Z, et al. Clinical use of a new nano-hydroxyapatite/Polyamide66 composite artificial Lamina in spinal decompression surgery: more than 4 Years’ follow-up. Med Sci Monit. (2018) 24:5573–9. doi: 10.12659/msm.907958
38. Sun B, Wu H, Xu Z, Lu J, Wang Y, Zhang K, et al. Is selective nerve root block necessary for learning percutaneous endoscopic lumbar discectomy: a comparative study using a cumulative summation test for learning curve. Int Orthop. (2020) 44(7):1367–74. doi: 10.1007/s00264-020-04558-1
Keywords: percutaneous endoscopic transforaminal discectomy, lumbar disc herniation, nomogram, prediction model, lower back pain
Citation: Jiang X, Gu L, Xu G, Cao X, Jiang J, Zhang D, Xu M and Yan Y (2023) Nomogram for predicting the unfavourable outcomes of percutaneous endoscopic transforaminal discectomy for lumbar disc herniation: a retrospective study. Front. Surg. 10:1188517. doi: 10.3389/fsurg.2023.1188517
Received: 17 March 2023; Accepted: 19 May 2023;
Published: 2 June 2023.
Edited by:
Alessandro Di Rienzo, Marche Polytechnic University, ItalyReviewed by:
Erika Carrassi, Azienda Ospedaliero Universitaria Ospedali Riuniti, Italy© 2023 Jiang, Gu, Xu, Cao, Jiang, Zhang, Xu and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mu Xu MzAzODQxODE3QHFxLmNvbQ== Yi Yan ODA3Nzg5MjE0QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.