
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 26 April 2023
Sec. Surgical Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1178394
Background: Mucosa-associated lymphoid tissue (MALT) lymphoma is a group of extranodal lymphomas that originate from B cells. Primary colonic MALT lymphoma is a rare disease, and there is no consensus on its endoscopic features and standard therapies. It is essential to raise awareness of colonic MALT lymphoma and choose the appropriate treatment.
Case presentation: In this case report, we describe a 0-IIb-type lesion that was found by electronic staining endoscopy and magnifying endoscopy. The patient underwent a definitive diagnostic ESD for diagnosis. The patient was evaluated for lymphoma after diagnostic ESD according to the Lugano 2014 evaluation criteria, which are divided into imaging remission on the basis of CT and/or magnetic resonance imaging (MRI) evaluation and metabolic remission on the basis of PET-CT evaluation. Based on the PET-CT results suggesting increased glucose metabolism in the sigmoid colon, the patient underwent additional surgical treatment. According to the pathological results of the surgery, we found that ESD could treat such lesions, which may provide a new option for colorectal MALT lymphoma.
Conclusion: The low incidence of colorectal MALT lymphoma, especially for 0-IIb lesions, which are difficult to detect, requires the use of electronic staining endoscopy to improve the detection rate. The combination with magnification endoscopy can improve the understanding of colorectal MALT lymphoma, which ultimately requires pathological support for diagnosis. According to our experience with the present patient case, ESD seems to be a feasible and economical choice for the treatment of massive colorectal MALT lymphoma. However, the combined application of ESD and another therapy scheme needs further clinical investigation.
Mucosa-associated lymphoid tissue (MALT) lymphoma is a subtype of non-Hodgkin's lymphoma derived from B cells in the peripheral follicular area of lymph nodes. Colorectal MALT lymphomas account for only 1.6% of all MALT lymphomas, which suggests a low incidence of colorectal MALT lymphoma. Therefore, the clinical characteristics, especially the endoscopic features, and standard therapy of colorectal MALT lymphoma have not been clearly established. In this case report, we describe a 0-IIb-type lesion that was found by electronic staining endoscopy and magnifying endoscopy. The patient underwent a definitive diagnostic ESD for diagnosis. According to the primary gastrointestinal lymphoma Lugano staging system (1), the case was classified as IE1 stage. The patient was evaluated for lymphoma after diagnostic ESD according to the Lugano 2014 evaluation criteria (2), which are divided into imaging remission on the basis of CT and/or magnetic resonance imaging (MRI) evaluation and metabolic remission on the basis of PET-CT evaluation. Based on the PET-CT results suggesting increased glucose metabolism in the sigmoid colon, the patient underwent additional surgical treatment. According to the pathological results of the surgery, we found that ESD could treat such lesions, which may provide a new option for colorectal MALT lymphoma.
Herein, we describe a case of colonic MALT lymphoma in a 55-year-old man. The patient showed no clinical symptoms. He was routinely examined at Jinhua People's Hospital on 23 June 2021. The electronic gastroscopy suggested chronic gastritis, and the electronic colonoscopy revealed a mucosal redness in the sigmoid colon under white light (Figure 1A). A 0-IIb mucosal lesion of approximately 4.5 cm*5.0 cm with a red surface and clear borders was seen after indigo carmine staining (Figure 1B). The narrow-band imaging magnifying (NBI-M) endoscopy detected some branched abnormal blood vessels and the disappearance of the glandular structure (Figures 1C,D). In order to further define the nature of the lesion, the patient was hospitalized and underwent diagnostic endoscopic submucosal dissection (ESD). Preoperative routine blood tests, routine biochemical tests, hepatitis B virus, hepatitis C virus (HCV), human immunodeficiency virus (HIV), and syphilis tests showed no significant abnormalities. After full communication with the patient and obtaining his informed consent, diagnostic endoscopic submucosal dissection (ESD) was performed on him on 02 July 2021 (Figure 1E). Postoperative pathology suggested that the sigmoid colon was consistent with B-cell non-Hodgkin's lymphoma, with focal hyperplastic polyp formation in the extra-nodal marginal zone of MALT. Immunohistochemistry showed CD20, CD79a, and BCL2 (+), CD21 lymphocytes and FDC (+), CD23FDC (+), P53 partial (+), CD3, CD5, CD10, BCL6, and CyclinD1 (−), Ki67 (+, extra follicular 5%), and EBER (−) (Figure 2).
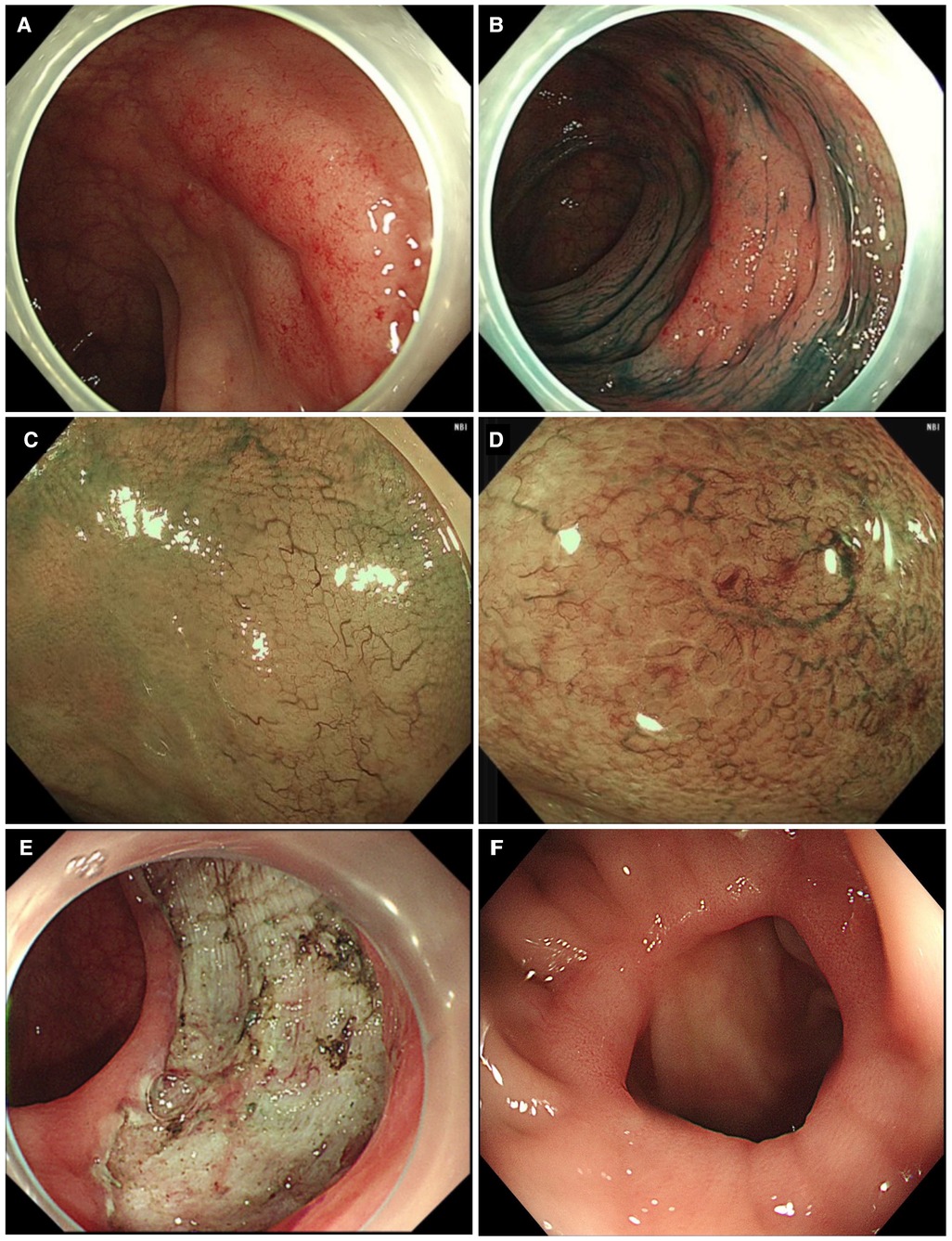
Figure 1. Clinical data of the patient with colonic mucosa–associated lymphoid tissue lymphoma. (A) Normal colonoscopy shows a 0-IIb-type lesion; (B) indigo carmine staining; (C) narrow-band imaging magnifying endoscopy shows an irregular branching vasculature; (D) disappeared glandular structure; (E) diagnostic ESD resection of lesions; (F) colonoscopy results after the operation.
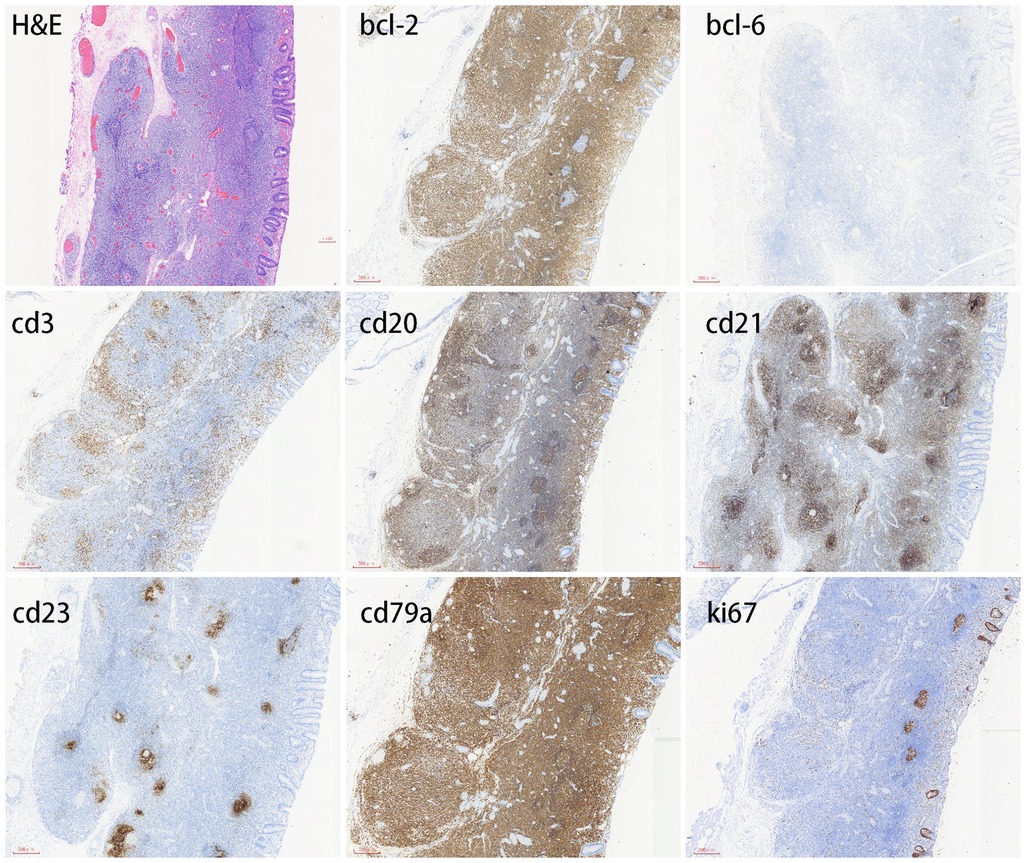
Figure 2. Immunohistochemical profile of colonic mucosa–associated lymphoid tissue lymphoma. MALT lymphoma is positive for CD20 and CD79a but negative for CD3 and Bcl-6. CD21 lymphocytes and FDC are positive, and CD23 FDC is also positive. Bcl-2 is usually positive. The Ki-67 proliferation index is typically low.
To further evaluate the staging of lymphoma and systemic metastasis, bone marrow aspiration and positron emission computed tomography (PET-CT) were performed on 15 July 2021. PET-CT showed a thickening of the sigmoid canal wall (Figure 3A), increased glucose metabolism, and a maximum SUV of approximately 7.8 (Figure 3B). The bone marrow aspiration report suggested no invasion of the bone marrow. Based on the PET-CT results suggesting increased glucose metabolism in the sigmoid colon, two possibilities were considered. First, it may be a false-positive due to the inflammatory response after colon ESD. Second, it is possible that the lymphoma may persist after colon ESD. After the patient was fully communicated and informed about his condition, he opted to undergo additional surgical treatment with partial colectomy and clearance of the peri-intestinal lymph nodes. Postoperative pathology showed no residual tumor in the original surgical field, negative surgical margins, and reactive pericolonic lymph nodes. The patient recovered well after surgery.
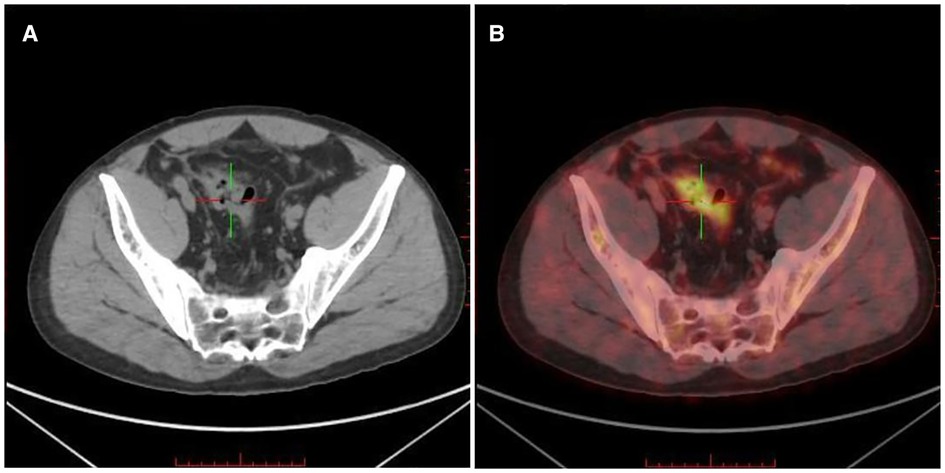
Figure 3. Positron emission computed tomography (PET-CT) of the lesion. (A) PET-CT showed a thickening of the sigmoid canal wall; (B) increased FDG uptake and a maximum SUV of approximately 7.8.
On 17 January 2022, the chest CT was repeated, which showed no significant abnormality. The abdominal CT examination suggested postoperative changes in the sigmoid colon, and no significant metastases were found. A repeat colonoscopy 1 year after surgery showed postsurgical scar formation and no recurrent lesions (Figure 1F).
Most non-Hodgkin's lymphoma (NHL) patients have superficial lymph node enlargement as the first symptom, and some patients have primary extranodal lymphoid tissue or organs. The MALT type of lymphoma in the extralymph node marginal zone can be found throughout the body, with the gastrointestine being the most common site of involvement, accounting for approximately 50% of all MALT lymphomas, followed by the parotid gland, skin, conjunctiva, head and neck, lung, thyroid, and breast (3, 4). In contrast, colorectal MALT lymphomas account for only 1.6% of all MALT lymphomas (5), and colorectal lymphomas account for only 0.2% of all colorectal malignancies with an annual incidence of 1.6/1 million (6). These results suggest a low incidence of colorectal MALT lymphoma. Bone marrow invasion is present in approximately 15%–20% of patients. About one-third of MALT lymphomas present as disseminated, and most MALT lymphomas are limited (7).
The etiology of MALT is unclear and may be related to persistent immune stimulation due to chronic infection or inflammation. Gastric MALT lymphoma is associated with chronic infection with HP, and a proportion of patients can be in remission after anti-HP treatment. Whether colonic MALT is associated with HP is inconclusive (8), which may be related to the small number of cases. Moreover, 22%–35% of non-gastric MALT lymphomas have HCV infection (9). Furthermore, chromosomal alterations t(11;18), t(1;14), t(14;18) are more common in MALT (10).
Relatively few cases of primary colonic MALT lymphoma have been reported in the literature, and common clinical manifestations are gastrointestinal bleeding, abdominal pain, perforation, and intussusception (11–13). The patient reported in this case was asymptomatic, and MALT was found during colonoscopy. The morphological changes of colonic MALT are diverse and can be single or multiple (14–16). Endoscopically, flat, elevated, polyp-like, or semipedunculated changes may be observed, and the surface may be smooth, granular, nodular, or ulcerative. The size can be up to 4–5 cm in a single lesion or in the form of multiple centimeter or subcentimeter nodules or polyps (17–20). If the endoscopic presentation of the lesion is atypical and susceptible to missed diagnosis or misdiagnosis, electronic staining endoscopy, combined with magnifying endoscopy, can improve the detection rate. Histologically, MALT lymphoma is a low-grade B-cell lymphoma that lacks pathomorphological and immunophenotypic features, and the small, shallow tissue of routine biopsies can cause diagnostic difficulties in pathology (21, 22). Diagnostic ESD procedures allow whole lesions to be excised and improve the diagnostic rate.
There is no standardized treatment protocol for colorectal MALT lymphoma. Treatment modalities include surgical resection, chemotherapy, radiotherapy, and endoscopic resection (14, 18, 23, 16). Rituximab is currently considered a treatment option for non-gastric MALT lymphoma, with an efficiency of approximately 80% (24, 25). ISRT also has good efficacy (26, 27). For extracolonic MALT lymphoma in certain specific sites such as the thyroid, lung, small intestine, and colon, surgical resection is an option. If the margins are positive, local regional ISRT should be administered after surgery, while follow-up observation can be an option for negative margins.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DC, D-FZ, YY, S-SC, and DL collected and analyzed the data. DC and DL drafted the manuscript. DL critically revised and gave final approval for the publication of the paper. All authors contributed to the article and approved the submitted version.
This study was supported by Jinhua City Science and Technology Plan Projects “Improve the Polarization of Cancer-related Fatigue: Clinical Efficacy Study” (2018-4-049).
The authors sincerely thank the participant for his willingness to participate in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rohatiner A, d′Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. AnnOncol. (1994) 5(5):397–400. doi: 10.1093/oxfordjournals.annonc.a058869
2. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Lawrence HS, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
3. Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. (1983) 52(8):1410–6. doi: 10.1002/1097-0142(19831015)52:8%3C1410::aid-cncr2820520813%3E3.0.co;2-3
4. Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: analysis of the surveillance, epidemiology, and end results database. Cancer. (2013) 119:629–38. doi: 10.1002/cncr.27773
5. Hahn JS, Kim YS, Lee YC, Yang WI, Lee SY, Suh CO. Elevenyear experience of low grade lymphoma in Korea (based on REAL classification). Yonsei Med J. (2003) 44:757–70. doi: 10.3349/ymj.2003.44.5.757
6. Fan CW, Changchien CR, Wang JY, Chen JS, Hsu KC, Tang R, et al. Primary colorectal lymphoma. Dis Colon Rectum. (2000) 43:1277–82. doi: 10.1007/BF02237436
7. Thieblemont C, Berger F, Dumontet C, Moullet I, Bouafia F, Felman P, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. (2000) 95(3):802–6. doi: 10.1007/s002770050019
8. Nakase H, Okazaki K, Ohana M, Ikeda K, Uchida K, Uose S, et al. The possible involvement of micro-organisms other than Helicobacter pylori in the development of rectal MALT lymphoma in H. pylori-negative patients. Endoscopy. (2002) 34(4):343–6. doi: 10.1055/s-2002-23643
9. Arcaini L, Burcheri S, Rossi A, Paulli M, Bruno R, Passamonti F, et al. Prevalence of HCV infection in nongastric marginal zone B-cell lymphoma of MALT. Ann Oncol. (2007) 18(2):346–50. doi: 10.1093/annonc/mdl388
10. Troppan K, Wenzl K, Neumeister P, Deutsch A. Molecular pathogenesis of MALT lymphoma. Gastroenterol Res Pract. (2015) 2015:102656. doi: 10.1155/2015/102656
11. Kanneganti K, Balar B. Mucosa-associated lymphoid tissue lymphoma presenting as massive gastrointestinal bleeding: a case report. Case Rep Gastroenterol. (2008) 2(3):296–300. doi: 10.1159/000154814
12. Adams KM, Roe NM. A rare case of MALT lymphoma underlying ileocecal intussusception. J Am Osteopath Assoc. (2016) 116(8):e37–40. doi: 10.7556/jaoa.2016.108
13. Jain V, Misra S, Ahmad F, Rahul K, Singh A. MALT lymphoma of caecum presenting as acute intestinal obstruction: a case report. Indian J Surg. (2013) 75(Suppl 1):286–9. doi: 10.1007/s12262-012-0672-8
14. Kim MH, Jung JT, Kim EJ, Kim TW, Kim SY, Kwon JG, et al. A case of mucosa-associated lymphoid tissue lymphoma of the sigmoid colon presenting as a semipedunculated polyp. Clin Endosc. (2014) 47(2):192–6. doi: 10.5946/ce.2014.47.2.192
15. Fischbach W. Colorectal MALT lymphoma: a rare clinical entity. Z Gastroenterol. (2018) 56(8):905–11. doi: 10.1055/a-0581-5736
16. Ghimire P, Wu G-Y, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. (2011) 17(6):697–707. doi: 10.3748/wjg.v17.i6.697
17. Abbas H, Niazi M, Makker J. Mucosa-associated lymphoid tissue (MALT) lymphoma of the colon: a case report and a literature review. Am J Case Rep. (2017) 18:491–7. doi: 10.12659/AJCR.902843
18. Akasaka R, Chiba T, Dutta AK, Toya Y, Mizutani T, Shozushima T, et al. Colonic mucosa-associated lymphoid tissue lymphoma. Case Rep Gastroenterol. (2012) 6(2):569–75. doi: 10.1159/000342726
19. Hasegawa N, Kato K, Yamada K, Morita K, Kuroiwa M, Ito H, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) of the sigmoid colon. Gastrointest Endosc. (2000) 52(6):802–4. doi: 10.1067/mge.2000.110737
20. Esteban JM, Gutiérrez del Olmo A, Baki W, Fernández S, Soria MT, Mediavilla JD, et al. Colonic mucosa-associated lymphoid tissue lymphoma presenting as multiple polyposis. Gastrointest Endosc. (2005) 61(7):928–30. doi: 10.1016/s0016-5107(04)02841-x
21. Howell JM, Auer-Grzesiak I, Zhang J, Andrews CN, Stewart D, Urbanski SJ, et al. Increasing incidence rates, distribution and histological characteristics of primary gastrointestinal non-hodgkin lymphoma in a North American population. Can J Gastroenterol. (2012) 26(7):452–6. doi: 10.1155/2012/480160
22. Burke JS. Lymphoproliferative disorders of the gastrointestinal tract: a review and pragmatic guide to diagnosis. Arch Path Lab Med. (2011) 135(10):1283–97. doi: 10.5858/arpa.2011-0145-RA
23. Seo S-W, Lee S-H, Lee D-J, Kim K-M, Kang J-K, Kim D-W, et al. Colonic mucosa-associated lymphoid tissue lymphoma identified by chromoendoscopy. World J Gastroenterol. (2014) 20(48):18487–94. doi: 10.3748/wjg.v20.i48.18487
24. Conconi A, Martinelli G, Thieblemont C, Ferreri AJM, Devizzi L, Peccatori F, et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood. (2003) 102(8):2741–5. doi: 10.1182/blood-2002-11-3496
25. Martinelli G, Laszlo D, Ferreri AJ, Pruneri G, Ponzoni M, Conconi A, et al. Clinical activity of rituximab in gastric marginal zone non-Hodgkin's Lymphoma resistant to or not eligible for anti-Helicobacter pylori therapy. J Clin Oncol. (2005) 23(9):1979–83. doi: 10.1200/JCO.2005.08.128
26. Goda JS, Gospodarowicz M, Pintilie M, Wells W, Hodgson DC, Sun A, et al. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer. (2010) 116(16):3815–24. doi: 10.1002/cncr.25226
Keywords: colonic mucosa–associated lymphoid tissue lymphoma, electronic staining endoscopy, pigmented endoscopy, magnifying endoscopy, treatment
Citation: Chen D, Zhong D-F, Yang Y, Chen S-S and Liu D (2023) Colonic mucosa–associated lymphoid tissue lymphoma: A case report. Front. Surg. 10:1178394. doi: 10.3389/fsurg.2023.1178394
Received: 2 March 2023; Accepted: 22 March 2023;
Published: 26 April 2023.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Natale Calomino, University of Siena, Italy© 2023 Chen, Zhong, Yang, Chen, Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Liu bGkzNzVkb25nQDE2My5jb20=
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.