
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg., 24 May 2023
Sec. Surgical Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1171234
Introduction: An intraductal papillary mucinous neoplasm (IPMN) is a potentially malignant cystic tumor that is characterized by an excessive papillary proliferation of mucin-producing epithelial cells. The IPMN usually exhibits different degrees of dysplasia and is accompanied by cystic dilation of the main pancreatic duct (MPD) or side branch. We report a case of an IPMN that has penetrated the stomach and has differentiated into an adenocarcinoma.
Case presentation: A 69-year-old female, suffering from chronic pancreatitis of unknown etiology, visited our outpatient clinic with complaints of sudden weight loss, diarrhea, and abdominal pain. She underwent several examinations to evaluate the reasons for her sudden onset of symptoms. A gastroscopy showed an ulcerated lesion covered with mucus. CT and magnetic resonance cholangiopancreatography images revealed that the MPD was dilated to 1.3 cm with a fistula formation between the MPD and the stomach. After a multidisciplinary discussion of this case, a total pancreatectomy was proposed. An en bloc total pancreatectomy with gastric wedge resection including the fistula together with splenectomy was carried out. A Roux-en-Y choledochojejunostomy and gastrojejunostomy were performed. Histology results revealed the association of IPMN with invasive carcinoma.
Discussion: Many reports on IPMN of the pancreas have been published recently. Fistula formation between IPMN and adjacent organs is possible. Given the CT and endoscopic ultrasonography findings, it shows that in our case a main duct IPMN (MD-IPMN) formed a pancreatico-gastric fistula. We point out that the adherence of invasive cancer cells contributed to the fistula formation between the pancreas and the stomach.
Conclusion: This case report provides evidence for the possibility of IPMN becoming complicated with pancreatico-gastric fistula. Thus, we suggest that surgical resection should be considered in the case of MD-IPMN because of its high propensity for malignant transformation.
Pancreatic intraductal papillary mucinous neoplasms (IPMNs) are characterized by cystic tumors with papillary epithelial cells that produce excessive amounts of mucus (1, 2). Usually, IPMN exhibits different degrees of dysplasia (1, 3). There are three types of IPMNs, i.e., main duct, branch duct, and mixed (1). Histologically, IPMNs are subdivided into four groups, i.e., IPMN with low-grade dysplasia (LGD), IPMN with intermediate-grade dysplasia, IPMN with high-grade dysplasia (HGD), and IPMN with an associated invasive carcinoma (4). Fistulation of IPMN of the pancreas into adjacent organs has been previously reported with incidence rates ranging from 1.9% to 6.6% (3). It may involve several adjacent organs, most frequently the duodenum, but sometimes, it may even involve several organs at the same (5). Here, we report a case of a pancreatico-gastric fistula as a complication of IPMN associated with ductal adenocarcinoma of the pancreas.
A 69-year-old female suffering from chronic pancreatitis of unknown etiology since the year 2018 experienced sudden weight loss, diarrhea, and abdominal pain. Her medical history includes diabetes mellitus, hypertension, and pancreatic insufficiency. She is also a heavy smoker. She underwent medical testing to evaluate her sudden onset of symptoms. Blood tests revealed no signs of inflammation, with normal liver functions, a normal rheumatological panel, and a normal IGG4 level. A gastroscopy showed an ulcerative lesion covered with mucus (Figure 1). CT scans revealed signs of chronic pancreatitis with calcifications. The main pancreatic duct (MPD) was dilated to 1.3 cm, with the presence of a fistula between the main pancreatic duct and the stomach (Figure 2). Magnetic resonance cholangiopancreatography (MRCP) images showed no additional findings when compared with the CT scan (Figure 3). Endoscopic ultrasound (EUS) showed a non-homogenous pancreas with diffused calcifications and the MPD with a diameter of approximately 1.3 cm (Figure 3). Multiple biopsies from the edges of the fistula via a gastroscopy yielded main-branch IPMN.
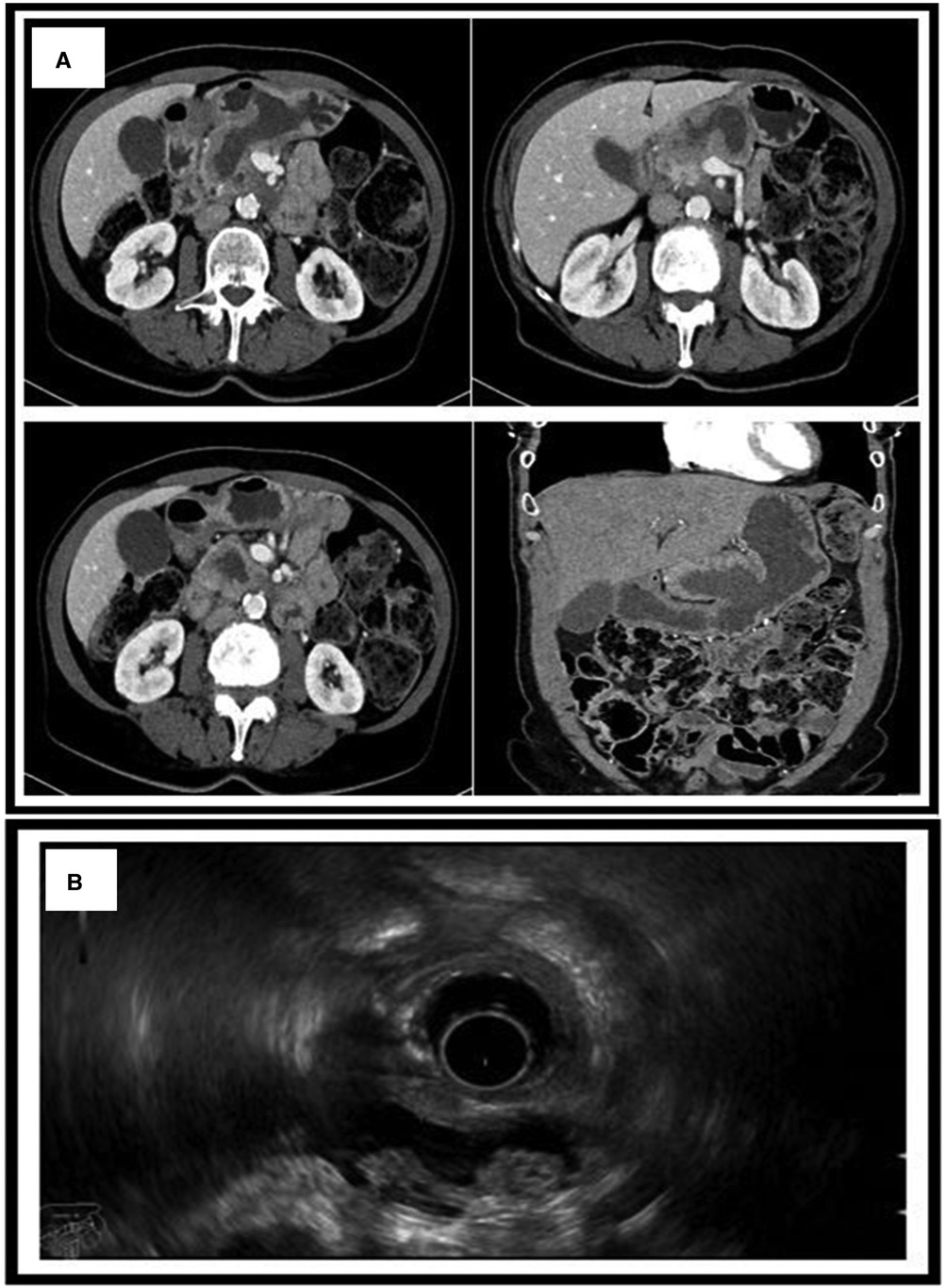
Figure 2. (A) CT scan showing the MPD dilated to 1.3 cm with the presence of a fistula between the main pancreatic duct and the stomach. (B) Endoscopic ultrasound showing a non-homogenous pancreas with diffused calcifications with a dilated MPD of 1.3 cm. MPD, main pancreatic duct.
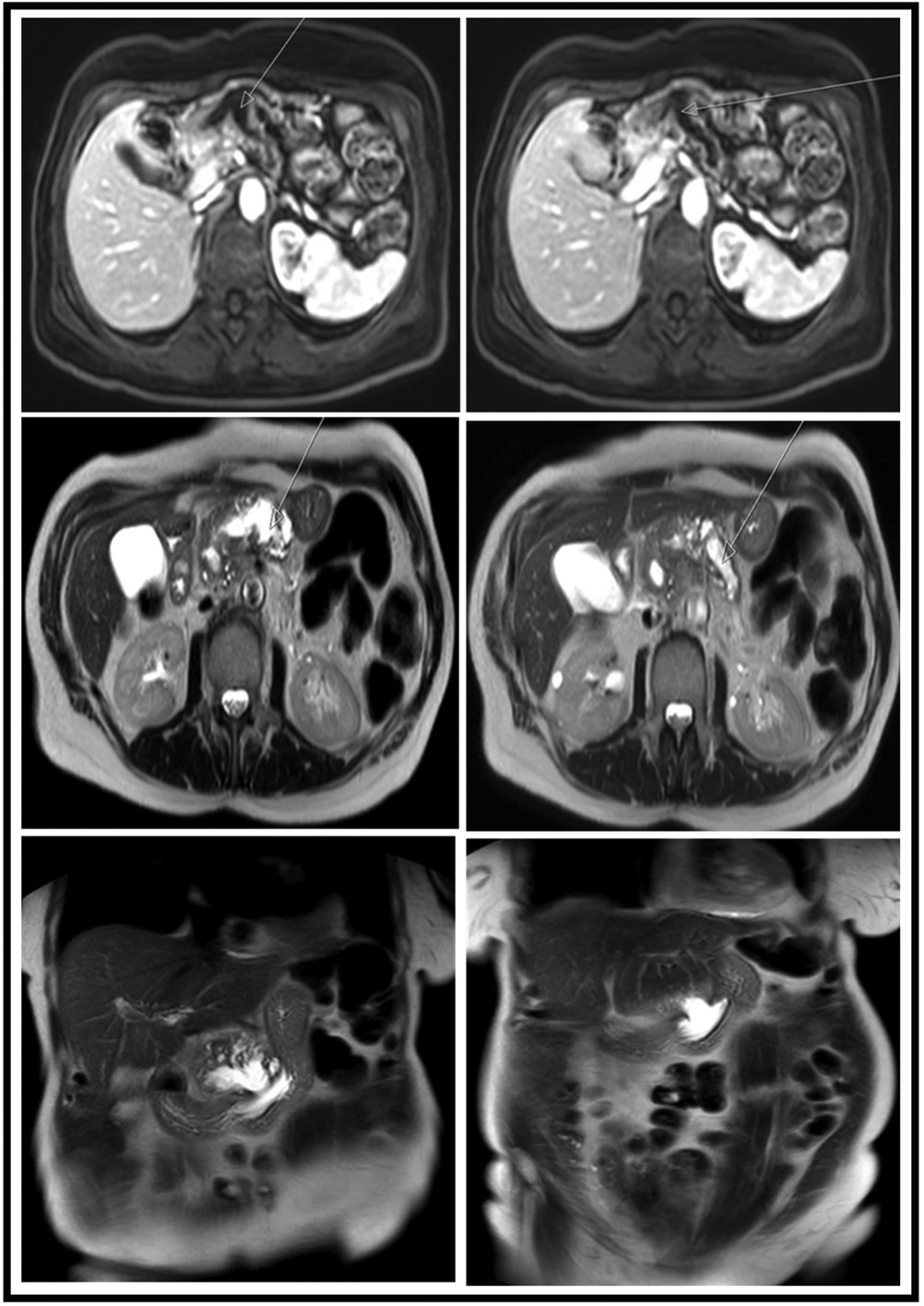
Figure 3. MRI images showing different views and sequences of the fistulation between the MPD and the stomach. Upper row, axial T1 + gadolinium; middle row, axial T2-weighted images; lower row, coronal T2-weighted images. MPD, main pancreatic duct.
Since both chronic pancreatitis and IPMN are considered risk factors for pancreatic malignancy, and also considering the high rate of malignancy due to main duct IPMN (MD-IPMN), a total pancreatectomy was proposed after a multidisciplinary discussion. An en bloc total pancreatectomy with wedge resection of the stomach including the fistula, splenectomy, and Roux-en-Y choledochojejunostomy and gastrojejunostomy were performed (Figure 4A). During the operation, there were no signs of peritoneal metastasis, and the surgery was uneventful. The postoperative course was complicated with bleeding from the stapler line of the gastric wedge resection that was treated with adrenaline injections via a gastroscopy. The neoplasm involved the head, body, and tail of the pancreas with a fistulation between the stomach and the MPD (Figure 4B). Pathological results revealed (1) a ductal adenocarcinoma of the pancreas that is G2 moderately differentiated and (2) IPMN with an associated invasive carcinoma. All margins were tumor-free, with no lymphovascular invasion (0/30) (pT3 pN0) (Figures 4C,D). The patient was discharged 2 weeks after the procedure. During a follow-up visit 10 days later, the physical examination was normal. The patient was referred to our oncology department and was scheduled for chemotherapy (the FOLFIRINOX regimen). No further complications were noted.
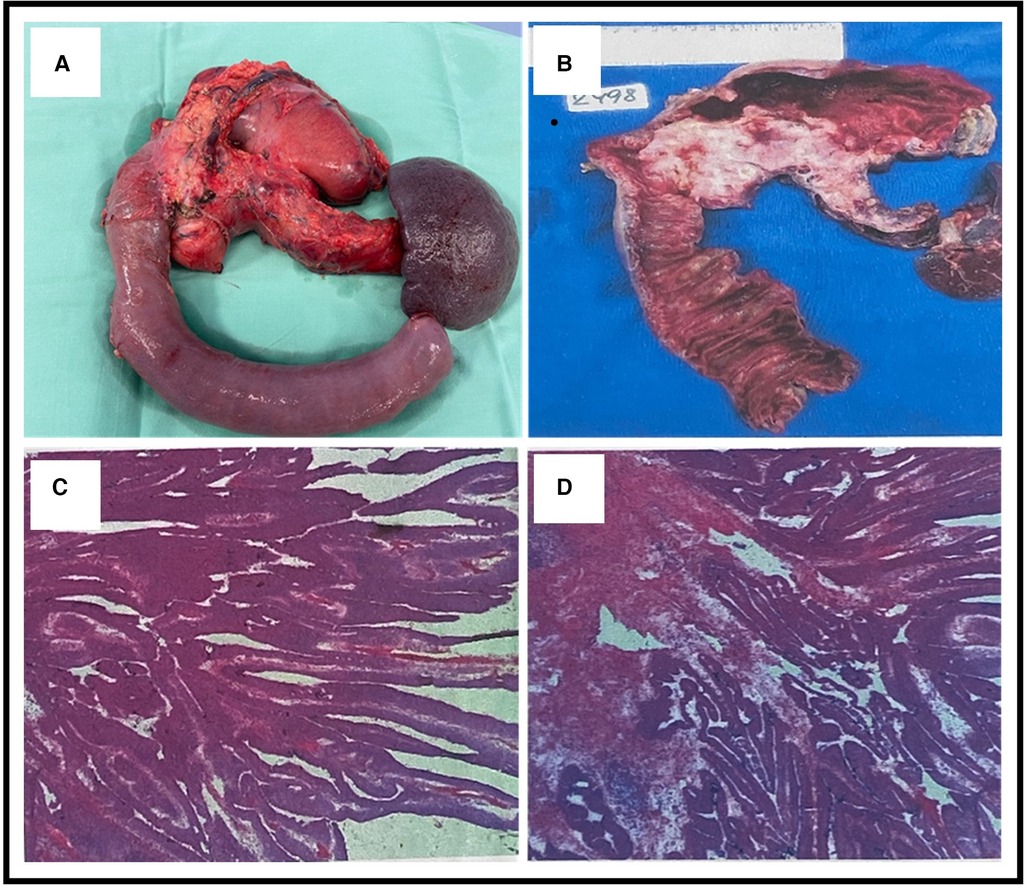
Figure 4. (A) An en bloc total pancreatectomy with wedge resection from the stomach including the fistula and splenectomy. (B) Gross sectional specimen showing a neoplasm involving the head, body, and tail of the pancreas and also showing the fistulation between the stomach and the main pancreatic duct (MPD). (C,D) Microscopic pathological pictures showing (1) ductal adenocarcinoma of the pancreas and intestinal-type morphology and (2) intraductal papillary mucinous neoplasm with an associated invasive carcinoma.
IPMN accounts for approximately 5% of the majority of cystic pancreatic lesions and is predominant in older male patients (3). In 1982, Ohashi was the first one to report on IPMN (6). Of the patients, 33% are symptomatic and present with non-specific symptoms including recurrent pancreatitis, diabetes, weight loss, and steatorrhea (7). Although IPMN and invasive ductal adenocarcinoma originate from the ductal cells of the pancreas, they possess different features. IPMN is characterized by excessive secretion of mucin and slow and expansive growth associated with a low malignancy potential for metastasis (8).
Several radiological imaging methods have been reported in diagnosing intraductal papillary mucinous neoplasm. Those methods include CT (a pancreatic protocol), MRI/MRCP, EUS, contrast-enhanced EUS (CE-EUS), and needle-based confocal laser endomicroscopy. To increase the diagnostic yield of IPMN, a biopsy via an endoscopic ultrasound–guided through-the-needle biopsy has been helpful in certain cases (9). Studies proved that CE-EUS, when conducted with a dedicated harmonic mode, presented an optimal sensitivity, high specificity, and positive predictive value through its ability to provide information on tissue micro vascularization by differentiating between enhanced mural nodules and other non-enhanced solid components (10).
The Fukuoka and Sendai consensus guidelines demonstrate the risk factors for high-grade dysplasia or malignancy transformation of IPMN. The Fukuoka consensus guidelines provided higher positive and negative values to predict high-risk IPMN than those provided by the Sendai consensus guidelines, i.e., 88% vs. 67% and 92.5% vs. 88%, respectively (11). According to the modified Fukuoka consensus guidelines, enhanced mural nodules, MPD ≥10 mm, and jaundice were classified as high-risk stigmata features (12). On the other hand, MPD dilation between 5 and 9.9 mm, cystic growth rate ≥5 mm/year, increased level of serum CA 19-9 (>37 U/mL), symptoms, enhanced mural nodules (<5 mm), cyst diameter ≥40 mm, and finally, adjacent lymphadenopathy were classified as worrisome features (12).
Whether benign or malignant, IPMN has complication potentials. Fistulation to adjacent organs occurred in 6.6% of IPMN cases (3). The duodenum (most common), common bile duct, stomach, and spleen (rarest) are the organs commonly involved in IPMN fistulation (3). Given the MRI, CT, and EUS findings, our case had MD-IPMN with a pancreatico-gastric fistula.
In the last three decades, several reports of pancreatic IPMN fistulation to adjacent organs have been reported in the literature (Table 1). Of the 27 cases reviewed (Table 1), the majority had adenomas (30%) and adenocarcinomas (30%). Only 10% of the patients had IPMN, of which 4% had borderline carcinoma. In 30% of the cases, the pathology was not mentioned. With regard to surgical management, 18% of the patients underwent pancreatoduodenectomy. While 15% had total pancreatectomy together with resection of the fistulated adjacent organs, only 4% underwent distal pancreatectomy together with resection of the fistulated adjacent organs. Only one patient had conservative treatment, and one died before surgical intervention. It is noteworthy to mention that in 44% of the cases reported, surgical management was not described.
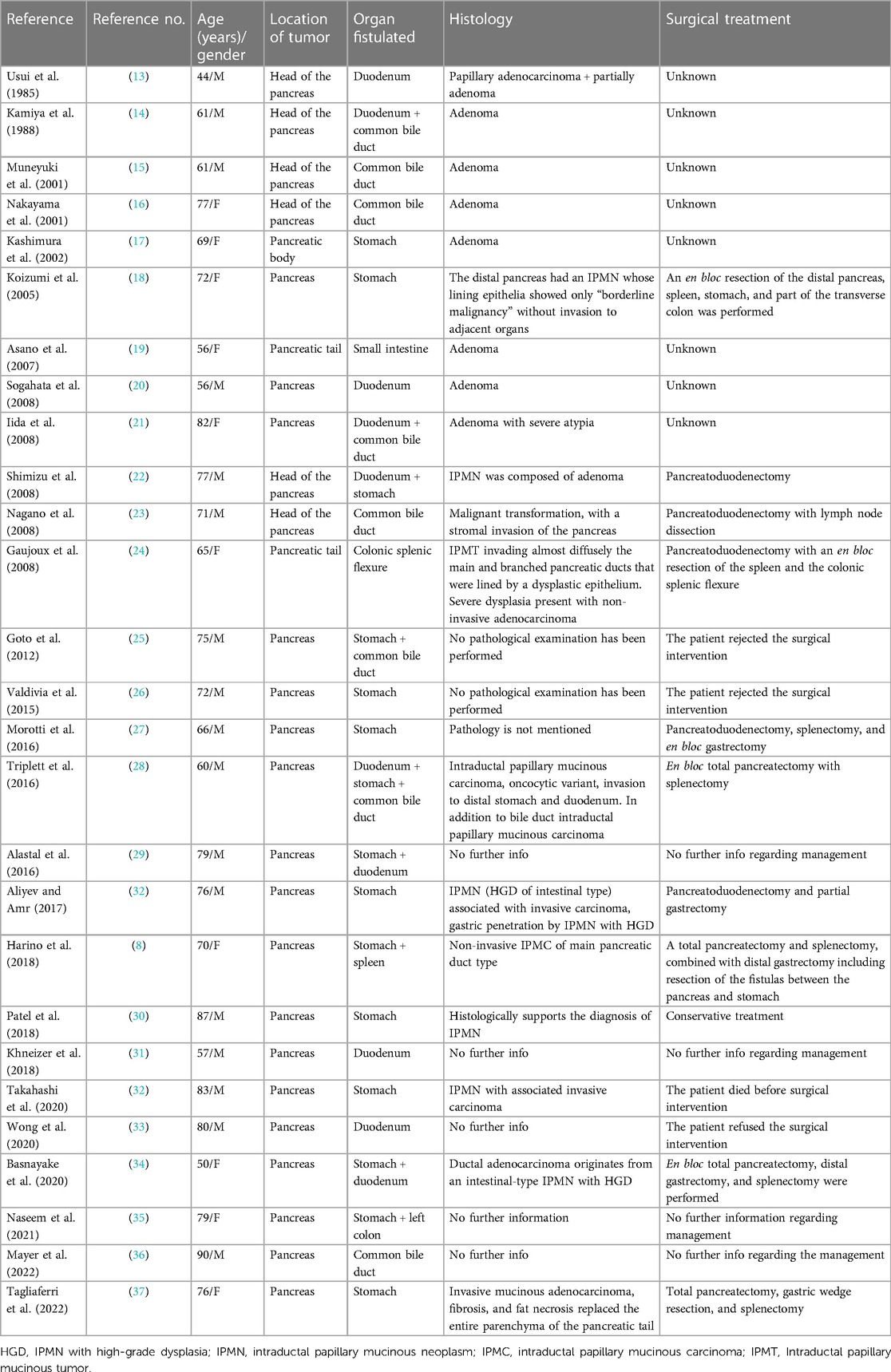
Table 1. Previously reported pancreatic intraductal papillary mucinous neoplasms with penetration to adjacent organs.
Pathologically, MD-IPMN has a substantial potential for progression into an aggressive invasive carcinoma (7). Two factors have been considered for the pathogenesis of fistula formation in IPMN, i.e., invasive penetration of cancer cells and mechanical penetration caused by elevated pressure in the mucus-filled pancreatic duct (8, 32). The first mechanism is seen in malignant tumors, while the second one is mostly seen in benign tumors; nevertheless, a large malignant IPMN would mechanically fistulate into adjacent organs by the effect of direct contact and high pressure on the surrounding organs. In our case, we point out that invasive penetration of the cancerous tumor contributed to the fistula formation.
In this paper, we reported a main duct IPMN that has differentiated into adenocarcinoma and, in the process, fistulated the stomach. In the treatment of IPMN with fistulation to adjacent organs, en bloc resection of the IPMN together with the fistula should be done to avoid possible malignant dissemination. Typically, the extent of resection depends on the extent of cancer invasion. In addition, the main duct IPMN should be considered for surgical resection because of its high risk for malignant transformation.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
MA wrote the paper. YM and GO revised the manuscript critically and provided academic guidance. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jabłońska B, Szmigiel P, Mrowiec S. Pancreatic intraductal papillary mucinous neoplasms: current diagnosis and management. World J Gastrointest Oncol. (2021) 13(12):1880. doi: 10.4251/wjgo.v13.i12.1880
2. Jabłońska B. Pancreatic cysts: etiology, diagnosis and management. Cent Eur J Med. (2014) 9(1):92–107. doi: 10.2478/s11536-013-0244-8
3. Aliyev KV, Amr B. Pancreatico-gastric fistula: a rare complication of intraductal papillary mucinous neoplasm. J Pancreas. (2017) 18(4):362–4.
4. Komo T, Oishi K, Kohashi T, Hihara J, Kanou M, Nakashima A, et al. Pancreatobiliary fistula associated with intraductal papillary mucinous carcinoma accompanying obstructive jaundice: a case report. Int J Surg Case Rep. (2018) 48:126–30. doi: 10.1016/j.ijscr.2018.05.019
5. Maubert A, Vanbiervliet G, Benizri EI. Pancreatico-gastric fistula complicating an intraductal papillary mucinous neoplasm. J Visc Surg. (2017) 154(2):137–8. doi: 10.1016/j.jviscsurg.2016.11.006
6. Ohashi K. Four cases of “mucin-producing” cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. (1982) 20:348–52.
7. Rao V, Sarkar R, Smithies A, Razack A, Wedgwood K. Spontaneous gastro-pancreatic fistula—a hitherto unknown natural history of IPMN? J Surg Case Rep. (2012) 2012(6):9–9. doi: 10.1093/jscr/2012.6.9
8. Harino T, Tomimaru Y, Noguchi K, Nagase H, Ogino T, Hirota M, et al. A case of intraductal papillary-mucinous neoplasm of the pancreas penetrating into the stomach and spleen successfully treated by total pancreatectomy. Surg Case Rep. (2018) 4(1):1–5. doi: 10.1186/s40792-018-0525-1
9. Facciorusso A, Kovacevic B, Yang D, Vilas-Boas F, Martínez-Moreno B, Stigliano S, et al. Predictors of adverse events after endoscopic ultrasound-guided through-the-needle biopsy of pancreatic cysts: a recursive partitioning analysis. Endoscopy. (2022) 54(12):1158–68. doi: 10.1055/a-1831-5385
10. Lisotti A, Napoleon B, Facciorusso A, Cominardi A, Crinò SF, Brighi N, et al. Contrast-enhanced EUS for the characterization of mural nodules within pancreatic cystic neoplasms: systematic review and meta-analysis. Gastrointest Endosc. (2021) 94(5):881–9. doi: 10.1016/j.gie.2021.06.028
11. Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. (2012) 12(3):183–97. doi: 10.1016/j.pan.2012.04.004
12. European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. (2018) 67(5):789–804. doi: 10.1136/gutjnl-2018-316027
13. Usui H, Tomoda J, Watanabe A, Nagashima H, Harada H, Kin H, et al. A case of mucin-producing pancreas carcinoma. J Jpn Soc Gastroenterol. (1985) 82:685–90 [Japanese].
14. Kamiya Y, Goto K, Noguchi Y, et al. A case of mucin-producing pancreas tumor leading to obstructive icterus. J Jpn Soc Gastroenterol Surg. (1988) 85:338 [abstract] [Japanese].
15. Muneyuki T, Okada Y, Choushi H, et al. A case of branched-type IPMT penetrating to the common bile duct leading to obstructive icterus and cholangitis. J Jpn Surg Assoc. (2001) 62:2083 [abstract] [Japanese].
16. Nakayama S, Inoue S, Kaneko T, et al. A resected case of intraductal papillary tumor of the pancreas penetrating to the bile duct leading to obstructive icterus. J Jpn Surg Assoc. (2001) 62:766 [abstract] [Japanese].
17. Kashimura J, Inoue A, Uno K, et al. A case of intraductal papillary tumor of the pancreas penetrating to the stomach. Progr Dig Endosc. (2002) 62:116 [abstract] [Japanese].
18. Koizumi M, Sata N, Yoshizawa K, Tsukahara M, Kurihara K, Yasuda Y, et al. Post-ERCP pancreatogastric fistula associated with an intraductal papillary-mucinous neoplasm of the pancreas—a case report and literature review. World J Surg Oncol. (2005) 3:70. doi: 10.1186/1477-7819-3-70
19. Asano T, Iwai K, Hazama K, et al. A resected case of intraductal papillary mucinous adenoma penetrating to the small intestine. J Jpn Surg Assoc. (2007) 68:2426 [abstract] [Japanese].
20. Sogahata K, Hata F, Miyamoto S, et al. A resected case of IPMN penetrating to the duodenum. J Jpn Soc Gastroenterol Surg. (2008) 41:1382 [abstract] [Japanese].
21. Iida M, Ueno T, Yoshida S, et al. Palliative hepaticojejunostomy for obstructive jaundice due to intraductal papillary mucinous neoplasm penetrating to the duodenum and common bile duct [Japanese]. Tan to Sui, 29 (2008), p. 793–6
22. Shimizu M, Kawaguchi A, Nagao S, Hozumi H, Komoto S, Hokari R, et al. A case of intraductal papillary mucinous neoplasm of the pancreas rupturing both the stomach and duodenum. Gastrointest Endosc. (2010) 71(2):406–12. doi: 10.1016/j.gie.2009.09.018
23. Nagano H, Koneri K, Honda K, Murakami M, Hirono Y, Maeda H, et al. Biliopancreatic fistula and abscess formation in the bursa omentalis associated with intraductal papillary mucinous cancer of the pancreas. Int J Clin Oncol. (2009) 14(5):460–4. doi: 10.1007/s10147-008-0864-1
24. Gaujoux S, Dokmak S, Deschamps L, Zappa M, Belghiti J, Sauvanet A. Pancreatocolonic fistula complicating noninvasive intraductal papillary mucinous tumor of the pancreas. Gastroenterol Clin Biol. (2008) 32(1 Pt. 1):79–82. doi: 10.1016/j.gcb.2007.12.003
25. Goto N, Yoshioka M, Hayashi M, Itani T, Mimura J, Hashimoto K. Intraductal papillary-mucinous neoplasm of the pancreas penetrating to the stomach and the common bile duct. J Pancreas. (2012) 13(1):61–5. doi: 10.6092/1590-8577/599
26. Valdivia C, Bruno P, Gaia M, Saracco GM, De Angelis C. A rare case of gastric fistulization of a main-duct intraductal papillary mucinous neoplasm. Minerva Gastroenterol Dietol. (2018) 64(4):383–5. doi: 10.23736/S1121-421X.18.02486-8
27. Morotti A, Busso M, Rappa Verona P, Guerrasio A. Gastropancreatic fistula in a patient with chronic pancreatitis and IPMN. BMJ Case Rep. (2016) 2016:bcr2016215375. doi: 10.1136/bcr-2016-215375
28. Triplett D, Musleh M, Agrawal S. Intraductal papillary mucinous neoplasm of the pancreas penetrating three adjacent organs. J Pancreas. (2016) 17(3):318–21.
29. Alastal Y, Hammad T, Khalil B, Ali F, Khan MA, Nawras A. Intraductal papillary mucinous neoplasm of the pancreas presented with dual, gastric, and duodenal fish mouth sign. Am J Gastroenterol. (2016) 111:S526.
30. Patel A, Allen A, Kuwahara J, Wadsworth T, Loeffler DM, Xie KL. Intraductal papillary mucinous neoplasm complicated by a gastropancreatic fistula. Radiol Case Rep. (2019) 14(3):320–3. doi: 10.1016/j.radcr.2018.11.015
31. Khneizer G, Reddy KM, Hammami MB, AlKaade S. Intraductal papillary mucinous neoplasm complicated by pancreatoduodenal fistula decreased frequency of recurrent acute pancreatitis. Gastroenterol Res. (2018) 113:S793–4. doi: 10.14740/gr1140
32. Takahashi H, Adachi Y, Nakahara K, Kikuchi T, Mita H, Nakamura M, et al. Intraductal papillary mucinous neoplasm with pancreatogastric fistula. Int Med. (2021) 60(8):1211–5. doi: 10.2169/internalmedicine.5889-20
33. Wong AH, Cho FK, Luk SY, Wong EM. Pancreaticoduodenal fistula: a rare presentation of intraductal papillary mucinous neoplasm (IPMN) of the pancreas.
34. Basnayake O, Wijerathne P, Jayarajah U, Fernandopulle N, Sivaganesh S. Total pancreatectomy for malignant intraductal papillary mucinous neoplasm (IPMN) complicated by gastropancreatic fistulae. Case Rep Surg. (2020) 2020. doi: 10.1155/2020/8547526
35. Naseem K, Sohail A, Memon A, Shah H. S1604 A case of intraductal papillary-mucinous neoplasm of the pancreas penetrating into the stomach and colon: a rare presentation. Am J Gastroenterol. (2021) 116:S722. doi: 10.14309/01.ajg.0000779948.28281.d4
36. Mayer P, Héroin L, Sosa-Valencia L, Dautrecque F, Pessaux P, Saviano A, et al. An impossible biliary drainage? Fistulization of a degenerated intraductal papillary mucinous pancreatic neoplasm to the common bile duct. Endoscopy. (2023) 55(S01):E39–41. doi: 10.1055/a-1930-5917
Keywords: case report, IPMN, adenocarcinoma, pancreatico-gastric fistula, total pancreatectomy
Citation: AbuDalu M, Munz Y and Ohana G (2023) Pancreatico-gastric fistula arising from IPMN associated with ductal adenocarcinoma of the pancreas: a case report and a literature review. Front. Surg. 10:1171234. doi: 10.3389/fsurg.2023.1171234
Received: 21 February 2023; Accepted: 2 May 2023;
Published: 24 May 2023.
Edited by:
Satvinder Singh Mudan, The London Clinic, United KingdomReviewed by:
Gunjan Desai, Lilavati Hospital and Research Centre, India© 2023 AbuDalu, Munz and Ohana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. AbuDalu bXVzdGFmYWFAYm1jLmdvdi5pbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.