- 1Department of Esophageal and Gastroenterological Surgery, Juntendo University Graduate School of Medicine, Tokyo, Japan
- 2Department of Thoracic Surgery, The Fourth Affiliated Hospital of China Medical University, Shenyang, China
- 3Medical Technology Innovation Center, Juntendo University, Tokyo, Japan
- 4Department of Gastrointestinal Surgery, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
Background: It remains controversial whether esophageal cancer patients may benefit from esophagectomy in specialized high-volume hospitals. Here, the effect of hospital volume on overall survival (OS) of esophageal cancer patients post esophagectomy was assessed.
Methods: PubMed, Embase, and Cochrane Library were systematically searched for relevant published articles between January 1990 and May 2022. The primary outcome was OS after esophagectomy in high- vs. low-volume hospitals. Random effect models were applied for all meta-analyses. Subgroup analysis were performed based on volume grouping, sample size, study country, year of publication, follow-up or study quality. Sensitivity analyses were conducted using the leave-one-out method. The Newcastle-Ottawa Scale was used to assess the study quality. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidance, and was registered (identifier: INPLASY202270023).
Results: A total of twenty-four studies with 113,014 patients were finally included in the meta-analysis. A significant improvement in OS after esophagectomy was observed in high-volume hospitals as compared to that in their low-volume counterparts (HR: 0.77; 95% CI: 0.71–0.84, P < 0.01). Next, we conducted subgroup analysis based on volume grouping category, consistent results were found that high-volume hospitals significantly improved OS after esophagectomy than their low-volume counterparts. Subgroup analysis and sensitivity analyses further confirmed that all the results were robust.
Conclusions: Esophageal cancer should be centralized in high-volume hospitals.
1. Introduction
Centralization of demanding cancer surgeries to improve the safety and effectiveness of cancer treatment is a topic of ongoing concern in many countries around the world (1–4). Esophagectomy is one of the most complex surgery with high morbidity and mortality, and whether it should be centralized in high-volume hospitals remains controversial (5–9).
Clinical long-term outcomes of esophageal cancer after surgery are usually affected by standardization of surgical procedures, chemotherapy, radiation therapy, molecular targeted therapy and immunotherapy (10–12); moreover, hospital volume also influences mortality after esophagectomy (13). Some previous studies have been reported that esophagectomy for cancer centralized in high-volume hospitals benefited long-term prognosis outcomes (6, 7, 14, 15), whereas, there are also some reports showing inconsistent results (5, 8, 9, 16). Therefore, whether a better long-term overall survival after esophagectomy showing high-volume hospitals remains to be established.
In the present study, we evaluated the influence of high- vs. low-volume hospitals on the long-term OS of patients with esophageal cancer after esophagectomy.
2. Materials and methods
2.1. Literature search strategy
This systematic review was registered in https://doi.org/10.37766/inplasy2022.7.0023 (identifier: INPLASY202270023) (17). We conducted a systematic search for all relevant articles on the relationship between hospital volume of esophagectomies and long-term OS (17). The search was performed in PubMed, Embase, and Cochrane Library. For example, we combined Medical Subject Headings (MeSH) terms and text terms for the search in PubMed. The following search terms were used: (“esophagectomy” OR “esophageal surgery “ OR “esophageal cancer surgery” OR “esophageal resection” OR “esophageal cancer resection”) AND (“hospital volume” OR “high volume” OR “low volume” OR “healthcare institution size” OR “surgical volume”). We also searched the references of the included studies to search for potentially eligible articles. The last search was completed on May 30, 2022. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidance (PRISMA) (17, 18).
2.2. Study selection and eligibility criteria
As we previously described, after the retrieval of the relevant articles, they were screened to remove the duplicates (17). All studies were published in English. Search results were screened by two authors (Q.W. and C.D.Z.) independently according to the titles and abstracts. To better reflect modern surgical practices and perioperative management, this study focuses only on articles published after 2002. Next, the retained studies were searched for their full text and further were screened according to the following eligibility criteria: publication in English language; surgery for esophageal carcinoma as the theme; primary outcomes included hospital volume and long-term OS; comparison of OS between high- and low-volume hospitals; original articles with informative data; articles reporting adjusted hazard ratios (HRs) in multivariate analysis; publication before 2002; and articles in which procedural volume was an exact cutoff. Any disagreements were resolved through consultation with the third author (17).
2.3. Data extraction
Two authors (QW and CDZ) independently extracted data from the included studies and collated the following information: author, published year, country, study period, population, the unit of exposure (hospital volume), volume classification for hospitals, volume grouping (dichotomies, tertiles, quartiles, quintiles or others) and the longest follow-up and clinical outcomes (OS) (17). Any disagreements were resolved by discussion with the third author. We further assessed the extent of risk adjustment (17).
2.4. Study quality evaluation
All included studies were rigorously assessed for methodological quality and risk of bias by two authors (QW and CDZ) by using the Newcastle-Ottawa Scale (17, 19). This scale assesses the quality of studies from three aspects: selection of study population (0–4 points), comparability between groups (0–2 points), and outcome measurement (0–3 points) (17). The total score is 9 points.
2.5. Data integration
High-volume hospitals or low-volume hospitals were defined by the authors of the included studies. We used hazard ratios (HRs) in low-volume groups as the reference. If an included study reported more than two surgical volume groups, only the lowest and highest volume groups were compared in the analysis. The primary outcome was OS at the last follow-up, excluding 30-day mortality, 90-day mortality, in-hospital mortality, and postoperative mortality (17).
2.6. Statistical analyses
The results were calculated by HRs with 95% confidence intervals (CIs) for long-term outcomes. Heterogeneity among the studies was quantified by the I2 test, and studies with a statistic of 25%–50% of I2 were regarded as low heterogeneous, 51%–75% as moderate, and more than 75% as highly heterogeneous (20). Regarding the clinical heterogeneity (inconsistency in pathological staging, therapeutic regimens, and other confounding factors among the studies), we applied random-effect models for all the analyses. To obtain adequate statistical power, subgroup analysis was conducted based on volume grouping category. Then meta-analyses of at least five included studies were performed for different cutoff values (high-volume hospital vs. low-volume hospital). In addition, subgroup analyses in relation to volume group, sample size, study country, year of publication, follow-up or study quality and sensitivity analyses of a leave-one-out method were conducted to verify the results. Funnel plots were used to evaluate potential publication bias. P < 0.05 was considered to be statistically significant. All statistical analyses were performed by Review Manager 5.4.1 and Stata 13.1.
3. Results
3.1. Study selection and characteristics
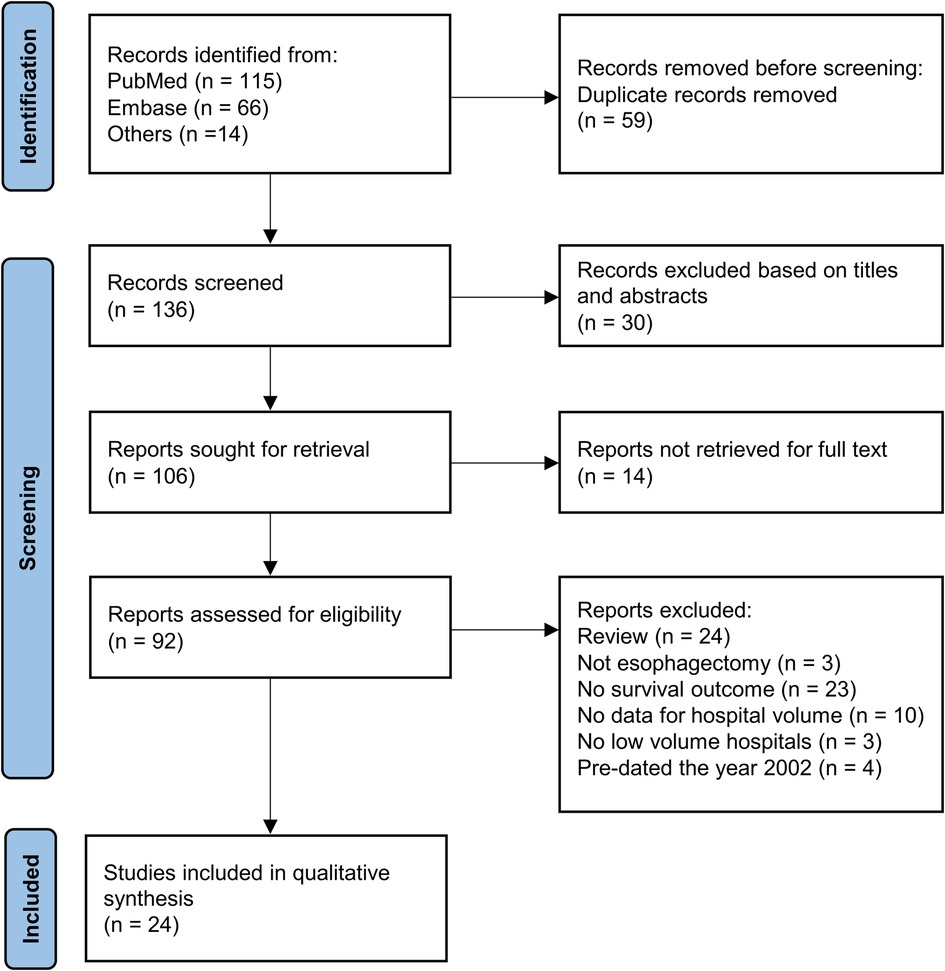
This systematic review was registered in https://doi.org/10.37766/inplasy2022.7.0023 (identifier: INPLASY202270023). Figure 1 shows the process of literature selection. We retrieved 115 articles from PubMed and 66 from Embase; of these, 136 studies were retained for primary selection after 59 duplicate studies were excluded. After screening of titles and abstracts, 30 studies were excluded. Among the remaining 106 articles, which were related to the volume-outcome relationship in esophageal cancer surgery, we further excluded 24 reviews without primary data, three articles not related to esophagectomy, 23 articles without data of long-term survival, 10 articles without data of hospital volume, three articles without data of low-volume hospitals, four articles published before 2002. Finally, 24 studies published from 2002 to May 2022 with 113,014 participants were included in the meta-analysis.
Among the 24 included studies, six were from the United States (6–8, 21–23), four from Sweden (9, 15, 24, 25), three each from Australia (26–28) and Netherlands (29–31), two each from Japan (32, 33) and England (14, 34), and one each from China (35), Korea (36), Brazil (37), and Canada (38) (Table 1). The longest follow-up period was 24 years.
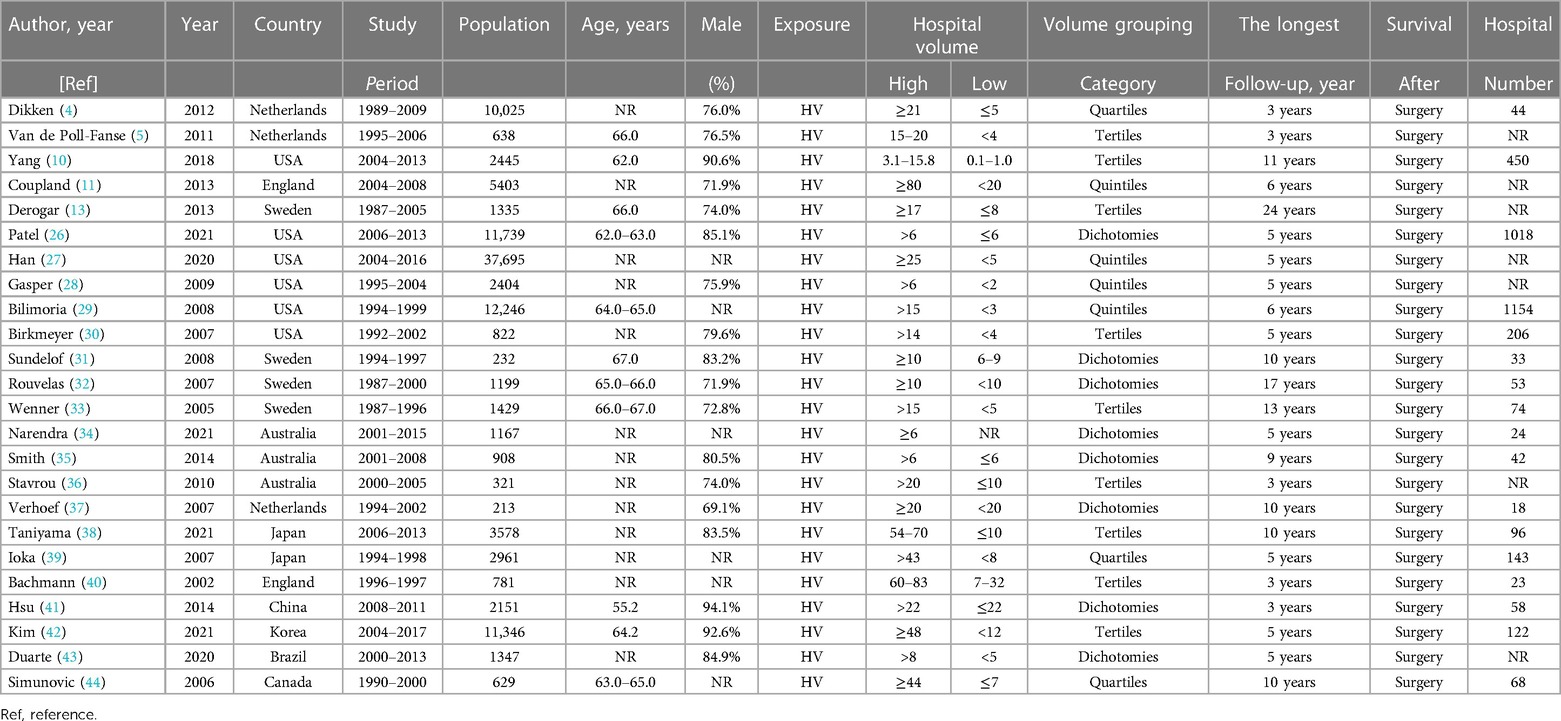
Table 1. Basic characteristics of all included studies for meta-analysis on the relation between hospital volume and outcome of esophagectomies for cancer.
3.2. Quality assessment
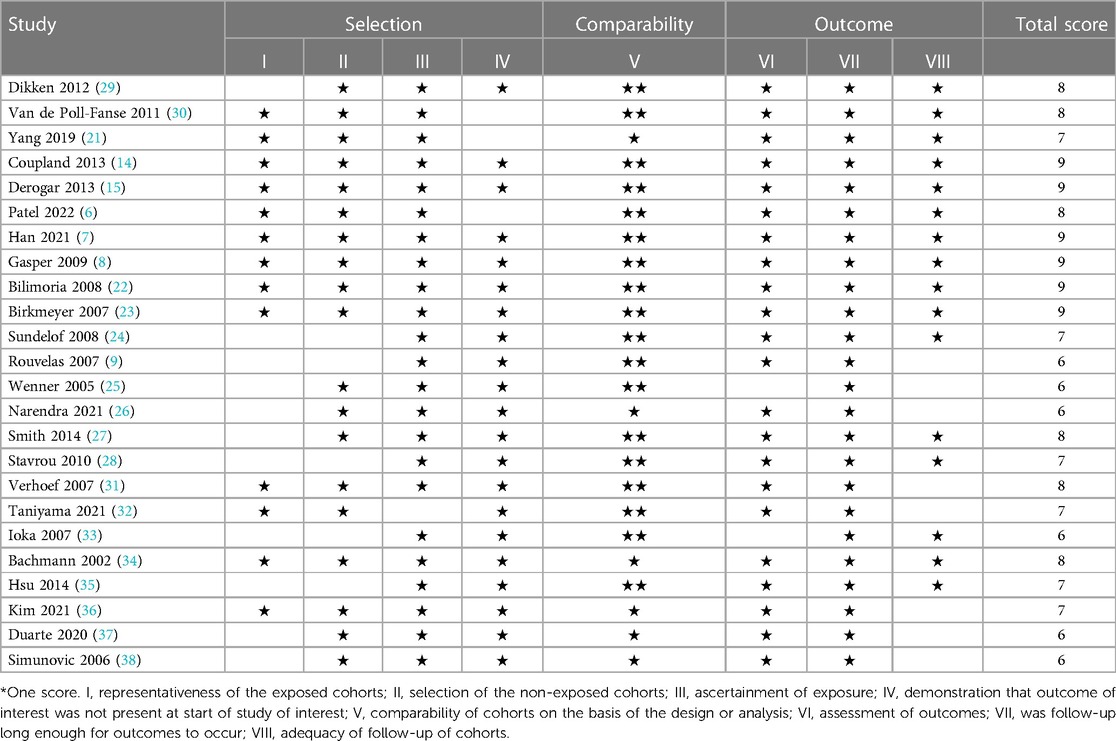
The quality of the included studies was assessed using the Newcastle-Ottawa Scale. The median Newcastle-Ottawa Scale score of the included studies was 7, with a range of 6–9 (Table 2).
3.3. Long-term os in relation to hospital volume
A total of 24 studies was included to assess the impact of high-volume vs. low-volume hospitals on long-term overall survival after esophagectomy. Regarding to the longest period of follow-ups, high-volume hospitals showed significantly better overall survival than low-volume hospitals (HR: 0.77; 95% CI: 0.71–0.84, P < 0.01) (Figure 2).
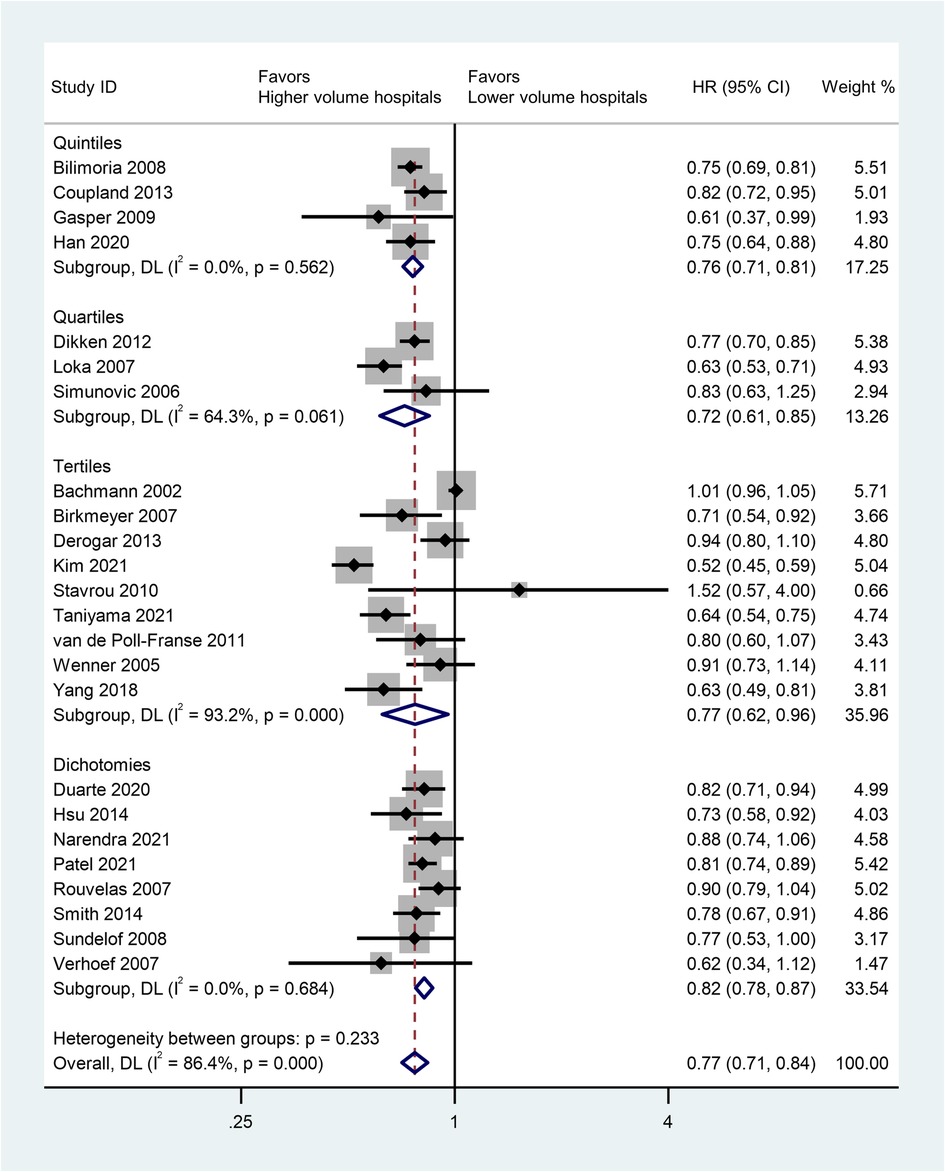
Figure 2. Forest plot of long-term survivals following esophagectomy comparing high- with low-volume hospitals (reference) according to volume grouping.
Next, we analyzed the pooled HRs of OS (high-volume hospital vs. low-volume hospital) for multiple cutoff values (Table 3). Consistent results were found that high-volume hospitals showed a significant improvement in OS after esophagectomy than their low-volume counterparts (all P ≤ 0.05).
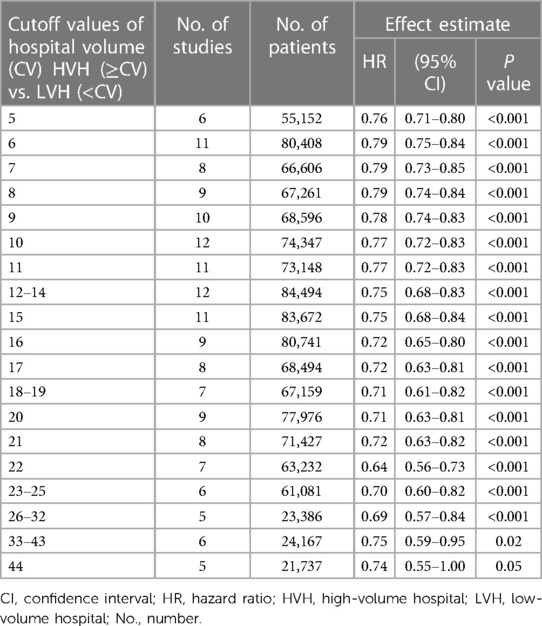
Table 3. Comparisons of the overall survivals between high- and low-volume hospitals by different cutoff values of hospital volume.
3.4. Subgroup analysis
Subgroup analysis was conducted based on volume grouping category in Figure 2. A significant improvement in OS after esophagectomy was observed in high-volume hospitals as compared to that in their low-volume counterparts in each volume grouping category. The pooled HRs were 0.76 (95% CI: 0.71–0.81) for quintiles, 0.72 (95% CI: 0.61–0.85) for quartiles, 0.77 (95% CI:0.62–0.96) for tertiles, and 0.82 (95% CI:0.78–0.87) for dichotomies, respectively (Figure 2, Table 4).
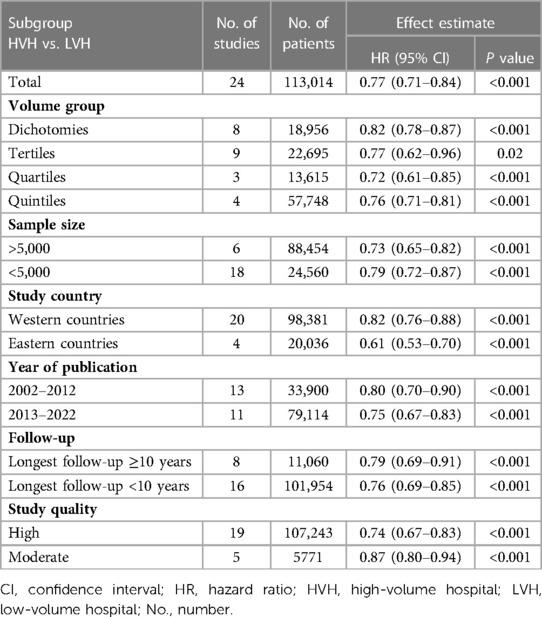
Table 4. Subgroup analyses of comparisons of the overall survivals between high- and low-volume hospitals.
In addition, we carried out subgroup analyses in relation to sample size, study country, year of publication, follow-up or study quality. Overall, the results were robust and that patients with esophagectomy significantly benefited from high-volume hospitals than from low-volume hospitals (Table 3).
3.5. Sensitivity analyses
Sensitivity analyses with the leave-one-out method further revealed the consistent results, which were observed a significant improvement in OS after esophagectomy in high-volume hospitals as compared to that in their low-volume counterparts, with HRs ranging from 0.75 (95% CI: 0.68–0.83) to 0.79 (95% CI: 0.73–0.85) (Table 5).
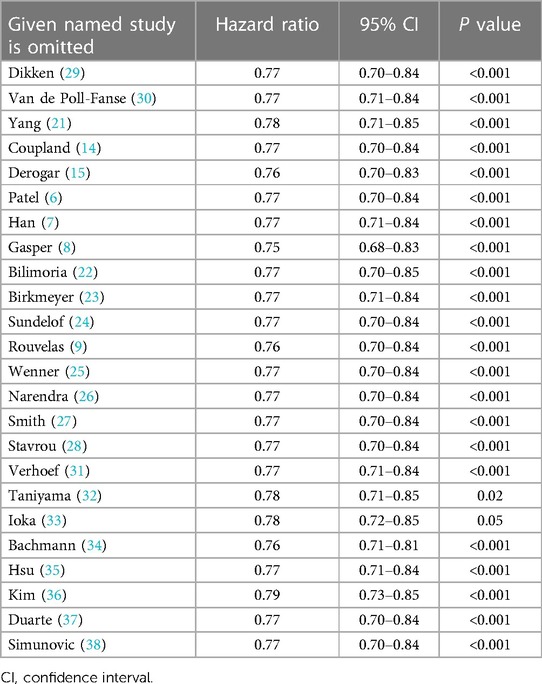
Table 5. Sensitivity analysis using leave-one-out method for overall survival of high-volume hospitals vs. low-volume hospitals.
3.6. Publication bias
We further assessed the publication bias (Figure 3). Because of the relatively small number of included studies in some volume grouping category meta-analyses, we consider that publication bias should exist.
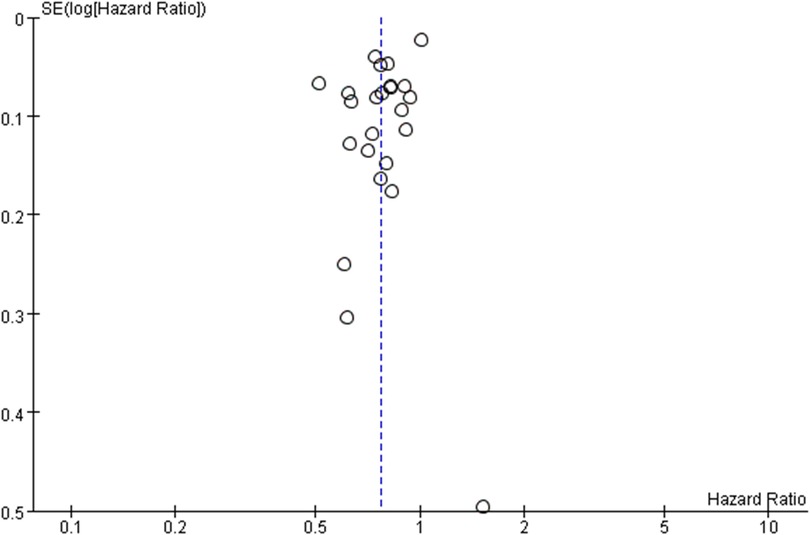
Figure 3. Funnel plot of survival benefit following esophagectomy comparing high- with low-volume hospital (reference).
4. Discussion
This meta-analysis outlined the most up-to-date evidence on the relationship between hospital volume and long-term survival outcomes in esophagectomy. We found for the first time that centralization of esophagectomy in high-volume hospitals improved OS as compared to that in low-volume hospitals and patients with esophageal cancer will benefit from an esophagectomy conducted in a higher volume hospital than in a lower one, whether in total or in volume grouping category. However, we were still unable to decide the optimal cutoff value of dividing high- and low-volume hospitals in current study.
Centralization of esophageal cancer surgery has been common in the Netherlands, England, and Canada (18, 39, 40), Comparing a centralized country (England) with a non-centralized country (U.S.), a previous study of 13,291 patients illustrated a lower in-hospital mortality in England hospitals than those in the U.S. (4.2% vs. 5.5%) (41). Regarding this, centralization is urgently required, in terms of high-volume hospitals with sufficient surgical volumes, skillful interdisciplinary teams, to provide the optimal treatment for patients with esophageal cancer.
Although the reasons why high-volume hospitals are associated with better long-term survival are still not fully understood, high-volume hospitals may provide patients with better multidisciplinary teams, more comprehensive preoperative examinations, more accurate preoperative diagnosis, perioperative management, and high-quality surgical care, more specialized surgeons who have more consistent skills of performing curable operations for esophageal cancer patients (42–45). Compared with low-volume hospitals, high-volume hospitals not only have a lower complication rate after esophagectomies, but also the ability of managing complications (46). In addition, the applications of neoadjuvant chemoradiation, perioperative chemotherapy, and postoperatively follow-up can improve long-term outcomes after esophagectomies; therefore, high-volume hospitals are more likely to provide a better overall cancer therapy and care, and the size of hospital volume may serve as a significant indicator of the overall medical quality and health care (47).
Unfortunately, it is difficult for patients to know the overall quality of nearby hospitals. Based on the main findings of current study, patients can select relatively higher volume hospitals nearby. Considering the importance of such knowledges, policy makers should make efforts to educate people for selecting the optimal hospitals for the treatments of specific diseases (e.g., esophagectomy for esophageal cancer), through public reporting systems.
Our study still has limitations. First, this study has the potential for selection bias of individual studies because of the original data, even with case mix adjustment. Second, all the included studies were observational and retrospective. Third, some of the included studies used the same database (e.g., Sweden), and some participants might be overlapped, even though the study period were different; however, sensitivity analyses of a leave-one-out method confirmed that all the current results were robust. Fourth, as some of the data in the included studies were obtained from the National Cancer Registry, some details of the surgery, such as surgical approach and the extent of lymph nodes dissection, were unknown. Fifth, the volume grouping categories of the annual hospital volumes across the included studies varied greatly, and there was still no optimal threshold, and the main findings of current study thus need to be verified in further studies.
5. Conclusion
In summary, high-volume hospitals significantly improved long-term OS of patients with esophageal cancer after esophagectomy as compared to their low-volume counterparts. Esophagectomy should be centralized in high-volume hospitals.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: QW, CDZ. Methodology: QW, SN. Software: QW. Validation: QW, MN, TF, SN, CDZ, SM. Formal analysis: QW, CDZ. Investigation: QW, MN, TF, SN, CDZ, SM. Resources: QW, CDZ. Data curation: QW, CDZ. Writing—original draft preparation: QW. Writing—review and editing: MN, SM. Visualization: QW. Supervision: SM. Reading and approving the final manuscript: QW, MN, TF, SN, CDZ, SM. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the members of the Department of Esophageal and Gastroenterological Surgery, Juntendo University Graduate School of Medicine, for their thoughtful discussion and helpful advice. The authors also thank all participants and study authors in this study. QW was partly supported by the Japan China Sasakawa Medical Fellowship. CDZ was partly supported by the China Scholarship Council (201908050148).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CI, confidence interval; HR, hazard ratio; HV, hospital volume; HVH, high-volume hospital; LVH, low-volume hospital; No., number; NR, not reported; ref, reference; USA, United States of America.
References
1. Sheetz KH, Dimick JB, Nathan H. Centralization of high-risk cancer surgery within existing hospital systems. J Clin Oncol. (2019) 37(34):3234–42. doi: 10.1200/JCO.18.02035
2. Balzano G, Guarneri G, Pecorelli N, Paiella S, Rancoita PMV, Bassi C, et al. Modelling centralization of pancreatic surgery in a nationwide analysis. Br J Surg. (2020) 107(11):1510–9. doi: 10.1002/bjs.11716
3. van Putten M, Nelen SD, Lemmens VEPP, Stoot JHMB, Hartgrink HH, Gisbertz SS, et al. Overall survival before and after centralization of gastric cancer surgery in The Netherlands. Br J Surg. (2018) 105(13):1807–15. doi: 10.1002/bjs.10931
4. Asplund J, Mattsson F, Plecka-Ostlund M, Markar SR, Lagergren J. Annual surgeon and hospital volume of gastrectomy and gastric adenocarcinoma survival in a population-based cohort study. Acta Oncol. (2022) 61(4):425–32. doi: 10.1080/0284186X.2022.2025612
5. Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. (2013) 63(4):232–48. doi: 10.3322/caac.21185
6. Patel DC, Jeffrey Yang CF, He H, Liou DZ, Backhus LM, Lui NS, et al. Influence of facility volume on long-term survival of patients undergoing esophagectomy for esophageal cancer. J Thorac Cardiovasc Surg. (2022) 163(4):1536–1546 e1533. doi: 10.1016/j.jtcvs.2021.05.048
7. Han S, Kolb JM, Hosokawa P, Friedman C, Fox C, Scott FI, et al. The volume-outcome effect calls for centralization of care in esophageal adenocarcinoma: results from a large national cancer registry. Am J Gastroenterol. (2021) 116(4):811–5. doi: 10.14309/ajg.0000000000001046
8. Gasper WJ, Glidden DV, Jin C, Way LW, Patti MG. Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference?: a follow-up analysis of another decade. Ann Surg. (2009) 250(3):472–83. doi: 10.1097/SLA.0b013e3181b47c79
9. Rouvelas I, Lindblad M, Zeng W, Viklund P, Ye W, Lagergren J. Impact of hospital volume on long-term survival after esophageal cancer surgery. Arch Surg. (2007) 142(2):113–7; discussion 118. doi: 10.1001/archsurg.142.2.113
10. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. (2021) 156(8):721–9. doi: 10.1001/jamasurg.2021.2373
11. Yu S, Zhang W, Ni W, Xiao Z, Wang Q, Zhou Z, et al. A propensity-score matching analysis comparing long-term survival of surgery alone and postoperative treatment for patients in node positive or stage III esophageal squamous cell carcinoma after R0 esophagectomy. Radiother Oncol. (2019) 140:159–66. doi: 10.1016/j.radonc.2019.06.020
12. He S, Xu J, Liu X, Zhen Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm Sin B. (2021) 11(11):3379–92. doi: 10.1016/j.apsb.2021.03.008
13. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. (2017) 390(10110):2383–96. doi: 10.1016/S0140-6736(17)31462-9
14. Coupland VH, Lagergren J, Luchtenborg M, Jack RH, Allum W, Holmberg L, et al. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004-2008. Gut. (2013) 62(7):961–6. doi: 10.1136/gutjnl-2012-303008
15. Derogar M, Sadr-Azodi O, Johar A, Lagergren P, Lagergren J. Hospital and surgeon volume in relation to survival after esophageal cancer surgery in a population-based study. J Clin Oncol. (2013) 31(5):551–7. doi: 10.1200/JCO.2012.46.1517
16. Gillison E. W., Powell J., McConkey C. C., Spychal R. T. Surgical workload and outcome after resection for carcinoma of the oesophagus and cardia. Br J Surg. (2002) 89(3):344–8. doi: 10.1046/j.0007-1323.2001.02015.x
17. Wang Q, Nasu M, Fukunaga T, Nojiri S, Zhang C-D, Mine S. Association of hospital volume and long-term survival after esophagectomy: a systematic review and meta-analysis. INPLASY. (2022). doi: 10.37766/inplasy2022.7.0023
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
20. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
21. Yang GQ, Mhaskar R, Rishi A, Naghavi AO, Frakes JM, Almhanna K, et al. Intensity-modulated radiotherapy at high-volume centers improves survival in patients with esophageal adenocarcinoma receiving trimodality therapy. Dis Esophagus. (2019) 32(8):doy124. doi: 10.1093/dote/doy124
22. Bilimoria KY, Bentrem DJ, Feinglass JM, Stewart AK, Winchester DP, Talamonti MS, et al. Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol. (2008) 26(28):4626–33. doi: 10.1200/JCO.2007.15.6356
23. Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. (2007) 245(5):777–83. doi: 10.1097/01.sla.0000252402.33814.dd
24. Sundelof M, Lagergren J, Ye W. Surgical factors influencing outcomes in patients resected for cancer of the esophagus or gastric cardia. World J Surg. (2008) 32(11):2357–65. doi: 10.1007/s00268-008-9698-2
25. Wenner J, Zilling T, Bladström A, Alvegård T. The influence of surgical volume on hospital mortality and 5-year survival for carcinoma of the oesophagus and gastric cardia. Anticancer Res. (2005) 25(1B):419–24.15816605
26. Narendra A, Baade PD, Aitken JF, Fawcett J, Leggett B, Leggett C, et al. Hospital characteristics associated with better “quality of surgery” and survival following oesophagogastric cancer surgery in Queensland: a population-level study. ANZ J Surg. (2021) 91(3):323–8. doi: 10.1111/ans.16397
27. Smith RC, Creighton N, Lord RV, Merrett ND, Keogh GW, Liauw WS, et al. Survival, mortality and morbidity outcomes after oesophagogastric cancer surgery in New South Wales, 2001-2008. Med J Aust. (2014) 200(7):408–13. doi: 10.5694/mja13.11182
28. Stavrou EP, Smith GS, Baker DF. Surgical outcomes associated with oesophagectomy in New South Wales: an investigation of hospital volume. J Gastrointest Surg. (2010) 14(6):951–7. doi: 10.1007/s11605-010-1198-7
29. Dikken JL, Dassen AE, Lemmens VE, Putter H, Krijnen P, van der Geest L, et al. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in The Netherlands between 1989 and 2009. Eur J Cancer. (2012) 48(7):1004–13. doi: 10.1016/j.ejca.2012.02.064
30. van de Poll-Franse LV, Lemmens VE, Roukema JA, Coebergh JW, Nieuwenhuijzen GA. Impact of concentration of oesophageal and gastric cardia cancer surgery on long-term population-based survival. Br J Surg. (2011) 98(7):956–63. doi: 10.1002/bjs.7493
31. Verhoef C, van de Weyer R, Schaapveld M, Bastiaannet E, Plukker JT. Better survival in patients with esophageal cancer after surgical treatment in university hospitals: a plea for performance by surgical oncologists. Ann Surg Oncol. (2007) 14(5):1678–87. doi: 10.1245/s10434-006-9333-0
32. Taniyama Y, Tabuchi T, Ohno Y, Morishima T, Okawa S, Koyama S, et al. Hospital surgical volume and 3-year mortality in severe prognosis cancers: a population-based study using cancer registry data. J Epidemiol. (2021) 31(1):52–8. doi: 10.2188/jea.JE20190242
33. Ioka A, Tsukuma H, Ajiki W, Oshima A. Hospital procedure volume and survival of cancer patients in Osaka, Japan: a population-based study with latest cases. Jpn J Clin Oncol. (2007) 37(7):544–53. doi: 10.1093/jjco/hym052
34. Bachmann M, Alderson D, Edwards D, Wotton S, Bedford C, Peters T, et al. Cohort study in south and west England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg. (2002) 89(7):914–22. doi: 10.1046/j.1365-2168.2002.02135.x
35. Hsu PK, Chen HS, Wu SC, Wang BY, Liu CY, Shih CH, et al. Impact of hospital volume on long-term survival after resection for oesophageal cancer: a population-based study in taiwandagger. Eur J Cardiothorac Surg. (2014) 46(6):e127–135; discussion e135. doi: 10.1093/ejcts/ezu377
36. Kim BR, Jang EJ, Jo J, Lee H, Jang DY, Ryu HG. The association between hospital case-volume and postoperative outcomes after esophageal cancer surgery: a population-based retrospective cohort study. Thorac Cancer. (2021) 12(18):2487–93. doi: 10.1111/1759-7714.14096
37. Duarte MBO, Pereira EB, Lopes LR, Andreollo NA, Carvalheira JBC. Chemoradiotherapy with or without surgery for esophageal squamous cancer according to hospital volume. JCO Glob Oncol. (2020) 6:828–36. doi: 10.1200/JGO.19.00360
38. Simunovic M, Rempel E, Thériault M, Coates A, Whelan T, Holowaty E, et al. Influence of hospital characteristics on operative death and survival of patients after major cancer surgery in Ontario. Can J Surg. (2006) 49(4):251–8.16948883
39. Varghese TK Jr., Wood DE, Farjah F, Oelschlager BK, Symons RG, MacLeod KE, et al. Variation in esophagectomy outcomes in hospitals meeting leapfrog volume outcome standards. Ann Thorac Surg. (2011) 91(4):1003–9; discussion 1009–1010. doi: 10.1016/j.athoracsur.2010.11.006
40. Chang AC. Centralizing esophagectomy to improve outcomes and enhance clinical research: invited expert review. Ann Thorac Surg. (2018) 106(3):916–23. doi: 10.1016/j.athoracsur.2018.04.004
41. Munasinghe A, Markar SR, Mamidanna R, Darzi AW, Faiz OD, Hanna GB, et al. Is it time to centralize high-risk cancer care in the United States? Comparison of outcomes of esophagectomy between England and the United States. Ann Surg. (2015) 262(1):79–85. doi: 10.1097/SLA.0000000000000805
42. Van den Broeck T, Oprea-Lager D, Moris L, Kailavasan M, Briers E, Cornford P, et al. A systematic review of the impact of surgeon and hospital caseload volume on oncological and nononcological outcomes after radical prostatectomy for nonmetastatic prostate cancer. Eur Urol. (2021) 80(5):531–45. doi: 10.1016/j.eururo.2021.04.028
43. Khanna A, Saarela O, Lawson K, Finelli A, Haber GP, Lee B, et al. Hospital quality metrics for radical cystectomy: disease specific and correlated to mortality outcomes. J Urol. (2019) 202(3):490–7. doi: 10.1097/JU.0000000000000282
44. Wright JD, Chen L, Hou JY, Burke WM, Tergas AI, Ananth CV, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. (2017) 130(3):545–53. doi: 10.1097/AOG.0000000000002164
45. Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. (2009) 250(6):1029–34. doi: 10.1097/sla.0b013e3181bef697
46. Ghaferi A, Birkmeyer J, Dimick J. Hospital volume and failure to rescue with high-risk surgery. Med Care. (2011) 49(12):1076–81. doi: 10.1097/MLR.0b013e3182329b97
Keywords: esophageal carcinoma, esophagectomy, hospital volume, overall survival, centralization
Citation: Wang Q, Mine S, Nasu M, Fukunaga T, Nojiri S and Zhang C-D (2023) Association of hospital volume and long-term survival after esophagectomy: A systematic review and meta-analysis. Front. Surg. 10:1161938. doi: 10.3389/fsurg.2023.1161938
Received: 13 February 2023; Accepted: 27 March 2023;
Published: 21 April 2023.
Edited by:
Atalel Fentahun Awedew, Addis Ababa University, EthiopiaReviewed by:
François Dépret, Assistance Publique Hopitaux De Paris, FranceYasuhiro Tsubosa, Shizuoka Cancer Center, Japan
© 2023 Wang, Mine, Nasu, Fukunaga, Nojiri and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shinji Mine cy5taW5lLmd2QGp1bnRlbmRvLmFjLmpw
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Qing Wang1,2
Qing Wang1,2 Shinji Mine
Shinji Mine Shuko Nojiri
Shuko Nojiri