
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 11 April 2023
Sec. Visceral Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1160149
Purpose: The purpose of this study is to assess the safety and clinical outcomes of transcatheter arterial embolization (TAE) via the cystic artery for treating patients with bleeding from the cystic artery.
Materials and Methods: This retrospective study included 20 patients who underwent TAE via the cystic artery between January 2010 and May 2022. Radiological images and clinical data were reviewed to evaluate causes of bleeding, procedure-related complications, and clinical outcomes. Technical success was defined as the disappearance of contrast media extravasation or pseudoaneurysm, as demonstrated on completion angiography. Clinical success was defined as discharge from the hospital without any bleeding-related issues.
Results: Hemorrhagic cholecystitis (n = 10) was the most common cause of bleeding, followed by iatrogenic (n = 4), duodenal ulcer (n = 3), tumor (n = 2), and trauma (n = 1). Technical success was achieved in all cases, and clinical success was achieved in 70% (n = 14) of patients. Three patients developed ischemic cholecystitis as a complication. Six patients with clinical failure died within 45 days after embolization.
Conclusion: TAE through the cystic artery has a high technical success rate in treating cystic artery bleeding, but clinical failure remains a common occurrence due to concurrent medical conditions and the development of ischemic cholecystitis.
Transcatheter arterial embolization (TAE) is a fundamental procedure within the realm of interventional radiology. Several studies involving patients with gastrointestinal bleeding, hemoptysis, post-partum bleeding, and musculoskeletal bleeding have reported favorable outcomes with respect to the safety and effectiveness of TAE (1–5). Bleeding originating from the cystic artery may be associated with surgical cholecystectomy, hemorrhagic cholecystitis, gallbladder cancer, and prior percutaneous drainage of the gallbladder (6–9). Several case studies have documented the successful use of TAE in treating cystic artery bleeding (6–9), and embolization procedures through the cystic artery have been safely attempted for the management of hepatocellular carcinoma (10–12).
Nonetheless, there is insufficient literature available on the use of TAE via the cystic artery to treat patients who suffer from bleeding originating from this vessel. The purpose of this study was to report on the safety and clinical outcomes of TAE through the cystic artery for the treatment of patients with cystic artery bleeding.
This retrospective study was approved by the institutional review board and the requirement for informed consent was waived. Between January 2010 and May 2022, 20 patients with bleeding from the cystic artery were treated with TAE via the cystic artery at two tertiary medical centers. The study population comprised 12 males and eight females [mean age, 64.2 ± 14.6 years (range, 34–85 years)].
The procedure for embolization via the cystic artery was conducted using a similar method to the previously described techniques (1–5). Access to the celiac trunk through the common femoral artery was achieved using a 5-Fr angiographic catheter. Digital subtraction angiography was performed using a 5-Fr catheter, followed by insertion of a 1.7–2.0 Fr microcatheter into the cystic artery. The embolic material used varied depending on the operator's decision.
A retrospective review of radiologic images and clinical data was conducted to identify causes of bleeding, procedure-related complications, and clinical outcomes. Technical success was defined as disappearance of contrast media extravasation or pseudoaneurysm demonstrated on completion angiography. Cessation of bleeding was defined as stabilization of hemoglobin level within 48 h of the embolization procedure, without the need for transfusion. Clinical success was defined as discharge from the hospital without any bleeding-related issues. Patients who met one of the following criteria were diagnosed with coagulopathy; prothrombin ratio > 1.5, partial thromboplastin time > 45 s, or platelet count of less than 80,000/㎕.
20 patients (male, 12; female, 8) presented with hemoperitoneum (n = 4), hematemesis (n = 4), melena (n = 4), bloody fluid from percutaneous cholecystostomy tube (n = 4), abdominal pain (n = 2), hematochezia (n = 1), and jaundice (n = 1) (Table 1). The causes of bleeding from the cystic artery were attributed to hemorrhagic cholecystitis (n = 10), iatrogenic (n = 4), duodenal ulcer (n = 3), tumor (n = 2), and trauma (n = 1). The iatrogenic factors contributing to bleeding were percutaneous cholecystostomy (n = 2), surgical cholecystectomy (n = 1), and radiofrequency ablation (n = 1). Nine patients had coagulopathy and six patients had liver cirrhosis. Three patients had underlying cancers [hepatocellular carcinoma (n = 1), gallbladder cancer (n = 1), and gastrointestinal stromal tumor (n = 1)].

Table 1. Summary of clinical and radiological findings who underwent cystic artery bleeding embolization.
Eleven patients presented to the hospital due to bleeding of the cystic artery, while nine patients were hospitalized for management of other medical problems. Six out of the nine hospitalized patients were admitted to the intensive care unit due to massive bleeding (n = 3), pneumonia (n = 1), heart failure (n = 1), and sepsis (n = 1) which were worsened by the development of cystic artery bleeding.
Embolization vessels were classified as the main cystic artery (n = 7) (Figure 1), deep cystic artery (n = 6), superficial cystic artery (n = 2) (Figure 2), and small bleeding twig (n = 5). Embolic materials used were n-butyl cyanoacrylate (NBCA) (n = 16), gelatin sponge particles (n = 2), coil (n = 1), and autologous blood clot (n = 1).
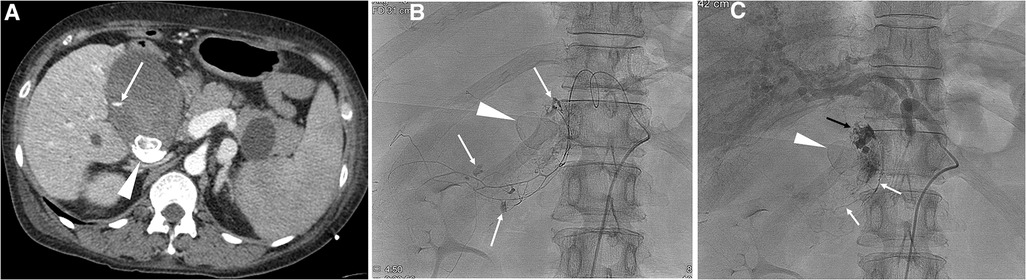
Figure 1. A 49-year-old female with liver cirrhosis and hemorrhagic cholecystitis. (A) CT scan shows extravasation of contrast media (arrow) in the gallbladder. Note gall stones (arrowhead). (B) Selective angiogram of the cystic artery shows multifocal extravasation (arrows) of contrast media. Note gall stones (arrowhead). A mixture of n-butyl cyanoacrylate and Lipiodol was used for embolization of the main cystic artery. (C) The final angiogram shows cessation of active bleeding. Note glue material in the cystic artery (white arrows) and in the lumen of the gallbladder (black arrow), and gall stones (arrowhead). Development of ischemic cholecystitis occurred after embolization of the cystic artery, resulting in sepsis and multiorgan failure. The patient died three days after cystic artery embolization.

Figure 2. 2 A 75-year-old male with hematochezia. (A) CT scan shows a pseudoaneurysm (arrow) located within the gallbladder. Note the presence of a blood clot with high attenuation in the stomach (S), duodenum (D), and gallbladder (arrowhead). (B) Right hepatic angiogram shows a pseudoaneurysm (arrow) from the superficial cystic artery. A mixture of n-butyl cyanoacrylate and Lipiodol was used for embolization of the bleeding point. (C) The final angiogram after embolization of the cystic artery shows a subtraction artifact of the pseudoaneurysm (white arrow) and superficial cystic artery (black arrow). Note intact perfusion of the deep cystic artery. The impacted gall stone was thought to induce development of a fistula between the gallbladder and duodenum, resulting in duodenal ulcer and hematochezia. The patient recovered without development of ischemic cholecystitis and was discharged two weeks after cystic artery embolization.
Technical success was achieved in all 20 (100%) patients. Cessation of bleeding was observed in 18 (90%) patients, and clinical success was achieved in 14 (70%) patients.
Median length of follow-up was 259 days (range, 2–3,669 days). Three patients who underwent embolization of the main cystic artery developed ischemic cholecystitis. Two patients with ischemic cholecystitis died due to sepsis and multiorgan failure resulting from ischemic cholecystitis. Four patients required subsequent treatment after cystic artery embolization, including one case of percutaneous cholecystostomy, and three cases of surgical cholecystectomy.
Six patients with clinical failure died in the hospital 3–45 days after embolization of the cystic artery. The causes of bleeding in these six patients were hemorrhagic cholecystitis (n = 5) and duodenal ulcer (n = 1). The causes of death were sepsis with multiorgan failure (n = 5) and pneumonia (n = 1). For these six patients, embolization vessels were classified as main cystic artery (n = 3), deep cystic artery (n = 2), and small bleeding twig (n = 1), and embolic materials used were NBCA (n = 5) and gelatin sponge particles (n = 1). Among the 11 patients who presented to the hospital due to bleeding of the cystic artery, clinical failure occurred in only one (9%) patient, while among the nine patients hospitalized for other medical problems, clinical failure occurred in five (56%) patients.
In this study, hemorrhagic cholecystitis (50%, 10 out of 20) was the most common cause of cystic artery bleeding. Hemorrhagic cholecystitis is a progression of cholecystitis, which ranges from simple to complicated cholecystitis, hemorrhagic cholecystitis, gangrenous cholecystitis, and gallbladder perforation (13). Surgical cholecystectomy remains the gold standard option for treatment of hemorrhagic cholecystitis (14), but some patients may not suitable candidate due to poor general condition and anesthesia risks. In these cases, embolization of the cystic artery may be considered as an alternative procedure with a high rate of technical success.
In this study, the technical success rate was 100% and cessation of bleeding was achieved in 90% of patients. Liquid embolic material has the advantage of being able to cast the bleeding point itself, which is not affected by a patient's coagulopathy. However, there is a potential risk of ischemic cholecystitis when casting the entire cystic artery. Temporary embolic materials such as gelatin sponge particle or autologous blood can be used to reduce this risk, but there may be a high re-bleeding rate. Based on the findings of this small retrospective study, it is suggested that main cystic artery embolization with liquid embolic material may be associated with clinical failure despite technical success. When embolization of the main cystic artery is unavoidable on angiographic findings, embolization with temporary embolic material or surgical cholecystectomy should be considered as alternative options.
Despite the high technical success rate, the clinical success rate, defined as discharge from the hospital without bleeding-related problems, was only 70%. This result may be explained by two points. First, clinical failure occurred in five out of nine patients who were hospitalized for other medical problems, as the development of hemorrhagic cholecystitis in seriously ill patients may not be salvaged by TAE through the cystic artery. Second, embolization of the main cystic artery may be inevitable in cases of multifocal bleeding or extravasation/pseudoaneurysm involving the main cystic artery, and this can lead to ischemic cholecystitis and subsequent deterioration of the patient's general condition, potentially causing sepsis and multiorgan failure.
Duodenal ulceration with bleeding from cystic artery is an uncommon but possible condition, which has been reported in several case reports (15–17). An anterior or postbulbar ulcer can cause a massive gastrointestinal bleeding by ulceration into cystic artery (17), in case of adhesion between gallbladder and duodenum with chronic inflammation or ulceration. Duodenal ulcer was the cause of bleeding in three patients in this study. After endoscopic hemostasis failed in all three patients, TAE was performed and bleeding from cystic artery was confirmed by angiography.
Ischemic cholecystitis developed in three patients who underwent main cystic artery embolization. Considering their poor general condition, the referring physicians chose conservative management rather than surgical cholecystectomy, which resulted in two cases of mortality. Hence, surgical cholecystectomy should be considered as a life-saving procedure when ischemic cholecystitis occurs after TAE.
This study has several limitations. First, the study population was relatively small due to the uncommon nature of the disease entity, and the long study period. Thus, statistical analysis could not be performed to identify risk factors for clinical failure. Second, the use of liquid embolic material, which may have a high technical success rate and a relatively low clinical success rate, may deter some practitioners from using TAE through the cystic artery. However, the authors believe that use of liquid embolic material may be appropriate when superselective embolization is feasible. Third, in some cases, evaluation of ischemic cholecystitis after TAE through the cystic artery may be inaccurate or impossible, as many patients already had serious underlying disease. Fourth, the retrospective nature of the study leaves room for selection bias, especially in two tertiary hospitals known for their high patient volumes, where embolization procedures may have been overused due to their quick implementation.
In conclusion, TAE through the cystic artery has a high technical success rate in treating cystic artery bleeding, but clinical failure remains a common occurrence due to concurrent medical conditions and the development of ischemic cholecystitis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Borad, Yonsei University Health System. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Guarantor of integrity of the entire study: GMK. Study concepts and design: H-CK. Literature research: H-CK, YJ, KH, GK. Clinical studies: H-CK, YJ, KH, GK. Data analysis: H-CK, GK. Manuscript preparation: H-CK. Manuscript editing: GK. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hur S, Jae HJ, Lee M, Kim HC, Chung JW. Safety and efficacy of transcatheter arterial embolization for lower gastrointestinal bleeding: a single-center experience with 112 patients. J Vasc Interv Radiol. (2014) 25(1):10–9. doi: 10.1016/j.jvir.2013.09.012
2. Hur S, Jae HJ, Lee H, Lee M, Kim HC, Chung JW. Superselective embolization for arterial upper gastrointestinal bleeding using N-butyl cyanoacrylate: a single-center experience in 152 patients. J Vasc Interv Radiol. (2017) 28(12):1673–80. doi: 10.1016/j.jvir.2017.07.027
3. Lee Y, Lee M, Hur S, Kim HC, Jae HJ, Chung JW, et al. Bronchial and non-bronchial systemic artery embolization with transradial access in patients with hemoptysis. Diagn Interv Radiol. (2022) 28(4):359–63. doi: 10.5152/dir.2022.201100
4. Wi JY, Kim HC, Chung JW, Jun JK, Jae HJ, Park JH. Importance of angiographic visualization of round ligament arteries in women evaluated for intractable vaginal bleeding after uterine artery embolization. J Vasc Interv Radiol. (2009) 20(8):1031–5. doi: 10.1016/j.jvir.2009.05.003
5. Yoo DH, Jae HJ, Kim HC, Chung JW, Park JH. Transcatheter arterial embolization of intramuscular active hemorrhage with N-butyl cyanoacrylate. Cardiovasc Intervent Radiol. (2012) 35(2):292–8. doi: 10.1007/s00270-011-0162-6
6. Rossini M, Bonati E, Cozzani F, Marcato C, Del Rio P. Hemobilia due to cystic artery pseudoaneurysm following cholecystectomy: diagnosis and management, a case report. Acta Biomed. (2019) 90(4):595–8. doi: 10.23750/abm.v90i4.7809
7. Maddineni S MMDL, McCabe S, Rozenblit G. Transcatheter embolization of a cystic artery pseudoaneurysm in a cirrhotic patient with perforated acute cholecystitis. Indian J Radiol Imaging. (2017) 27(4):521–3. doi: 10.4103/ijri.IJRI_358_16
8. Mahalingam S, Shaikh OH, Kumbhar US, Mohan A. Cystic artery pseudoaneurysm due to carcinoma of the gallbladder. BMJ Case Rep. (2021) 14(6):e241714. doi: 10.1136/bcr-2021-241714
9. Sada DM, Metwalli ZA. Cystic artery hemorrhage after cholecystostomy catheter exchange treated with transcatheter embolization. Semin Intervent Radiol. (2019) 36(2):108–10. doi: 10.1055/s-0039-1688429
10. Kang B, Kim HC, Chung JW, Hur S, Joo SM, Jae HJ, et al. Safety of chemotherapeutic infusion or chemoembolization for hepatocellular carcinoma supplied exclusively by the cystic artery. Cardiovasc Intervent Radiol. (2013) 36(5):1313–9. doi: 10.1007/s00270-012-0542-6
11. Chu HH, Kim HC, Chung JW, Lee JH, Yu SJ, Hur S, et al. Repeated intra-arterial therapy via the cystic artery for hepatocellular carcinoma. Cardiovasc Intervent Radiol. (2014) 37(5):1283–91. doi: 10.1007/s00270-013-0795-8
12. Kim HC, Miyayama S, Choi JW, Kim GM, Chung JW. Hepatocellular carcinoma supplied by the inferior phrenic artery or cystic artery: anatomic and technical considerations. Radiographics. (2023) 43(1):e220076. doi: 10.1148/rg.220076
13. Ng ZQ, Pradhan S, Cheah K, Wijesuriya R. Haemorrhagic cholecystitis: a rare entity not to be forgotten. BMJ Case Rep. (2018) 2018:bcr2018226469. doi: 10.1136/bcr-2018-226469
14. Parekh J, Corvera CU. Hemorrhagic cholecystitis. Arch Surg. (2010) 145(2):202–4. doi: 10.1001/archsurg.2009.265
15. Cooper SG, Morse SS, Strauss EB. Peptic erosion of the cystic artery with massive duodenal hemorrhage: therapeutic embolization. Cardiovasc Intervent Radiol. (1988) 11(5):278–80. doi: 10.1007/BF02577035
16. Ford GA, Simpson AH, Gear MW, Wilkinson SP. Duodenal ulceration into the cystic artery. Postgrad Med J. (1990) 66(772):144–6. doi: 10.1136/pgmj.66.772.144
Keywords: cystic artery, embolization, n-butyl cyanoacrylate, hemorrhagic cholecystitis, hemorrhage
Citation: Kim H-C, Jeong YS, Han K and Kim GM (2023) Transcatheter arterial embolization of cystic artery bleeding. Front. Surg. 10:1160149. doi: 10.3389/fsurg.2023.1160149
Received: 13 February 2023; Accepted: 23 March 2023;
Published: 11 April 2023.
Edited by:
Peter C. Ambe Universität Witten/Herdecke, GermanyReviewed by:
Stefania Cimbanassi, University of Milan, Italy© 2023 Kim, Jeong, Han and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gyoung Min Kim a2ltZ21AeW9uc2VpLmFjLmty
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.