
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg., 30 March 2023
Sec. Surgical Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1133335
 Yunxiang Zhou1,2,3
Yunxiang Zhou1,2,3 Linping Dong4,5
Linping Dong4,5 Linyun Dai6
Linyun Dai6 Sien Hu4
Sien Hu4 Yongji Sun4
Yongji Sun4 Yulian Wu2,3,4,5
Yulian Wu2,3,4,5 Tao Pan1,2,3*
Tao Pan1,2,3* Xiawei Li2,3,4*
Xiawei Li2,3,4*
Background: Hepatoid adenocarcinoma of the stomach (HAS) is a highly malignant subtype of gastric carcinoma with specific clinicopathological features and extremely poor prognosis. We present an exceedingly rare case of complete response after chemo-immunotherapy.
Case Description: A 48-year-old woman with highly elevated serum alpha-fetoprotein (AFP) level was found to have HAS verified by pathological examination based on gastroscopy. Computed tomography scan was done and TNM staging of the tumor was T4aN3aMx. Programmed cell death ligand-1 (PD-L1) immunohistochemistry was performed, revealing a negative PD-L1 expression. Chemo-immunotherapy including oxaliplatin plus S-1 and PD-1 inhibitor terelizumab was given to this patient for 2 months until the serum AFP level decreased from 748.5 to 12.9 ng/mL and the tumor shrank. D2 radical gastrectomy was then performed and histopathology of the resected specimen revealed that the cancerous cells had disappeared. Pathologic complete response (pCR) was achieved and no evidence of recurrence has been found after 1 year of follow-up.
Conclusions: We, for the first time, reported an HAS patient with negative PD-L1 expression who achieved pCR from the combined chemotherapy and immunotherapy. Although no consensus has been reached regarding the therapy, it might provide a potential effective management strategy for HAS patient.
As a rare malignant neoplasm with high aggressiveness and poor prognosis, hepatoid adenocarcinoma of the stomach (HAS) accounts for 1.6%–4.3% of all gastric cancers (1) and 63% of hepatoid adenocarcinoma manifesting outside the liver (2). Due to the lack of typical early clinical symptoms, the majority of HAS patients are diagnosed at an advanced stage of the disease, with lymphatic permeation, blood vessel, and regional lymph node metastasis (3). Similar to gastric cancer, HAS is usually diagnosed histologically after endoscopic biopsy and staged using CT, endoscopic ultrasound, PET, and laparoscopy (4). Pathology is the only golden standard for diagnosing HAS, which has similar tissue morphology comparable to hepatocellular carcinoma (HCC) and frequently expresses alpha-fetoprotein (AFP) on immunohistochemistry (5–7).
Given the limited understanding of the low-incident disease, no consensus has been reached regarding the therapy of HAS so far. Although surgical resection in combination with chemotherapy is recognized as the optimal treatment for this lethal malignancy, the long-term survival remains unsatisfactory owing to the late detection, early recurrence, and aggressive biological manner of the tumor (8). In recent years, immunotherapy with immune checkpoint inhibitors (ICIs) has shown beneficial effects and good safety for various solid cancers including non-small cell lung cancer (9), gallbladder carcinosarcoma (10), bladder urothelial carcinoma (11), colorectal adenocarcinoma (12), etc. For gastric cancer, immunotherapy has also drastically improved the treatment options in the advanced phase, especially for chemorefractory gastric cancer (4, 13). However, research on immunotherapy applied to HAS has barely been reported. In this study, we, for the first time, reported an HAS patient who achieved pathologic complete response (pCR) from the combined chemotherapy and immunotherapy with negative programmed cell death ligand-1 (PD-L1) expression.
As shown in the clinical time line of Figure 1, a 48-year-old woman was admitted to the local hospital due to highly elevated serum AFP level during a yearly routine physical examination. She underwent upper endoscopy and was found to have HAS in the cardia verified by pathological examination (Figure 2A). With no notable family history and no history of smoking or drinking, she did not complain of any obvious symptoms. After that, she was then referred to our hospital for further treatment. The results of blood routine, biochemical tests, and coagulation tests were within the normal ranges. Contrast-enhanced CT (CE-CT) scan showed that the wall of the gastric fundus and cardia was thickened and enhanced, and multiple lymph nodes were found with enlargement. The tumor was thus diagnosed as T4aN3aMx and Borrmann type III (Figure 3A). Immunohistochemistry (IHC) showed c-erbB-2(GC) 1+, c-ervB-2(GC)-Positive Control 3+, MSH2+, MSH6+, MLH1+, PMS2+, SALL4+, AFP+, GPC3+, Syn−, CgA−, CD56−, and Ki-67 80%+. Genome sequencing showed microsatellite stable (MSS) in tumor tissues. PD-L1 expression level was also tested using the PD-L1 IHC 22C3 pharmDx. A specimen with combined positive score (CPS) ≥1 is considered to have PD-L1 expression but the patient's tumor had a CPS of <1, thus revealing a negative PD-L1 expression.
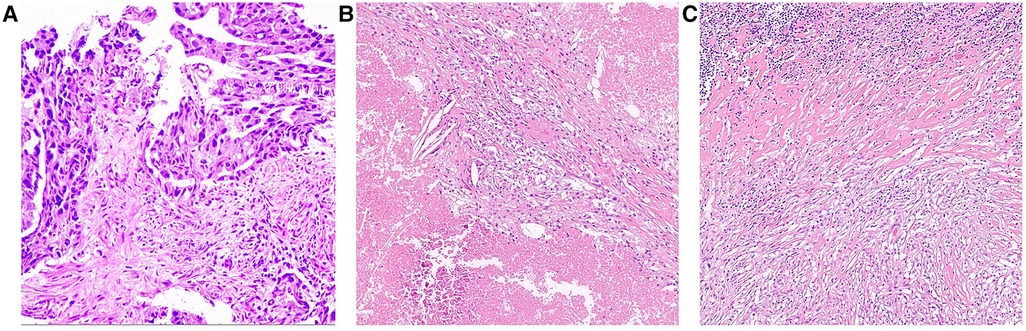
Figure 2. Pathological findings (A) Endoscopic biopsy before chemo-immunotherapy at local hospital (Hematoxylin-eosin staining, magnification ×10); (B) Tumor area and (C) lymph node after 3 courses of chemo-immunotherapy (Hematoxylin-eosin staining, magnification ×10).
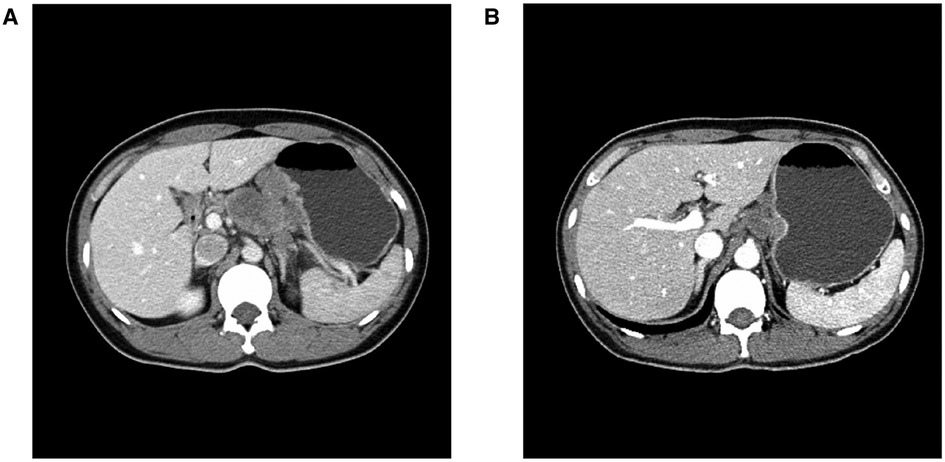
Figure 3. Abdominal CT imaging (A) Before chemo-immunotherapy; (B) After 3 courses of chemo-immunotherapy.
However, the high efficiency and low toxicity of ICIs have been confirmed by previous clinical studies (Table 1) and with respect to the patient's wishes, a multidisciplinary decision was made to trial a neoadjuvant therapy regimen including oxaliplatin plus S-1 (SOX) and PD-1 inhibitor terelizumab every 3 weeks (S-1, 100 mg/day, orally on days 1–14; oxaliplatin, 130 mg/m2, intravenously on day 1; terelizumab, 200 mg, intravenously on day 1; the patient had a weight of 45 kg and a height of 156 cm). After three courses of the combined therapy, the serum AFP level decreased from 748.5 to 12.9 ng/mL (Figure 1) and the tumor shrunk (Figure 3B). D2 radical gastrectomy was then performed and no residual tumor cells were found in resected tissue; most of the cells appeared to degenerate and die (Figures 2B,C). Hence, the disease might have been considered as a partial response (PR), but the postoperative pathology revealed that a pCR had been achieved. As for the adverse effects during the treatment, the patient presented some common symptoms like fatigue, loss of appetite, and nausea, which however could be tolerated well by adopting symptomatic therapies. To date, the patient in this study has been followed up for more than a year after the radical surgery. AFP level has been around 2.5–2.8 ng/mL during the past 10 months and no evidence of recurrence has been found by gastroenteroscopy or CE-CT.
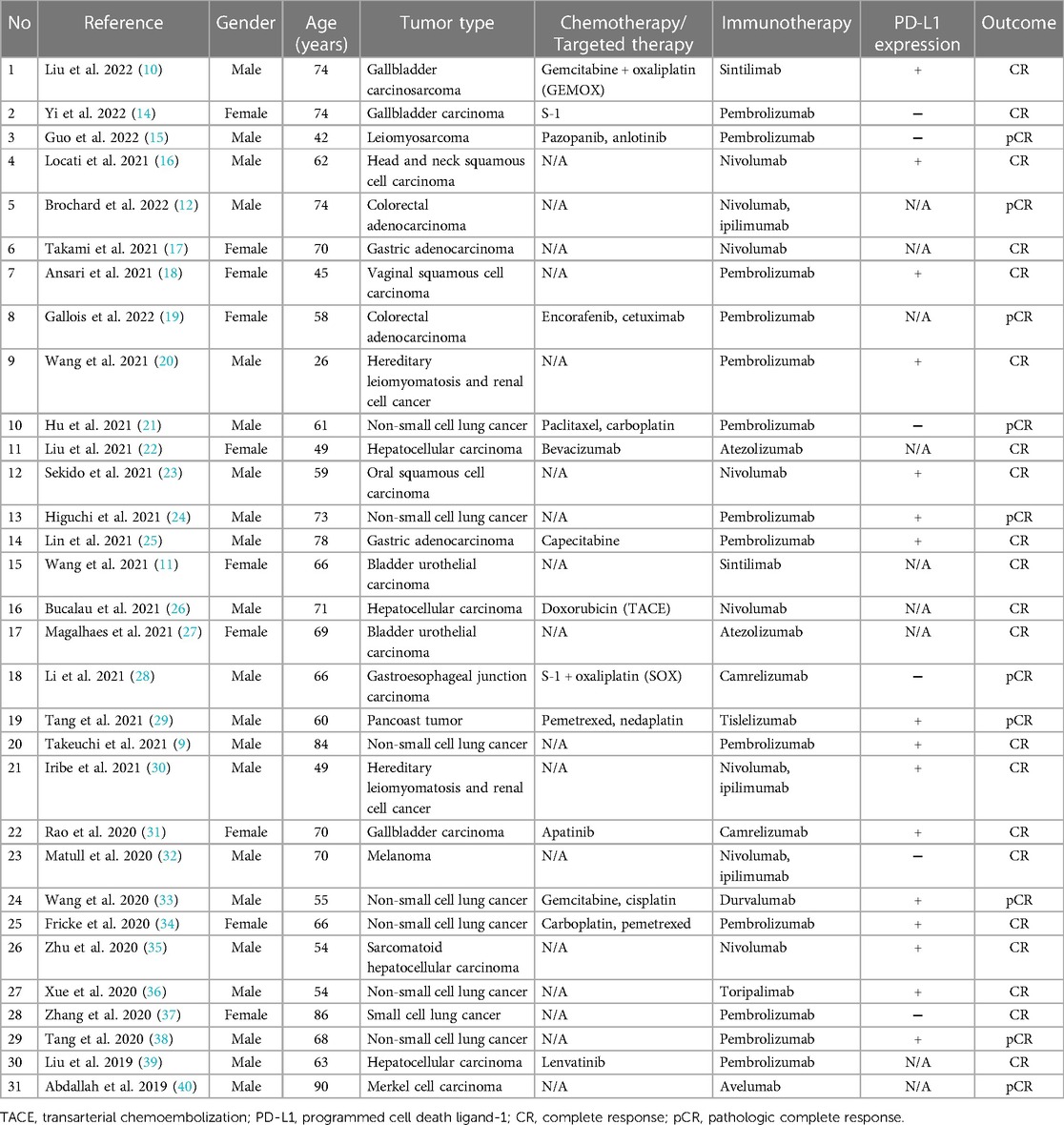
Table 1. The cases of complete response after immunotherapy in combination with/without chemotherapy/targeted therapy.
Ever since first identified as “AFP-producing gastric cancer” in 1970s (41), HAS has still been underrecognized due to its rarity in clinical practice (42). Although various cases and retrospective reports of small sample size regarding HAS have been reported over the years (3, 43–48), it has not been sufficiently understood, resulting in a high mortality and terrible prognosis. Herein, we presented a rare case of HAS that was successfully treated with combined SOX and terelizumab. To the best of our knowledge, this is the first report of pCR in HAS from combined chemotherapy and anti-PD-1 therapy.
Previous reports have discovered that the high-frequency gene alternations in HAS tumor samples including TP53, RPTOR, CD3EAP, CEBPA, WISP3, and MARK1, among which TP53 was the most frequent one. Apart from gene mutation, HAS is also a malignancy with a remarkable augment of copy number gains (CNGs) (3). In particular, patients with CNGs situated in 20q11.21–13.12 of a chromosome with a trend of increasing serum concentration of AFP have been reported to have worse prognosis (44). Here in this case, although the patient refused to perform further additional molecular analyses, it was speculated that she might be lucky enough to avoid such CNG due to the less aggressive bio-behavior of the tumor.
Given the limited randomized controlled trials (RCTs) regarding the HAS, no consensus on the ideal treatment approach for the disease has been reached. Based on previous published literature, most of the cases received radical surgery with adjuvant chemotherapy (1, 8, 49–53). Despite no recommended standard regimen of chemotherapy, adjuvant chemotherapy has been confirmed as one of the independent factors of long-term survival (7, 54). It has been reported that SOX (49) or FOLFOX (52) might be potentially favorable protocols for HAS. In addition to systemic chemotherapy, interventional therapy with relatively less toxic side effects could be used when a liver metastasis exists and targeted therapy might be an alternative when resistance to chemotherapy occurs (55).
In recent years, anti-PD-1/PD-L1 immunotherapy has attracted great attention and revolutionized the treatment landscape of various solid tumors. The general underlying mechanism of cancer immunotherapy is to stimulate an immunologic reaction to inhibit the tumor immune escape from the immunologic surveillance system (37). However, it has barely been reported in HAS, let alone pCR cases that benefited from immunotherapy. In this study, it has been suggested that neoadjuvant immunotherapy plus chemotherapy followed by surgery might offer an advantageous or alternative method for treating HAS. Further evaluation of ICIs and their combination treatments in the perioperative setting might be beneficial for patients with locally resectable disease (28).
Although immunotherapy is right now in the center of the spotlight of anticancer battlefield, there are still a number of patients who unfortunately failed to benefit from it (56). Usually upregulated in various tumors, PD-L1 expression is the most frequently used biomarker for predicting the potential response to ICIs (57). In this case, what was surprising was that the patient presented with MSS and negative PD-L1 expression who was supposed to be relatively insensitive to immunotherapy and found that she still had an excellent response to anti-PD-1 antibody combined with chemotherapy, indicating that other mechanisms than MSI and PD-L1 positive might account for the responsiveness to ICIs (28). As shown in Table 1, this phenomenon has also been reported in gallbladder carcinoma (14), leiomyosarcoma (15), non-small cell lung cancer (21), gastroesophageal junction carcinoma (28), melanoma (32), and small cell lung cancer (37). Previous studies demonstrated that chemotherapy might be able to enhance anticancer immunity by reactivating immune effector cells, stimulating tumor antigen presentation, and eliminating immune suppressor cells, thus resulting in a synergistic anticancer effect compared with the anti-PD-1 monotherapy (58, 59). Recently, a meta-analysis has also found that the early death rate upon immune checkpoint inhibitors across solid malignancies was not predictable by PD-L1 expression (60). This is an area that requires further exploration in future.
However, given the single-case retrospective nature of this study, the major limitation might be the insufficient evidence to support the benefit of the combined treatment. The understanding of the biological behavior of HAS is still limited due to its rare incidence. Moreover, the mechanisms of the current anti-PD1 therapeutic strategies have not been fully elucidated. Nonetheless, this case report sheds some new light on the treatment of HAS. It is anticipated that both clinical and basic research will continue to advance with the accumulation of future HAS cases.
To conclude, this study presented a rare case of HAS with negative PD-L1 expression which, however, achieved pCR following chemotherapy (SOX) in combination with immunotherapy (terelizumab), providing a novel perspective on the potential treatment strategies for this aggressive malignancy. Further studies are highly warranted to explore the underlying mechanisms of the combined chemo-immunotherapy and improve our understanding regarding the management of HAS.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Zhejiang University School of medicine. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YZ designed the work. LDo and LDa participated in the patients' care during their hospital course. XL, SH, and YS helped collect the data. YW guided the administration of the project and revised the manuscript. TP helped with the figures and approved the final version of the manuscript. XL drafted the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China under grants No. 82272954 (YW).
We thank for the support from the participant and her family.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nakao S, Nakata B, Tendo M, Kuroda K, Hori T, Inaba M, et al. Salvage surgery after chemotherapy with S-1 plus cisplatin for α-fetoprotein-producing gastric cancer with a portal vein tumor thrombus: a case report. BMC Surg. (2015) 15(1):1–4. doi: 10.1186/1471-2482-15-5
2. Yoshizawa J, Ishizone S, Ikeyama M, Nakayama J. Gastric hepatoid adenocarcinoma resulting in a spontaneous gastric perforation: a case report and review of the literature. BMC Cancer. (2017) 17(1):1–7. doi: 10.1186/s12885-017-3357-7
3. Xia R, Zhou Y, Wang Y, Yuan J, Ma X. Hepatoid adenocarcinoma of the stomach: current perspectives and new developments. Front Oncol. (2021) 11:633916. doi: 10.3389/fonc.2021.633916
4. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
5. Ishikura H, Kishimoto T, Andachi H, Kakuta Y, Yoshiki T. Gastrointestinal hepatoid adenocarcinoma: venous permeation and mimicry of hepatocellular carcinoma, a report of four cases. Histopathology. (1997) 31(1):47–54. doi: 10.1046/j.1365-2559.1997.5740812.x
6. Zhou K, Wang A, Ao S, Chen J, Ji K, He Q, et al. The prognosis of hepatoid adenocarcinoma of the stomach: a propensity score-based analysis. BMC Cancer. (2020) 20(1):1–10. doi: 10.1186/s12885-020-07031-9
7. Zhang JF, Shi SS, Shao YF, Zhang HZ. Clinicopathological and prognostic features of hepatoid adenocarcinoma of the stomach. Chin Med J. (2011) 124(10):1470–6. doi: 10.3760/cma.j.issn.0366-6999.2011.10.006
8. Zeng XY, Yin YP, Xiao H, Zhang P, He J, Liu WZ, et al. Clinicopathological characteristics and prognosis of hepatoid adenocarcinoma of the stomach: evaluation of a pooled case series. Curr Med Sci. (2018) 38(6):1054–61. doi: 10.1007/s11596-018-1983-1
9. Takeuchi E, Okamoto Y, Takahashi N, Morizumi S, Toyoda Y, Kuroda N, et al. Complete response of squamous cell carcinoma of the lung following treatment with pembrolizumab in an elderly patient: a case report. Thorac Cancer. (2021) 12(2):259–63. doi: 10.1111/1759-7714.13733
10. Liu QQ, Lin HM, Han HW, Yang CN, Liu C, Zhang R. Complete response to combined chemotherapy and anti-PD-1 therapy for recurrent gallbladder carcinosarcoma: a case report and literature review. Front Oncol. (2022) 12:803454. doi: 10.3389/fonc.2022.803454
11. Wang J, Li Q, Lv H, Nie C, Chen B, Xu W, et al. A PD-1 inhibitor induces complete response of advanced bladder urothelial carcinoma: a case report. Front Oncol. (2021) 11:671416. doi: 10.3389/fonc.2021.671416
12. Brochard C, Chicaud M, Colle R, Parc Y, Svrcek M. Pathological complete response of a metastatic MisMatch repair deficient/MicroSatellite instable colon cancer after immunotherapy: a case report. Ann Pathol. (2022) 42(2):172–6. doi: 10.1016/j.annpat.2021.12.008
13. Rihawi K, Ricci AD, Rizzo A, Brocchi S, Marasco G, Pastore LV, et al. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: novel translational implications. Int J Mol Sci. (2021) 22(8):3805. doi: 10.3390/ijms22083805
14. Yi B, Zhao Z, Dong H, Yuan L, Wu Y, Xu Y, et al. Case report: durable complete response after combined immunotherapy following resection of primary tumor in a gallbladder cancer patient with distant metastatic lymph nodes of favorable immune-microenvironment. Front Immunol. (2022) 13:820566. doi: 10.3389/fimmu.2022.820566
15. Guo X, Li S, Tong H, Zhang Y, Ji Y, Zhuang R, et al. Case report: complete response to antiangiogenesis and immune checkpoint blockade in an unresectable MMR-deficient leiomyosarcoma harboring biallelic loss of PTEN. Front Oncol. (2022) 12:802074. doi: 10.3389/fonc.2022.802074
16. Locati LD, Serafini MS, Carenzo A, Canevari S, Perrone F, Orlandi E, et al. Complete response to nivolumab in recurrent/metastatic HPV-positive head and neck squamous cell carcinoma patient after progressive multifocal leukoencephalopathy: a case report. Front Oncol. (2021) 11:799453. doi: 10.3389/fonc.2021.799453
17. Takami T, Yasuda K, Uozumi N, Musiake Y, Shintani H, Kataoka N, et al. Confirmed complete response to nivolumab for advanced gastric cancer with peritoneal dissemination: a case report. J Med Case Rep. (2021) 15(1):604. doi: 10.1186/s13256-021-03200-x
18. Ansari J, Eltigani Mohmmed Y, Ghazal-Aswad S, Ansari H, Akhter SMJ, Hassoun Hadid O, et al. Rare case of chemotherapy-refractory metastatic vaginal squamous cell carcinoma with complete response to concurrent pembrolizumab and radiotherapy—case report and literature review. Gynecol Oncol Rep. (2021) 38:100878. doi: 10.1016/j.gore.2021.100878
19. Gallois C, Taieb J, Sabouret A, Broudin C, Karoui M, Garinet S, et al. Upfront progression under pembrolizumab followed by a complete response after encorafenib and cetuximab treatment in BRAF V600E-mutated and microsatellite unstable metastatic colorectal cancer patient: a case report. Genes Chromosom Cancer. (2022) 61(2):114–8. doi: 10.1002/gcc.23012
20. Wang T, Huang Y, Huang X, Lv Z, Tian S, Ma X, et al. Complete response of hereditary leiomyomatosis and renal cell cancer (HLRCC)-associated renal cell carcinoma to pembrolizumab immunotherapy: a case report. Front Oncol. (2021) 11:735077. doi: 10.3389/fonc.2021.735077
21. Hu C, Ma Q, Li N, Luo N, Hao S, Jiang M, et al. Case report: pathological complete response in a brain-metastatic lung squamous cell carcinoma patient with long-term benefit from chemo-immunotherapy. Front Oncol. (2021) 11:693704. doi: 10.3389/fonc.2021.693704
22. Liu G, Zhou W, Li X, Guo L, He T, Zhao J, et al. Case report: complete response of primary massive hepatocellular carcinoma to anti-programmed death ligand-1 antibody following progression on anti-programmed death-1 antibody. Front Immunol. (2021) 12:712351. doi: 10.3389/fimmu.2021.712351
23. Sekido K, Imaue S, Tomihara K, Tachinami H, Yamagishi K, Okazawa S, et al. Durable complete response to immunotherapy with anti-PD-1 antibody nivolumab in a patient with oral squamous cell carcinoma presenting with lung metastasis: a case report. Clin Case Rep. (2021) 9(9):e04545. doi: 10.1002/ccr3.4545
24. Higuchi M, Kawamata T, Oshibe I, Soeta N, Saito T, Hojo H, et al. Pathological complete response after immune-checkpoint inhibitor followed by salvage surgery for clinical stage IV pulmonary adenocarcinoma with continuous low neutrophil-to-lymphocyte ratio: a case report. Case Rep Oncol. (2021) 14(2):1124–33. doi: 10.1159/000515509
25. Lin CY, Mehta P, Waters KM, Chang E, Hendifar A, Osipov A, et al. Complete response to neoadjuvant pembrolizumab and capecitabine in microsatellite stable, Epstein-Barr virus-positive, locally advanced gastric adenocarcinoma: case report. AME Case Rep. (2021) 5:30. doi: 10.21037/acr-21-11
26. Bucalau AM, Tancredi I, Pezzullo M, Covas A, Verset G. Complete response of a hepatocellular carcinoma with complex macrovascular invasion after combined treatment with chemoembolization and immunotherapy: a case report. Acta Gastroenterol Belg. (2021) 84(2):371–4. doi: 10.51821/84.2.371
27. Magalhães DST, Magalhães HM, Mesquita ASA. Long lasting complete response with immunotherapy in a metastatic bladder carcinoma: a case report. Porto Biomed J. (2021) 6(1):e127. doi: 10.1097/j.pbj.0000000000000127
28. Li X, Huang Q, Lei Y, Zheng X, Dai S, Leng W, et al. Locally advanced gastroesophageal junction cancer with pathological complete response to neoadjuvant therapy: a case report and literature review. Ann Transl Med. (2021) 9(6):513. doi: 10.21037/atm-21-434
29. Tang WF, Xu W, Huang WZ, Lin GN, Zeng YM, Lin JS, et al. Pathologic complete response after neoadjuvant tislelizumab and chemotherapy for pancoast tumor: a case report. Thorac Cancer. (2021) 12(8):1256–9. doi: 10.1111/1759-7714.13910
30. Iribe Y, Furuya M, Shibata Y, Yasui M, Funahashi M, Ota J, et al. Complete response of hereditary leiomyomatosis and renal cell cancer (HLRCC)-associated renal cell carcinoma to nivolumab and ipilimumab combination immunotherapy by: a case report. Fam Cancer. (2021) 20(1):75–80. doi: 10.1007/s10689-020-00195-0
31. Rao J, Xia J, Yang W, Wu C, Sha B, Zheng Q, et al. Complete response to immunotherapy combined with an antiangiogenic agent in multiple hepatic metastases after radical surgery for advanced gallbladder cancer: a case report. Ann Transl Med. (2020) 8(23):1609. doi: 10.21037/atm-20-4420
32. Matull J, Livingstone E, Wetter A, Zimmer L, Zaremba A, Lahner H, et al. Durable complete response in a melanoma patient with unknown primary, associated with sequential and severe multi-organ toxicity after a single dose of CTLA-4 plus PD-1 blockade: a case report. Front Oncol. (2020) 10:592609. doi: 10.3389/fonc.2020.592609
33. Wang Y, Yang X, Tian X, Jia Z, Bing Z, Cao L, et al. Neoadjuvant immunotherapy plus chemotherapy achieved pathologic complete response in stage IIIB lung adenocarcinoma harbored EGFR G779F: a case report. Ann Palliat Med. (2020) 9(6):4339–45. doi: 10.21037/apm-20-1692
34. Fricke J, Mambetsariev I, Pharaon R, Subbiah S, Rajurkar S, Salgia R. Hyperprogression on immunotherapy with complete response to chemotherapy in a NSCLC patient with high PD-L1 and STK11: a case report. Medicine. (2020) 99(46):e22323. doi: 10.1097/MD.0000000000022323
35. Zhu SG, Li HB, Yuan ZN, Liu W, Yang Q, Cheng Y, et al. Achievement of complete response to nivolumab in a patient with advanced sarcomatoid hepatocellular carcinoma: a case report. World J Gastrointest Oncol. (2020) 12(10):1209–15. doi: 10.4251/wjgo.v12.i10.1209
36. Xue W, Zhang T, Wang X, Duan J, Bi N. Complete response induced by anti-PD-1-based immunotherapy with toripalimab in a patient with locally advanced lung adenocarcinoma who failed rapidly after concurrent chemoradiotherapy: a case report. J Clin Pharm Ther. (2020) 45(6):1511–4. doi: 10.1111/jcpt.13248
37. Zhang N, Zhu J, Lv H. Complete response to pembrolizumab in a patient with extensive-stage small-cell lung cancer: a case report. Ann Palliat Med. (2020) 9(4):2347–52. doi: 10.21037/apm-19-590
38. Tang Y, Li Y, Zhang L, Tong G, Ou Z, Wang Z, et al. Pathologic complete response to preoperative immunotherapy in a lung adenocarcinoma patient with bone metastasis: a case report. Thorac Cancer. (2020) 11(4):1094–8. doi: 10.1111/1759-7714.13361
39. Liu Z, Li X, He X, Xu Y, Wang X. Complete response to the combination of lenvatinib and pembrolizumab in an advanced hepatocellular carcinoma patient: a case report. BMC Cancer. (2019) 19(1):1062. doi: 10.1186/s12885-019-6287-8
40. Abdallah N, Nagasaka M, Chowdhury T, Raval K, Hotaling J, Sukari A. Complete response with neoadjuvant avelumab in Merkel cell carcinoma—a case report. Oral Oncol. (2019) 99:104350. doi: 10.1016/j.oraloncology.2019.06.031
41. Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med. (1970) 78(28):1277–8.
42. Zhang ZR, Wu J, Li HW, Wang T. Hepatoid adenocarcinoma of the stomach: thirteen case reports and review of literature. World J Clin Cases. (2020) 8(6):1164–71. doi: 10.12998/wjcc.v8.i6.1164
43. Ilyas W, Jain P, Goody R, Swinson D, Hingorani M. The potential role of radiotherapy in the management of hepatoid carcinomas of the stomach: a case report. Oncol Res Treat. (2020) 43(4):170–3. doi: 10.1159/000505375
44. Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, et al. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer. (2019) 22(6):1183–92. doi: 10.1007/s10120-019-00965-5
45. Søreide JA. Therapeutic approaches to gastric hepatoid adenocarcinoma: current perspectives. Ther Clin Risk Manag. (2019) 15:1469–77. doi: 10.2147/TCRM.S204303
46. Kwon MJ, Byeon S, Kang SY, Kim KM. Gastric adenocarcinoma with enteroblastic differentiation should be differentiated from hepatoid adenocarcinoma: a study with emphasis on clear cells and clinicopathologic spectrum. Pathol Res Pract. (2019) 215(9):152525. doi: 10.1016/j.prp.2019.152525
47. Fu Y, Zhu H, Peng WJ. Gastric hepatoid adenocarcinoma: differentiation from gastric adenocarcinoma with dynamic contrast-enhanced computed tomographic findings. J Comput Assist Tomogr. (2019) 43(6):887–91. doi: 10.1097/RCT.0000000000000924
48. Chen EB, Wei YC, Liu HN, Tang C, Liu ML, Peng K, et al. Hepatoid adenocarcinoma of stomach: emphasis on the clinical relationship with alpha-fetoprotein-positive gastric cancer. Arch Pathol Lab Med. (2016) 140(6):508–23. doi: 10.5858/arpa.2015-0173-CP
49. Xiao C, Wu F, Jiang H, Teng L, Song F, Wang Q, et al. Hepatoid adenocarcinoma of the stomach: nine case reports and treatment outcomes. Oncol Lett. (2015) 10(3):1605–9. doi: 10.3892/ol.2015.3430
50. Liu XM, Chen GQ, Li SL, Zai TS. Hepatoid adenocarcinoma of the stomach: a case report and literature review. Exp Ther Med. (2015) 9(6):2133–6. doi: 10.3892/etm.2015.2393
51. Lin YY, Chen CM, Huang YH, Lin CY, Chu SY, Hsu MY, et al. Liver metastasis from hepatoid adenocarcinoma of the stomach mimicking hepatocellular carcinoma: dynamic computed tomography findings. World J Gastroenterol. (2015) 21(48):13524–31. doi: 10.3748/wjg.v21.i48.13524
52. Velut G, Mary F, Wind P, Aparicio T. Adjuvant chemotherapy by FOLFOX for gastric hepatoid adenocarcinoma. Dig Liver Dis. (2014) 46(12):1135–6. doi: 10.1016/j.dld.2014.08.036
53. Ye MF, Tao F, Liu F, Sun AJ. Hepatoid adenocarcinoma of the stomach: a report of three cases. World J Gastroenterol. (2013) 19(27):4437–42. doi: 10.3748/wjg.v19.i27.4437
54. Qu BG, Bi WM, Qu BT, Qu T, Han XH, Wang H, et al. PRISMA-compliant article: clinical characteristics and factors influencing prognosis of patients with hepatoid adenocarcinoma of the stomach in China. Medicine. (2016) 95(15):e3399. doi: 10.1097/MD.0000000000003399
55. Doi Y, Takii Y, Mitsugi K, Kimura K, Mihara Y. The effectiveness of hepatic arterial infusion chemotherapy with 5-fluorouracil/cisplatin and systemic chemotherapy with ramucirumab in alpha-fetoprotein-producing gastric cancer with multiple liver metastases. Case Rep Oncol Med. (2018) 2018:5402313. doi: 10.1155/2018/5402313
56. Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. (2021) 14(1):156. doi: 10.1186/s13045-021-01164-5
57. Bai RL, Chen NF, Li LY, Cui JW. A brand new era of cancer immunotherapy: breakthroughs and challenges. Chin Med J. (2021) 134(11):1267–75. doi: 10.1097/CM9.0000000000001490
58. Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. (2020) 17(12):725–41. doi: 10.1038/s41571-020-0413-z
59. Wu J, Waxman DJ. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett. (2018) 419:210–21. doi: 10.1016/j.canlet.2018.01.050
60. Viscardi G, Tralongo AC, Massari F, Lambertini M, Mollica V, Rizzo A, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. (2022) 177:175–85. doi: 10.1016/j.ejca.2022.09.031
Keywords: hepatoid adenocarcinoma of the stomach, pathologic complete response, chemo-immunotherapy, PD-1 inhibitor, terelizumab, immune checkpoint inhibitor (ICIs)
Citation: Zhou Y, Dong L, Dai L, Hu S, Sun Y, Wu Y, Pan T and Li X (2023) Pathologic complete response of hepatoid adenocarcinoma of the stomach after chemo-immunotherapy: A rare case report and literature review. Front. Surg. 10:1133335. doi: 10.3389/fsurg.2023.1133335
Received: 28 December 2022; Accepted: 13 March 2023;
Published: 30 March 2023.
Edited by:
Alessandro Rizzo, University of Bologna, ItalyReviewed by:
Angela Dalia Ricci, “Saverio de Bellis” Research Hospital, Italy© 2023 Zhou, Dong, Dai, Hu, Sun, Wu, Pan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Pan MjMxMTMxOEB6anUuZWR1LmNu Xiawei Li bGl4aWF3ZWlAemp1LmVkdS5jbg==
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.