- 1Department of Neurosurgery, University of California San Francisco, San Francisco, CA, United States
- 2Department of Neurosurgery, San Francisco Veterans Affairs Medical Center, San Francisco, CA, United States
Objectives: Spine surgery is associated with early impairment of gastrointestinal motility, with postoperative ileus rates of 5–12%. A standardized postoperative medication regimen aimed at early restoration of bowel function can reduce morbidity and cost, and its study should be prioritized.
Methods: A standardized postoperative bowel medication protocol was implemented for all elective spine surgeries performed by a single neurosurgeon from March 1, 2022 to June 30, 2022 at a metropolitan Veterans Affairs medical center. Daily bowel function was tracked and medications were advanced using the protocol. Clinical, surgical, and length of stay data are reported.
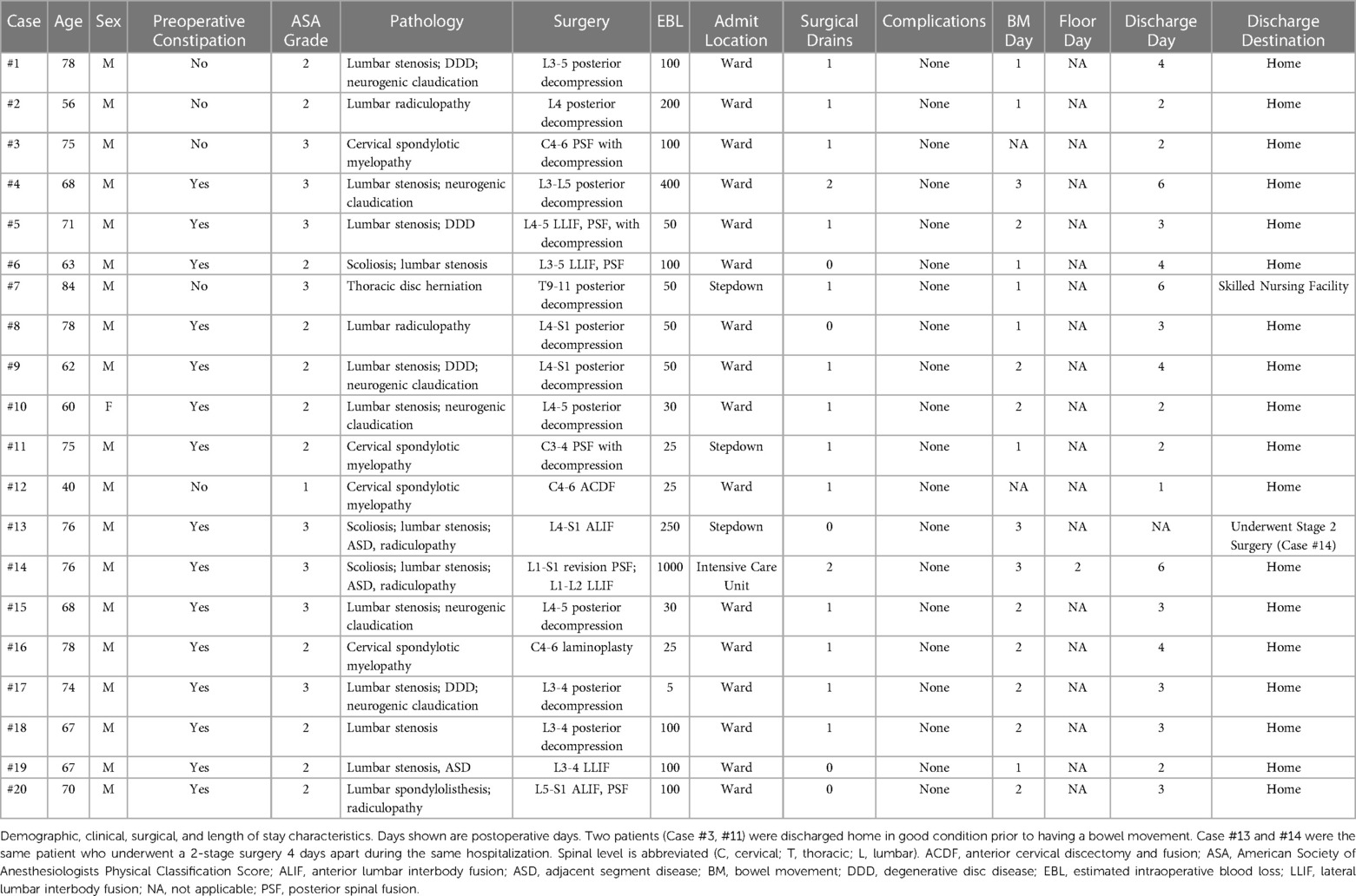
Results: Across 20 consecutive surgeries in 19 patients, mean age was 68.9 years [standard deviation (SD) = 10; range 40–84]. Seventy-four percent reported preoperative constipation. Surgeries consisted of 45% fusion and 55% decompression; lumbar retroperitoneal approaches constituted 30% (10% anterior, 20% lateral). Two patients were discharged in good condition prior to bowel movement after meeting institutional discharge criteria; the other 18 cases all had return of bowel function by postoperative day (POD) 3 (mean = 1.8-days, SD = 0.7). There were no inpatient or 30-day complications. Mean discharge occurred 3.3-days post-surgery (SD = 1.5; range 1–6; home 95%, skilled nursing facility 5%). Estimated cumulative cost of the bowel regimen was $17 on POD 3.
Conclusions: Careful monitoring of return of bowel function after elective spine surgery is important for preventing ileus, reducing healthcare cost, and ensuring quality. Our standardized postoperative bowel regimen was associated with return of bowel function within 3 days and low costs. These findings can be utilized in quality-of-care pathways.
Introduction
Spine deformity affects 30%–70% of the elderly population (1), and spine surgery for degenerative conditions has continued to rise over the past two decades (2–4). Optimizing outcomes and reducing complications in this growing population is paramount. Age-related factors, medical comorbidities, and perioperative opioid use can impact return of bowel function after spine surgery. Ileus, an extreme form of constipation associated with loss of bowel motility, usually manifests in 3–5 days after surgery (5–7), and constitutes a severe complication due to its morbidity, increased hospital length of stay (HLOS), and cost (8–10). Given rates of postoperative ileus at 5%–12% after spine surgery (11, 12), return of bowel function has become an important determinant of healthcare outcomes and cost.
The etiology of postoperative ileus is typically multifactorial. Risk factors include predisposing conditions (e.g., gastrointestinal disorders), perioperative fluid and electrolytes imbalance, stress and inflammation, and narcotic administration for pain control (13). In spine surgery, gastrointestinal morbidities are associated with fusion surgeries, longer intraoperative time, higher blood loss, and higher postoperative opioid doses (14). In addition, direct exposure and retraction of the abdominal viscera during anterior approaches to the thoracolumbar spine are thought to exacerbate postoperative bowel paresis and dysfunction (15). Ileus has been found to occur more frequently in patients undergoing ≥3-level posterior fusion (35% vs. 58%) (11). While not formally evaluated in spine surgery, the acute care impact of postoperative ileus has been extensively examined in abdominal surgery with significantly increased HLOS by 6 days (11.5 vs. 5.5 days) and per-patient costs by $9,000 ($18,877 vs. $9,460) (16).
The high cost of morbidity for postoperative ileus to both patients and healthcare institutions directly contrasts with the relatively low cost of prophylactic medications needed to improve gastrointestinal motility. While patients recovering from spine surgery often receive motility-promoting medications, standardized protocols for an optimized bowel regimen remain sparse. Recently, Enhanced Recovery After Surgery (ERAS) protocols have described general bowel regimens as part of integrated postoperative recovery (17, 18), however specific/stepwise management is rarely reported in detail. To date, only one report exists in the pediatric orthopedic literature regarding the implementation of a dedicated bowel regimen after spine surgery (19).
Herein, we report findings from a single-institution study of elective spine surgery patients after implementation of a standardized bowel regimen protocol with specific details regarding tiered management. We discuss the nuances of careful monitoring for return of bowel function in tandem with our protocol in order to improve outcomes and cost.
Materials and methods
Rationale and patient selection
A standardized postoperative bowel medication protocol (Figure 1) was implemented as part of standard clinical care for all elective spine surgeries performed by a single neurosurgeon from March 1, 2022 to June 30, 2022 at a metropolitan Veterans Affairs medical center (San Francisco Veterans Affairs Medical Center (SFVAMC), California, United States (US)). The rationale for the implementation and close follow-up of return to bowel function at our institution originated from anecdotal observations of elective spine cases with persistent constipation and ileus. Further review revealed that practice variations existed in bowel medication administration and documentation of return of bowel function. As constipation and ileus have been reported after all types of spine surgery, the tiered bowel regimen protocol was implemented as part of standard care for our elective spine surgery patients. We did not restrict the current analysis by anatomic location (cervical/thoracic/lumbar), surgical approach (anterior/lateral/posterior), or technique (fusion/decompression). To focus on a more uniform cohort, patients who required emergent spine surgery and existing inpatients who developed an indication for spine surgery were not included in this analysis.

Figure 1. Standardized postoperative bowel regimen protocol. Standardized, tiered bowel regimen protocol implemented at our institution based on return of bowel function and postoperative day (POD). Additive medications beyond the POD 0 regimen should be withdrawn once the patient has return of bowel function. BID = twice per day; BM = bowel movement; PO = per oral (by mouth).
Our study was conducted in accordance with the Medical Association's Declaration of Helsinki, national and institutional guidelines. The study protocol was reviewed and approved by the University of California, San Francisco Committee on Human Research (UCSF CHR; study #22-37541), which is the SFVAMC Institutional Review Board of record. Our study consisted of retrospective medical record review without patient contact, and a waiver of informed consent was granted by the UCSF CHR.
Standard multimodal postoperative care
All patients received standard of care multimodal pain management consisting of acetaminophen 1,000 mg every 6 h (Q6H), gabapentin 300 three times per day (TID), baclofen 5 mg twice per day (BID) as needed (PRN) for muscle spasms, oxycodone 2.5 mg Q4H PRN for moderate pain, oxycodone 5 mg Q4H PRN for severe pain, and hydromorphone 0.2 mg Q2H PRN for breakthrough pain. Patients sat up to chair for meals, underwent foley catheter removal on POD 1 morning, and underwent early mobility protocols including physical/occupational therapy starting POD 1. The exception was one patient who required a two-stage surgery on separate dates (stage 1: L4-S1 anterior interbody fusion; stage 2: L1-S1 posterior fusion with hardware revision 4 days later). He was mobilized in the interim and began physical/occupational therapy after completing his second surgery, in concordance with institutional guidelines. Standard dietary modifications at our institution include a clear liquid diet until bowel movement for anterior approach lumbar surgeries, and a soft diet until bowel movement for lateral approach lumbar surgeries, due to relatively increased risks of bowel paresis secondary to indirect manipulation from these retroperitoneal approaches. Once return of bowel function occurred, patients were advanced to a regular, heart-healthy diet.
Standardized bowel regimen protocol
Daily bowel function was evaluated and bowel medications were advanced using the protocol as part of standard care. Starting POD 0, all patients received oral docusate 250 mg BID, oral senna 17.2 mg BID, and oral polyethylene glycol 17 g daily, and in addition, oral bisacodyl 5 mg daily for 3 days. On POD 1, bisacodyl suppository 10 mg was added to the regimen if bowel function had not returned. On POD 2, oral magnesium citrate 148 ml or 296 ml (per patient tolerance) was added to the regimen if bowel function had not returned. On POD 3, sodium phosphate (1 standard bottle of 118 ml contains 19 g monobasic sodium phosphate and 7 g dibasic sodium phosphate) or alternative enema daily or BID was added (per patient tolerance) if bowel function had not returned, a chemistry panel was sent, and electrolytes were repleted to normal values. If there was no return of bowel movement on POD 4, the existing protocol would be evaluated to consider an alternative oral agent (e.g., lactulose), in conjunction with a detailed clinical and abdominal exam, consideration of abdominal imaging, and appropriate specialist consultation for further evaluation (e.g., internal medicine, gastrointestinal, acute care surgery).
Our bowel regimen protocol was verified with institutional pharmacy guidelines to be within the daily safe maximum dosages for each medication. Once there was return of bowel function, the protocol reverted back to the POD 0 regimen of docusate/senna/polyethylene glycol, with oral bisacodyl if within 3 days post-surgery (per patient tolerance), and other additive medications were withdrawn.
Pharmacology of bowel regimen medications
A feature of our protocol was the careful selection of pharmacological agents with distinct and complementary mechanisms of action. The medications utilized in the bowel regimen protocol fall into the categories of stool softeners (docusate), stimulant laxatives (senna, bisacodyl), and osmotic agents (polyethylene glycol, magnesium citrate, fleet enema). Docusate is an anionic surfactant that lowers the surface tension between the oil-water interface of stool, increasing water and lipid penetration of stool to facilitate excretion (20). Senna and bisacodyl act through direct contact with the submucosal and myenteric plexuses to increase intestinal motility and secretions, thereby increasing peristalsis (21). Polyethylene glycol, magnesium citrate, and sodium phosphate/fleet enema are osmotic preparations that draw water into the intestinal lumen to stimulate motility through increased volume (20).
Statistical analysis
Detailed clinical, surgical, and postoperative data are reported, including American Society of Anesthesiologists Physical Status Classification (ASA), surgery indication/approach, postoperative care level, estimated intraoperative blood loss (EBL), complications, and discharge destination. As part of standard care, patients were queried on admission whether they had preoperative constipation, and daily postoperatively regarding whether they experienced discomfort attributable to their bowel regimen. Inpatient and 30-day complications were assessed by chart review. Day of return of bowel function and day of hospital discharge are reported.
As medication costs differ by institution, the estimated daily and cumulative costs of our bowel regimen protocol were calculated using two widely-utilized sources of medication pricing in the public domain. The Drugs.com database (https://www.drugs.com/price-guide/) is powered by several independent leading medical-information suppliers. GoodRx (https://www.goodrx.com) is a price comparison resource that aggregates prescription drug prices across 8–10 leading US pharmacies. Each medication was entered onto these two sources, and costs were calculated based on per-unit pricing; for GoodRx, costs were averaged across available leading pharmacies. We then calculated the average between Drugs.com and GoodRx to provide the final estimated price for each medication.
Descriptive statistics are reported as mean and standard deviation (SD) for continuous variables, and proportions for categorical variables. A limited exploratory analysis was conducted to identify factors that may be associated with time to return of bowel function. Age (divided into 10-year subgroups), history of constipation, ASA grade, surgical approach (cervical, posterior thoracolumbar, anterior or lateral thoracolumbar) (11, 15, 22), and EBL (0–299 ml vs. ≥300 ml; approximate volume of 1 unit of packed red blood cells) were considered, with days to first bowel movement as the dependent variable. Univariate linear regressions were performed, and variables statistically significant at p < 0.05 were entered onto a multivariable linear regression. Mean differences (B) and 95% confidence intervals (CI) are reported. Analyses were performed using Statistical Package for Social Sciences, version 29 (IBM Corporation, Armonk, NY, US).
Results
Baseline and surgical characteristics
Our study included 20 consecutive surgeries in 19 patients. One patient had a 2-stage surgery on different dates. Mean age was 68.9 years (SD 10; range 40–84), 94.7% were male, and 73.7% reported preoperative constipation. By ASA grade, 5.2% were ASA 1, 52.7% ASA 2, and 42.1% ASA 3. Detailed patient characteristics are presented in Table 1.
Surgeries consisted of 20% cervical (anterior fusion = 1, posterior fusion = 2, posterior decompression = 1), 5% thoracic (posterior decompression = 1), and 75% lumbar (anterior fusion = 1, combined anterior/posterior fusion = 1, lateral fusion = 1, combined lateral/posterior fusion = 3, posterior decompression = 9). Of the 6 lumbar fusion surgeries, 3 were one-level, 2 were two-level, and 1 was five-level. By EBL, 50% were 0–99 ml, 40% were 101–299 ml, and 10% were ≥300 ml. Ninety-five percent were admitted to floor and 5% to ICU.
Postoperative course and return of bowel function
Eighteen patients had return of bowel function by POD 3 (mean 1.8 days, SD 0.7). Two patients were discharged in good condition after uncomplicated cervical fusion prior to bowel movement (Table 1; case #3, C4–6 posterior fusion and decompression; case #12, C4–6 anterior discectomy and fusion) as they reported no subjective complaints or gastrointestinal discomfort, were tolerating a regular diet with flatus, and met institutional discharge criteria on POD 2 and 1, respectively. Mean HLOS was 3.3 days post-surgery (SD 1.5, range 1–6). Overall, 94.7% (18 of 19 patients) were discharged to home, and 5.3% (1 of 19) to skilled nursing facility (SNF). There were no documented inpatient or 30-day complications, and no patient reported subjective discomfort due to their bowel regimen.
On exploratory analysis of the 18 patients with documented return of bowel function, history of constipation (No vs. Yes: mean 1.0 vs. 1.9 days; p = 0.040), ASA (Grade 2 vs. 3: 1.5 vs. 2.3 days; p < 0.001), and EBL (0–299 vs. ≥300 ml: 1.6 vs. 3.0 days; p = 0.008) were associated with time to return of bowel function on univariate analyses, while age and surgical approach were not (Supplementary Table S1). On multivariable analysis, history of constipation [B: + 0.8 days, 95% CI (0.1–1.4); p = 0.023] and ASA Grade 3 [B: + 0.6 days (0.0–1.1); p = 0.049] remained statistically significant factors, while a nonsignificant statistical trend was observed for EBL ≥300 ml [B: + 0.8 days (0.0–1.7); p = 0.057].
Bowel regimen protocol cost analysis
The estimated per-patient cost of the implemented bowel regimen was $1–2 on POD 0, $1–2 on POD 1, $5 on POD 2, and $9–13 on POD 3 (Figure 2). No patient required advancement of their bowel regimen protocol beyond POD 3, nor exceeded a total estimated bowel regimen medication cost of $35 (for discharge on POD 6).

Figure 2. Estimated daily cost of postoperative bowel regimen protocol. Estimated costs of the standardized bowel regimen for each postoperative day were calculated using two widely utilized sources of medication pricing in the public domain: the Drugs.com database (https://www.drugs.com/price-guide/) and GoodRx (https://www.goodrx.com). $ = United States dollar; BID = twice per day; btl = bottle; PO = per oral (by mouth).
Discussion
Postoperative ileus is a common complication of spine surgery and carries significant morbidity. Patients who experience ileus require sustained medical management and monitoring, which can prolong hospitalization and increase healthcare costs. Ileus can compound deleterious effects, e.g., poor nutritional intake and increased risks of infection, which can be life-threatening and further impact recovery (23). Ensuring expeditious recovery of bowel function after spine surgery is therefore paramount to improving postoperative outcomes and reducing overall costs.
Advantages of a standardized bowel regimen protocol
The bowel regimen described here promotes recovery of gastrointestinal motility through a tiered protocol utilizing additive, multimodal medications with distinct mechanisms of action. In our report, no patient experienced ileus despite the prevalence of risk factors such as older age, male gender, and anterior/lateral approaches (12, 22). Surgeries performed on patients in the cohort include a representative mixture of case types by level (cervical, thoracic, lumbar), approaches (anterior, posterior), and procedures (fusions, decompressions).
Features of this bowel regimen protocol ensure ease of implementation. All specified medications have a low side-effect profile and are easily administered orally, in pill or liquid form, or rectally with the assistance of care providers. These supportive features helped with obtaining a 100% regimen adherence rate for patients in the cohort. Moreover, these medications are available over the counter and are expected to be widely accessible in inpatient settings. The low technical barrier and high accessibility of the included medications support its implementation at other sites of care. Relative to the estimated per-patient cost of ileus, the total cost of bowel prophylaxis for all patients in our study was considerably less. A majority (63%) of patients were discharged on or prior to POD 3, which is the day the regimen reaches its maximum daily cost of $8.21. Indirect costs were likely minimized by the short average HLOS. Anecdotally speaking, if the ileus rate after spine surgery is estimated at 5% (1 in 20 cases) (11, 12), and the estimated average per-patient cost of our bowel regimen is $25 (for POD 3–4 discharge), the regimen's aggregate cost over 20 patients would be $500 to offset over $9,000 in cost per ileus (16).
A growing body of evidence is exploring the development and evaluation of standardized protocols to minimize postoperative gastrointestinal dysmotility. In 2022, Bansal et. al. described the application of ERAS protocols in spine surgery (24), which offers comprehensive guidance to prevent and reduce a range of potential postoperative complications. In our anecdotal experience, implementation of a bowel regimen requires considerable attention to detail. ERAS bowel regimens have limitations, as the pathway does not provide guidance on next steps if return of bowel function has not occurred after 48–72 h. Through meticulous tracking of our data after implementation, we found that patients tolerated the bowel regimen well without adverse events (e.g., loose bowel movements, abdominal discomfort). Our protocol also outlines that additive medications beyond docusate/senna/polyethylene glycol should be withdrawn once the patient has return of bowel function to reduce the risk of adverse effects and minimize unnecessary costs. With careful attention from providers and standardization of an optimized bowel regimen protocol, gastrointestinal complications can be increasingly prevented in order to improve HLOS, morbidity, cost, and patient satisfaction metrics.
While limited in scope and generalizability, our exploratory analysis identified preoperative constipation and higher ASA grade as multivariable factors associated with increased time to return of bowel function. Higher ASA grade is a known predictor of gastrointestinal dysfunction after spine surgery (14). History of preoperative constipation, however, is not routinely queried in spine surgery patients, nor part of preoperative documentation in most spine ERAS protocols (17, 18, 24). A 2015 study of 612 orthopedic surgery patients found that severe preoperative constipation (≥2 laxatives from different classes for ≥6 months) was an independent risk factor for postoperative ileus (25). While preliminary, our findings suggest that assessment of history of constipation has relevance in the early identification of patients at risk for postoperative gastrointestinal dysmotility.
Limitations
Our study has several limitations. Our sample size was small and under the care of a single neurosurgeon in a well-resourced, tertiary referral hospital, which enabled close monitoring of every patient and access to all necessary medications. The demographic and comorbidity characteristics of our sample are representative of the unique patient population served by our metropolitan Veterans Affairs medical system, and may not be generalizable to care settings with less access to resources and greater demographic diversity. Observations from our study were drawn from an uncontrolled case series, which precludes definitive and generalizable conclusions. As stated previously, the multivariable regressions performed in our study were exploratory given our limited sample size, and require validation in larger cohorts. Patients undergoing emergency spine surgery and/or inpatients referred to neurosurgery non-electively were not included. Future studies that employ rigorous epidemiological study designs and ability to adjust for covariates and/or confounders would increase the power of our observations.
US medication pricing is complex and varies by care setting. Recent US and Canadian reports have shown increasing rates of prescription medication spending in both inpatient and outpatient settings (26–28). A US health insurance organization reported that for 10 specialty medications within the top 25 medications by 2019 Medicare Part B spending (9 intravenous immunotherapy agents, 1 intramuscular neuromuscular blocker), hospital and physician office charges were 108% and 22% higher, respectively, compared to specialty pharmacies (29). Our cost analysis was limited to publicly-available data, and likely reflects outpatient rather than true inpatient costs, which may be higher. However, the medications in our protocol are relatively inexpensive and readily available over the counter, and may not incur the same cost discrepancies as specialty medications.
Conclusions
Careful monitoring of return of bowel function after elective spine surgery is important for preventing gastrointestinal symptoms and ileus, reducing healthcare cost, and ensuring quality of care. Our standardized postoperative bowel regimen protocol was associated with return of bowel function within 3 days and low costs. These findings can be utilized in quality of care and quality improvement pathways.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by University of California San Francisco Committee on Human Research, the institutional review board of record for the San Francisco Veterans Affairs Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conception or design of work (JKY, PET); Acquisition, analysis, or interpretation of data for the work (JKY, NK, ASW, JEC, LLE, GTM, PET); Drafting the work or revising it critically for important intellectual content (JKY, NK, ASW, JEC, LLE, GTM, PET); Providing approval for publication of the content (JKY, NK, ASW, JEC, LLE, GTM, PET); Agree to be held accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (JKY, NK, ASW, JEC, LLE, GTM, PET). All authors contributed to the article and approved the submitted version.
Acknowledgements
We thank the patients, providers, nurses, and institutional support staff at the San Francisco Veterans Affairs Medical Center for their time, dedication, and support of our clinical initiatives.
Conflict of interest
Disclosure of Funding Not Directly Applicable to the Current Work: GTM received grant funding from the National Institute of Neurological Disorders and Stroke (#RC2NS069409, #U01NS086090, #U01NS1365885), and the United States Department of Defense (#W81XWH-13-1-0441, #W81XWH-14-2-0176, #W81XWH-18-2-0042), awarded to the institution (University of California, San Francisco). JKY received grant funding from the Neurosurgery Research and Education Foundation & Bagan Family Foundation (#A139203), awarded to the institution (University of California, San Francisco). All other authors declare no conflicts of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1130223/full#supplementary-material.
References
1. Smith C, Lamba N, Ou Z, Vo Q-A, Araujo-Lama L, Lim S, et al. The prevalence of complications associated with lumbar and thoracic spinal deformity surgery in the elderly population: a meta-analysis. J Spine Surg. (2019) 5:223–35. doi: 10.21037/jss.2019.03.06
2. Grotle M, Småstuen MC, Fjeld O, Grøvle L, Helgeland J, Storheim K, et al. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open. (2019) 9:e028743. doi: 10.1136/bmjopen-2018-028743
3. Rajaee SS, Bae HW, Kanim LEA, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. (2012) 37:67–76. doi: 10.1097/BRS.0b013e31820cccfb
4. Sivasubramaniam V, Patel HC, Ozdemir BA, Papadopoulos MC. Trends in hospital admissions and surgical procedures for degenerative lumbar spine disease in England: a 15-year time-series study. BMJ Open. (2015) 5:e009011. doi: 10.1136/bmjopen-2015-009011
5. Venara A, Neunlist M, Slim K, Barbieux J, Colas PA, Hamy A, et al. Postoperative ileus: pathophysiology, incidence, and prevention. J Visc Surg. (2016) 153:439–46. doi: 10.1016/j.jviscsurg.2016.08.010
6. Livingston EH, Passaro EP Jr. Postoperative ileus. Dig Dis Sci. (1990) 35:121–32. doi: 10.1007/BF01537233
7. Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. (2000) 87:1480–93. doi: 10.1046/j.1365-2168.2000.01595.x
8. Khan M, Joyce E, Horn J, Scoville JP, Ravindra V, Menacho ST. Postoperative bowel complications after non-shunt-related neurosurgical procedures: case series and review of the literature. Neurosurg Rev. (2022) 45:275–83. doi: 10.1007/s10143-021-01609-y
9. Jaber A, Hemmer S, Klotz R, Ferbert T, Hensel C, Eisner C, et al. Bowel dysfunction after elective spinal surgery: etiology, diagnostics and management based on the medical literature and experience in a university hospital. Orthopade. (2021) 50:425–34. doi: 10.1007/s00132-020-04034-z
10. Oh CH, Ji GY, Yoon SH, Hyun D, Park H-C, Kim YJ. Paralytic ileus and prophylactic gastrointestinal motility medication after spinal operation. Yonsei Med J. (2015) 56:1627–31. doi: 10.3349/ymj.2015.56.6.1627
11. Yilmaz E, Benca E, Patel AP, Hopkins S, Blecher R, Abdul-Jabbar A, et al. What are risk factors for an ileus after posterior spine surgery?-A case control study. Global Spine J. (2022) 12:1407–11. doi: 10.1177/2192568220981971
12. Fineberg SJ, Nandyala SV, Kurd MF, Marquez-Lara A, Noureldin M, Sankaranarayanan S, et al. Incidence and risk factors for postoperative ileus following anterior, posterior, and circumferential lumbar fusion. Spine J. (2014) 14:1680–5. doi: 10.1016/j.spinee.2013.10.015
13. Sindell S, Causey MW, Bradley T, Poss M, Moonka R, Thirlby R. Expediting return of bowel function after colorectal surgery. Am J Surg. (2012) 203:644–8. doi: 10.1016/j.amjsurg.2011.12.007
14. Stienen MN, Smoll NR, Hildebrandt G, Schaller K, Tessitore E, Gautschi OP. Constipation after thoraco-lumbar fusion surgery. Clin Neurol Neurosurg. (2014) 126:137–42. doi: 10.1016/j.clineuro.2014.08.036
15. Choy W, Barrington N, Garcia RM, Kim RB, Rodriguez H, Lam S, et al. Risk factors for medical and surgical complications following single-level ALIF. Global Spine J. (2017) 7:141–7. doi: 10.1177/2192568217694009
16. Goldstein JL, Matuszewski KA, Delaney CP, Senagore A, Chiao EF, Shah M, et al. Inpatient economic burden of postoperative ileus associated with abdominal surgery in the United States. P T. (2007) 32:82–90. https://researchexperts.utmb.edu/en/publications/inpatient-economic-burden-of-postoperative-ileus-associated-with-.
17. Porche K, Samra R, Melnick K, Brennan M, Vaziri S, Seubert C, et al. Enhanced recovery after surgery (ERAS) for open transforaminal lumbar interbody fusion: a retrospective propensity-matched cohort study. Spine J. (2022) 22:399–410. doi: 10.1016/j.spinee.2021.10.007
18. Debono B, Corniola MV, Pietton R, Sabatier P, Hamel O, Tessitore E. Benefits of enhanced recovery after surgery for fusion in degenerative spine surgery: impact on outcome, length of stay, and patient satisfaction. Neurosurg Focus. (2019) 46:E6. doi: 10.3171/2019.1.FOCUS18669
19. Seilhamer C, Di Lorenzo C, Holstine J, Samora JB. Creating a bowel management plan for pediatric orthopaedic spine surgery patients. Spine Deform. (2021) 9:365–71. doi: 10.1007/s43390-020-00212-3
20. Liu LWC. Chronic constipation: current treatment options. Can J Gastroenterol. (2011) 25(Suppl B):22B–8B. doi: 10.1155/2011/360463
21. Tack J, Müller-Lissner S. Treatment of chronic constipation: current pharmacologic approaches and future directions. Clin Gastroenterol Hepatol. (2009) 7:502–8. quiz 496. doi: 10.1016/j.cgh.2008.12.006
22. Park SC, Chang SY, Mok S, Kim H, Chang B-S, Lee C-K. Risk factors for postoperative ileus after oblique lateral interbody fusion: a multivariate analysis. Spine J. (2021) 21:438–45. doi: 10.1016/j.spinee.2020.10.002
23. Weledji EP. Perspectives on paralytic ileus. Acute Med Surg. (2020) 7:e573. doi: 10.1002/ams2.573
24. Bansal T, Sharan AD, Garg B. Enhanced recovery after surgery (ERAS) protocol in spine surgery. J Clin Orthop Trauma. (2022) 31:101944. doi: 10.1016/j.jcot.2022.101944
25. Lee TH, Lee JS, Hong SJ, Jang JY, Jeon SR, Byun DW, et al. Risk factors for postoperative ileus following orthopedic surgery: the role of chronic constipation. J Neurogastroenterol Motil. (2015) 21:121–5. doi: 10.5056/jnm14077
26. Sherman M, Curfman GD, Parent J, Wagner AK. Prescription medications account for one in four dollars spent by A commercial health plan. Health Affairs Forefront. (2018) doi: 10.1377/forefront.20180821.820628
27. Hofmeister M, Sivakumar A, Clement F, Hayes KN, Law M, Guertin JR, et al. Trends in Canadian prescription drug purchasing: 2001-2020. J Pharm Policy Pract. (2022) 15:20. doi: 10.1186/s40545-022-00420-4
28. Tichy EM, Hoffman JM, Suda KJ, Rim MH, Tadrous M, Cuellar S, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. (2022) 79:1158–72. doi: 10.1093/ajhp/zxac102
29. New study: hospitals charge double for drugs—specialty pharmacies more affordable. AHIP. (2022). https://www.ahip.org/news/press-releases/new-study-hospitals-charge-double-for-drugs-specialty-pharmacies-more-affordable [Accessed February 16, 2023].
Keywords: clinical protocol, cost effectiveness, gastrointestinal motility, ileus, postoperative care, quality of care, spine, standard of care
Citation: Yue JK, Krishnan N, Wang AS, Chung JE, Etemad LL, Manley GT and Tarapore PE (2023) A standardized postoperative bowel regimen protocol after spine surgery. Front. Surg. 10:1130223. doi: 10.3389/fsurg.2023.1130223
Received: 23 December 2022; Accepted: 27 February 2023;
Published: 17 March 2023.
Edited by:
Harvinder Singh Chhabra, Indian Spinal Injuries Centre, IndiaReviewed by:
Srikumar Venkataraman, All India Institute of Medical Sciences, IndiaMahmoud Elguindy, University of Texas Southwestern Medical Center, United States
Shane Shahrestani, University of Southern California, United States
© 2023 Yue, Krishnan, Wang, Chung, Etemad, Manley and Tarapore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John K. Yue am9obi55dWVAdWNzZi5lZHU=
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 John K. Yue
John K. Yue Nishanth Krishnan
Nishanth Krishnan Albert S. Wang1,2
Albert S. Wang1,2 Jason E. Chung
Jason E. Chung