
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 15 March 2023
Sec. Thoracic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1127627
This article is part of the Research Topic Current Trends in Endoscopic Thoracic Surgery: Insights From the XXI SIET National Meeting View all 18 articles
Objective: We report our experience of transition to robotic-assisted thoracic surgery (RATS) for lung resections with the da Vinci Xi surgical system, exposing short-term results.
Materials and methods: This is a single-center, retrospective analysis of RATS lung resections performed between April 2021 and September 2022 during our new robotic program. The surgical approach evolved over time, starting from a four-arm approach with four incisions. Alternative RATS approaches were subsequently evaluated, such as uniportal and biportal.
Results: During a 17-month period, 29 lung resections were performed. Of them, 16 were lobectomies, 7 were segmentectomies, and 6 were wedge resections. The most common indication for anatomical lung resection was non-small cell lung cancer. A uniportal approach was used for two simple segmentectomies and a biportal RATS was performed in five lobectomies and two segmentectomies. A mean number of 8.1 lymph nodes and a mean of 2.6 N2 and 1.9 N1 stations were resected during surgery, and no nodal upstaging was observed. Negative resection margins were 100%. There were two (7%) conversions, one to open surgery and one to video-assisted thoracic surgery (VATS). Eight (28%) patients experienced complications with no 30-day mortality.
Discussion: High-ergonomic and high-quality views were immediately observed. After some procedures, we abandoned uniportal RATS because of the possibility of arm collisions and the necessity of a VATS-skilled surgeon at the operating table.
Conclusion: RATS for lung resections was safe and effective, and from the surgeon's standpoint, several practical advantages over VATS were observed. Further analysis on outcomes will help better understand the value of this technology.
The application of robotic surgical systems in thoracic surgery is still rapidly increasing. Robotic-assisted thoracic surgery (RATS) is believed to offer specific advantages: enhanced and 3D view, instruments articulation, higher ergonomics, and movement filtering. The transition to RATS in lung resections has been suggested to differ when starting from a precedent open surgery experience rather than starting from video-assisted thoracic surgery (VATS). The approach used (e.g., uniportal, biportal) may also have a significance. In April 2021, our thoracic surgery department started a RATS program, using the da Vinci Xi surgical system (Intuitive Surgical, California, United States). Both pulmonary and mediastinal procedures were performed. Previously, lung resections were routinely performed with a uniportal VATS approach. In this brief report, we expose our real-world experience of transition to RATS for lung resections, along with obstacles and challenges met.
A single-center, retrospective analysis was conducted. Data from patients who underwent RATS lung resections with the da Vinci Xi from April 2021 to September 2022 in our institution (Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico of Milan, Italy) were retrieved. All patients provided informed consent prior to surgery, and the study was approved by the ethical review board of our institution (approval no. 3.11/2022-273). A dedicated weekly session was established for the RATS program. Initially, mediastinal and simple lung procedures were performed, in order to get familiar with the robotic system. The selected anatomical lung resections were lobectomies and simple segmentectomies. Pneumonectomies, bilobectomies, and sleeve lobectomies were excluded, as well as lung resections after neoadjuvant treatment. The previous diagnostic and therapeutic pathway for non-small cell lung cancer (NSCLC) was not changed by the RATS program. Prior to surgery, all patients affected by a diagnosed or suspected NSCLC received a contrast-enhanced computed tomography (CT) scan of the thorax and a total body fluorodeoxyglucose positron emission tomography (FDG-PET) scan. If a pathological diagnosis was not available, a frozen section analysis on a wedge resection was performed prior to an eventual anatomical resection. During segmentectomies, an N1 lymph node was resected for intraoperative frozen section analysis, and if positive, a lobectomy would have been performed. The intersegmental plane was identified using indocyanine green venous injection after arterial stapling. Preoperative functional tests (mainly respiratory) were performed in accordance with international guidelines (1, 2).
In the early phase of the program, the robotic-assisted (RA) approach with four arms described by Veronesi et al. was employed. The anterior mini-thoracotomy, typically in the fourth intercostal space, was used for both a robotic arm and the space for the assistant activity. A soft tissue retractor (Alexis®) was positioned here. Additionally, three robotic ports were positioned along the seventh and eighth intercostal space, with the camera located in the midaxillary line port (3, 4). Subsequently, alternative RATS approaches were applied. The three-arm biportal approach consisted in a mini-thoracotomy, usually performed in the sixth to seventh intercostal space, on the anterior axillary line, and an additional robotic port positioned in the sixth to seventh intercostal space, on the posterior axillary line. The camera port, an arm port, and the space for the assistant activity were located in the mini-thoracotomy, and the other arm port was positioned in the second access. The three-arm uniportal approach was based on a 5-cm mini-thoracotomy in the sixth intercostal space, on the midaxillary line, from which both the robotic arms and the assistant could work. Finally, after this experience, a triportal approach was attempted, with a mini-thoracotomy in the fourth-fifth intercostal space, on the anterior axillary line, to accommodate both the arm port and the assistant. The camera port was positioned in the seventh to eighth intercostal space, midaxillary line, and the arm port in the seventh to eighth intercostal space. The patient position was always the same, in the lateral decubitus. A schematic representation of approaches can be found in Figures 1, 2. Manual staplers were used by the assistant. No CO2 was insufflated and a 30° camera was used.
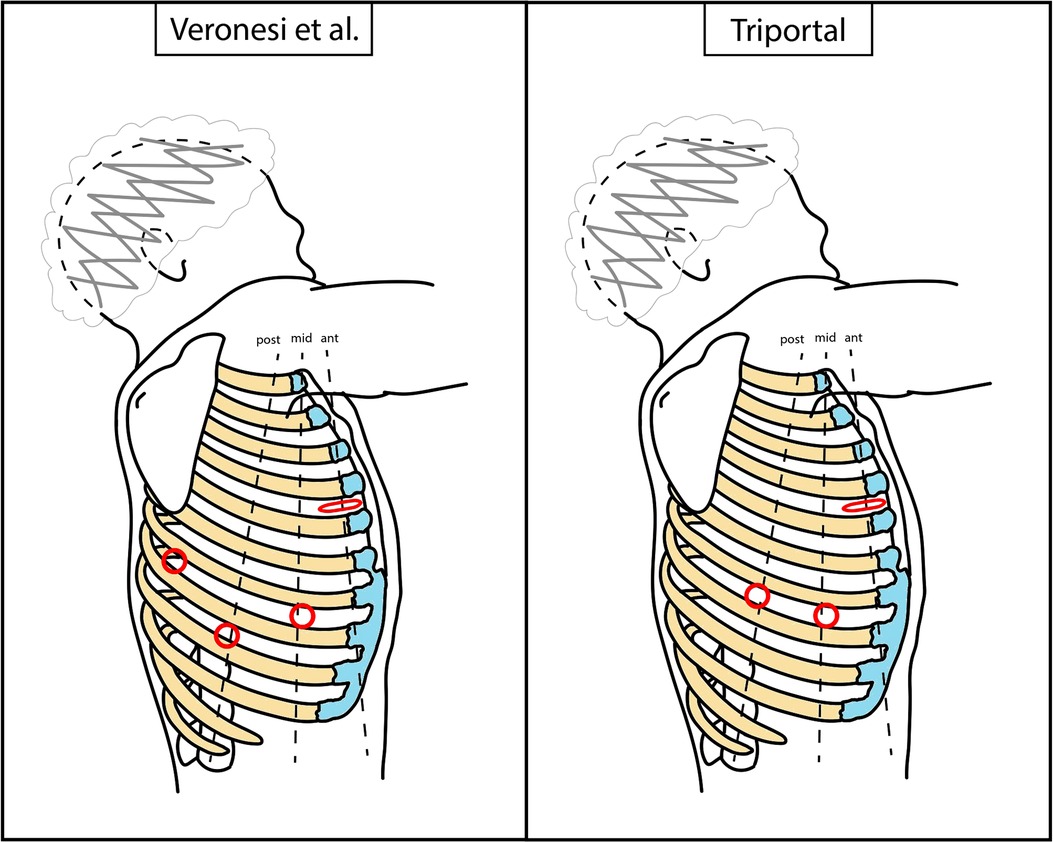
Figure 1. Schematic representation of RATS accesses positioning. Dimensions and distances are not to scale, but only indicative. Red circle, robotic port; red flattened circle, mini-thoracotomy (valid as assistant access too). RATS, robotic-assisted thoracic surgery.
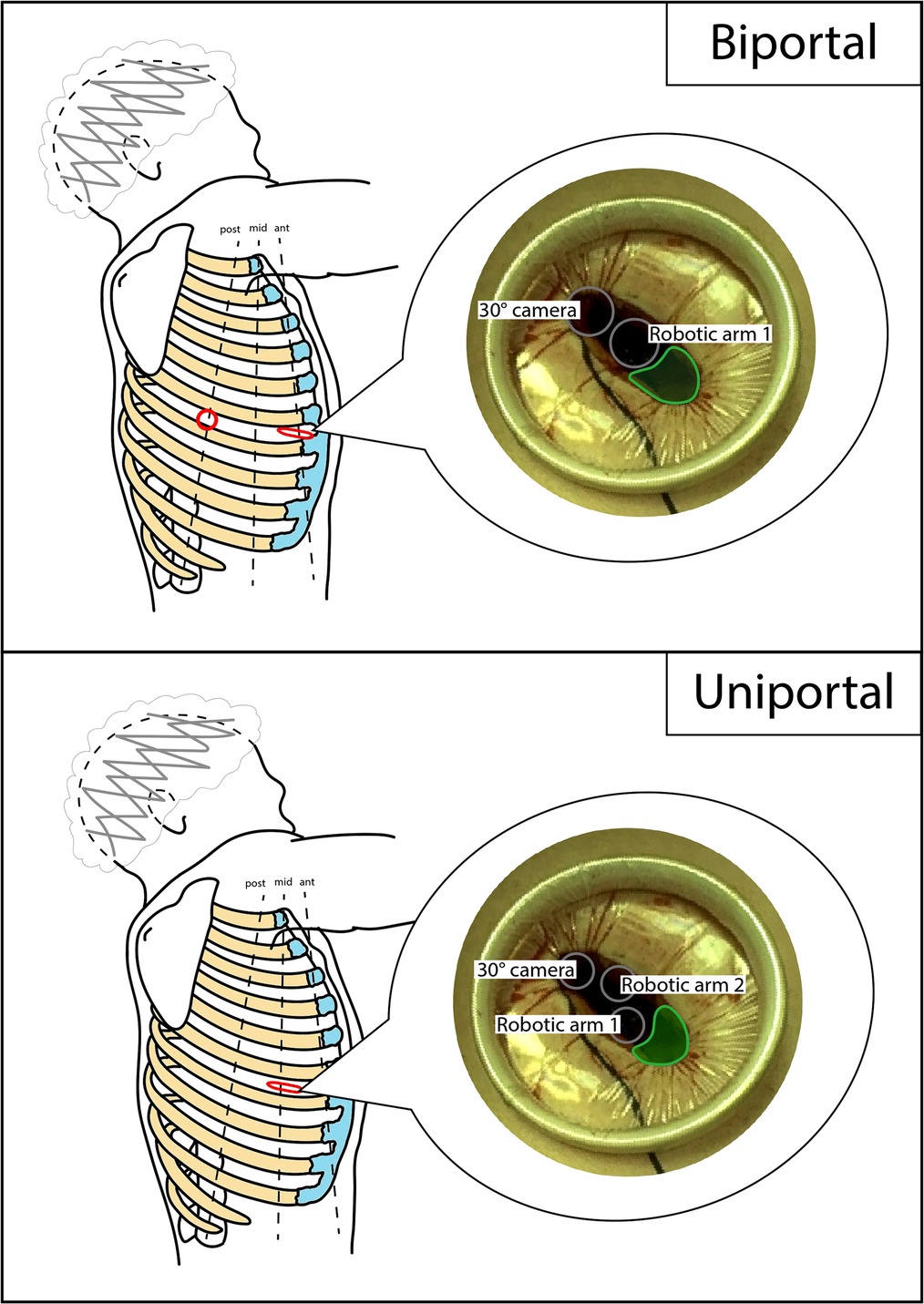
Figure 2. Schematic representation of biportal and uniportal RATS accesses positioning. Dimensions and distances are not to scale, but only indicative. Red circle, robotic port; red flattened circle, mini-thoracotomy (valid as assistant access too); gray circle, robotic trocar; green area, assistant area/access. RATS, robotic-assisted thoracic surgery.
During a 17-month period of RATS program, 29 lung resections were performed with the da Vinci Xi robotic surgical system. Of them, 16 were lobectomies, 7 were segmentectomies, and 6 were wedge resections. All procedures were performed by two surgeons (DT and AP). The most common indication for anatomical lung resection was NSCLC. Only one hamartoma was treated with segmentectomy due to its position, impeding a wedge resection. Mean age of patients was 64 (±12) years, and 20 (69%) were female. Twelve (41%) were never smokers, whereas 11 (38%) were former smokers and 6 (21%) were active smokers. Twenty-three (79%) had at least one polymorbidity (e.g., systemic arterial hypertension, diabetes), and 10 (34%) at least two. Concerning preoperative respiratory function, mean % predicted (%p) forced expiratory volume 1 s (FEV1) was 103% (±0.21), mean %p forced vital capacity (FVC) was 109% (±0.18), and mean %p diffusing capacity of the lungs for carbon monoxide (DLCO) was 77% (±0.16). Details concerning disease and procedure characteristics and perioperative outcomes are reported in Table 1. Mean operative time was 238 min (median 232) for lobectomy, 230 min (median 212) for segmentectomy, and 98 min (median 99) for wedge. Even if a formal statistical analysis was not conducted (given the small number of procedures), a difference in operative times between the different approaches of lobectomy was noted. In particular, the biportal lobectomies carried an additional mean of 101 min than multiport lobectomies. There were two conversions (7%). One was a planned RATS segmentectomy for a cT1cN0 stage NSCLC that was converted to VATS lobectomy for technical reasons. The other one was a RATS lobectomy in a cT3N1 stage NSCLC that was converted to open surgery due to bleeding from a pulmonary artery branch. Regarding lymphadenectomy, we found that a mean number of 8.1 lymph nodes were retrieved during surgery. A mean number of 2.6 N2 and 1.9 N1 stations were resected. No nodal upstaging was observed. Negative resection margins were obtained in all cases (100%). The mean postoperative length of stay was 6.8 days for lobectomy, 7.2 for segmentectomy, and 3 for wedge resection. Mean chest tube duration was 5.3 days for lobectomy, 5.7 for segmentectomy, and 1.8 for wedge resection. Globally, eight (28%) patients experienced complications. Of these, six were grade I and three were grade II [Clavien–Dindo classification (5)]. Further details are available in Table 2. No 30-day readmission in hospital nor 30-day death were recorded.
The first lobectomy was performed after 8 procedures with a standard four-arm approach, whereas the first segmentectomy after 21 cases and with a uniportal approach. Overall, four uniportal (two segmentectomies and two uniportal pleurodesis with wedge resection), and eight biportal (five lobectomies, two segmentectomies, and one pleurodesis with wedge resection) procedures were performed. The first uniportal operation was a pleurodesis and wedge resection, whereas the first biportal procedure was a simple segmentectomy.
Our study represents a real-world report of a thoracic surgery unit transitioning to RATS for lung resections. During a transition to a new technique or approach, it is legit to question if it will achieve better results. Four major objectives should be pursued: higher, or at least equal, safety, reduced time, lower costs, and increased results. In this case, increased results concerns both surgical and oncological outcomes. However, all these four may not be always obtained simultaneously. In the case of lung resections, the transition to RATS can happen either from open surgery or VATS. Differences between these two transitions are thought to exist. It has been suggested that transition from open surgery to RATS is easier than from open surgery to VATS (4). The hypothesized reason is the similarity of surgical steps in lung resections between open and RATS. However, it is also common to believe that an experienced VATS surgeon has less difficulties in approaching RATS than an open surgeon. This idea could be supported by the similarity of RATS to VATS because of the reduced to absent tactile feedback and the visualization of the thoracic cavity through the screen (6). Results from single-surgeon experiences of transition to RATS suggest similar performances between RATS and VATS. Initially, RATS operative times are longer probably due to docking time and familiarization with instruments (7, 8).
At the beginning of our RATS experience, we had some concerns on performing multiple accesses for a robotic lung resection, rather than a single one as in uniportal VATS. However, after some operations, we acquired confidence with the multiport approach. Some practical advantages over VATS were immediately observed after the first procedures. First of all, the high ergonomics resulted in a less tiring and more comfortable surgery for the console operator. In addition, the quality of the view was significantly higher, thanks to the enhanced quality of the video, the 3D vision, and the stability of the camera. This somehow helped compensating the absence of haptic feedback, especially during dissection of hilar elements. As a consequence, we expected that RATS would result in a higher number of resected lymph nodes than VATS. Nevertheless, even if a formal analysis and comparison were not made, the results did not favor this hypothesis. We are still in an early phase and more cases are needed to make our results more robust. In our experience, staplers were used by the assistant, thus reducing the independence of the console operator. However, we believe autonomy was higher compared to VATS, given that all the instruments, camera included, were easily controlled by the operating surgeon.
Our previous uniportal VATS experience eventually led our team to experiment alternative RATS approaches, with the objective of reducing the incisions. Therefore, both biportal and uniportal RATS approaches were performed. The time required for setting the robotic arms, and for adjusting them during the operation to avoid collision, inevitably determined longer operative times. Collisions were significantly higher with the uniportal approach, and as reported in recent papers, it required the presence of a uniportal VATS-skilled surgeon at the operating table (9). Collisions between instruments are potentially harmful for the patient, and the assistant's help revealed to be important during several steps. Given these issues, we decided to abandon the uniportal approach for major lung resections. In addition, it should be kept in mind that to date this type of technique is not approved by the manufacturer, so medical–legal issues could also arise in case of major complications. We believe that in the future, once the technology for the uniportal approach is developed, this may be a viable option under conditions of greater patient safety.
At present, our preferred approach for RATS lung resections is the triportal one. In general, a certain degree of freedom of choice on the number and location of the incisions is accepted, based on surgeon's preference and case characteristics. No superior study between one approach and another has yet been published. Still, one interesting issue is multiple nerve damage as a possible cause of more pain. Some authors believe this may cause more pain compared to approaches that are performed accessing only one or two intercostal spaces (10–12). In our experience, multiport VATS was thought to be more painful than uniportal VATS (13). During our RATS program we did not systematically collect quality data concerning postoperative pain; thus, we were not able to make any comparison or analysis. From a theoretical standpoint, damaging only one intercostal nerve rather than more than one, when positioning multiple ports, would logically result in less pain. However, to date, a reliable systematic analysis and comparison is still not available and is likely to be particularly complex given the number of factors involved in postoperative pain. On the other hand, our experience taught us that practical advantages of accessing the thorax through different intercostal spaces are ensuring more possible directions for instruments and a wider triangulation.
In our experience, we preferred not to use CO2 insufflation. We considered that the benefit of CO2 in lung resections was not worth the need for dedicated devices (e.g., Alnote-Lapsingle©), given the presence of the mini-thoracotomy. We believed it would be probably simpler to use CO2 with a robotic portal (RP) approach (3). Of course, we are aware that CO2 pressure would result in a better exposure of structures, mainly by compression of lung and diaphragm. In fact, during our thymic RATS procedures, CO2 insufflation was routinely used, thanks to the application of an RP approach.
We believe that RATS is an interesting technology that may be beneficial in lung resections. However, given its cost, it is expected to bring benefits not only to surgeons but also to patients in order to be justified. Thus, we will monitor outcomes of RATS procedures in our center. It may reveal to perform better in determined surgical gestures, as suturing. In fact, it resembles the open surgery experience, and this may facilitate procedures as sleeve resections, as reported by several authors (14–16). Thus, the positive impact of RATS may appear more significant in this kind of procedures, rather than in routine ones.
Some limits of this study can be identified. First, the cohort of patients is small and from a single center, limiting the power of our results. In addition, we are still in the learning curve phase, thus requiring more time to produce definitive data from both involved surgeons.
We found that RATS lung resections were safe and effective, and from the surgeon's standpoint, several practical advantages over VATS were observed. Results are probably premature to be correctly interpreted and, of course, we are still in the learning curve phase. At present time, we believe that the uniportal approach is not advisable because of possible conflicts between the robotic arms and the resulting risks to the patient. Further analysis of outcomes will help better understand the value of this technology.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The present study involving human participants was reviewed and approved by the ethical review board of our institution. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DT and AP contributed to the study conception. Material preparation, data collection, and analysis were performed by GM. The first draft of the manuscript was written by GM, AP, and DT, and all authors commented on the previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
The publication costs for this manuscript were covered by the fund Current research—IRCCS from the Italian Ministry of Health dedicated to the IRCCS Foundation Ca’ Granda Ospedale Maggiore Policlinico.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J. (2009) 34(1):17–41. doi: 10.1183/09031936.00184308
2. Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143(5 Suppl):e166S–90S. doi: 10.1378/chest.12-2395
3. Cerfolio R, Louie BE, Farivar AS, Onaitis M, Park BJ. Consensus statement on definitions and nomenclature for robotic thoracic surgery. J Thorac Cardiovasc Surg. (2017) 154(3):1065–9. doi: 10.1016/j.jtcvs.2017.02.081
4. Veronesi G, Galetta D, Maisonneuve P, Melfi F, Schmid RA, Borri A, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg. (2010) 140(1):19–25. doi: 10.1016/j.jtcvs.2009.10.025
5. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
6. Feczko AF, Wang H, Nishimura K, Farivar AS, Bograd AJ, Vallières E, et al. Proficiency of robotic lobectomy based on prior surgical technique in the society of thoracic surgeons general thoracic database. Ann Thorac Surg. (2019) 108(4):1013–20. doi: 10.1016/j.athoracsur.2019.04.046
7. Lee BE, Korst RJ, Kletsman E, Rutledge JR. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg. (2014) 147(2):724–9. doi: 10.1016/j.jtcvs.2013.10.002
8. Gómez-Hernández MT, Fuentes MG, Novoa NM, Rodríguez I, Varela G, Jiménez MF. The robotic surgery learning curve of a surgeon experienced in video-assisted thoracoscopic surgery compared with his own video-assisted thoracoscopic surgery learning curve for anatomical lung resections. Eur J Cardiothorac Surg. (2022) 61(2):289–96. doi: 10.1093/ejcts/ezab385
9. Gonzalez-Rivas D, Bosinceanu M, Motas N, Manolache V. Uniportal robotic-assisted thoracic surgery for lung resections. Eur J Cardiothorac Surg. (2022) 62(3):ezac410. doi: 10.1093/ejcts/ezac410
10. Wei B, Cerfolio RJ. Robotic lobectomy and segmentectomy: technical details and results. Surg Clin North Am. (2017) 97(4):771–82. doi: 10.1016/j.suc.2017.03.008
11. Zhao G, Jiang X, Wang F, Chu M, Zhang C, Zhao W, et al. Lobectomy with high-position single-intercostal two-port video-assisted thoracoscope for non-small cell lung cancer is a safe and effective surgical procedure. J Thorac Dis. (2020) 12(12):7346–54. doi: 10.21037/jtd-20-3469
12. Young R, McElnay P, Leslie R, West D. Is uniport thoracoscopic surgery less painful than multiple port approaches? Interact Cardiovasc Thorac Surg. (2015) 20(3):409–14. doi: 10.1093/icvts/ivu391
13. Louis SG, Gibson WJ, King CL, Veeramachaneni NK. Uniportal video-assisted thoracoscopic surgery (VATS) technique is associated with decreased narcotic usage over traditional VATS lobectomy. J Vis Surg. (2017) 3:117. doi: 10.21037/jovs.2017.08.05
14. Veronesi G, Cerfolio R, Cingolani R, Rueckert JC, Soler L, Toker A, et al. Report on first international workshop on robotic surgery in thoracic oncology. Front Oncol. (2016) 6:214. doi: 10.3389/fonc.2016.00214
15. Elliott IA, Yanagawa J. Can the robot overcome technical challenges of thoracoscopic bronchial anastomosis? J Thorac Dis. (2019) 11(Suppl 9):S1123–5. doi: 10.21037/jtd.2019.04.100
Keywords: robotic-assisted thoracic surgery, RATS, biportal, uniportal, lung cancer, brief report
Citation: Palleschi A, Mattioni G, Mendogni P and Tosi D (2023) A real-world experience of transition to robotic-assisted thoracic surgery (RATS) for lung resections. Front. Surg. 10:1127627. doi: 10.3389/fsurg.2023.1127627
Received: 19 December 2022; Accepted: 20 February 2023;
Published: 15 March 2023.
Edited by:
Federico Raveglia, ASST-Monza, ItalyReviewed by:
Andrea De Vico, Azienda Usl Teramo, Italy© 2023 Palleschi, Mattioni, Mendogni and Tosi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Mattioni Zy5tYXR0aW9uaUBvdXRsb29rLml0
†ORCID Alessandro Palleschi orcid.org/0000-0003-4510-5167 Giovanni Mattioni orcid.org/0000-0003-4136-8293 Paolo Mendogni orcid.org/0000-0002-4303-6244 Davide Tosi orcid.org/0000-0003-3767-0449
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.