- Department of Thoracic Surgery, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China
Background: Primary tracheal or bronchial tumors are relatively uncommon, whether benign or malignant. Sleeve resection is an excellent surgical technique for most primary tracheal or bronchial tumors. However, depending on the size and location of the tumor, thoracoscopic wedge resection of trachea or bronchus can be performed with the assistance of a fiberoptic bronchoscope for some malignant and benign tumors.
Case Description: We performed a single incision video-assisted bronchial wedge resection in a patient with a left main bronchial hamartoma with a size of 7 × 5 × 5 mm. The patient was discharged from the hospital six days after the surgery with no postoperative complications. There was no obvious discomfort during the 6-month postoperative follow-up, and the reexamination of fiberoptic bronchoscopy revealed no evident stenosis of the incision.
Conclusions: Through the detailed case study and literature review, we believe that tracheal or bronchial wedge resection is a significantly superior technique under the appropriate conditions. Video-assisted thoracoscopic wedge resection of trachea or bronchus should be a new and excellent development direction of minimally invasive bronchial surgery.
Introduction
Primary tracheal or bronchial tumors are relatively uncommon, whether benign or malignant. Tracheal or bronchial tumors are classified into three types: malignant, low-grade, and benign on their degree of differentiation. Tracheal or bronchial segmental resection with end-to-end anastomosis is currently the standard surgical treatment for tracheal or bronchial tumors. Sleeve resection is an excellent surgical technique for most primary tracheal or bronchial tumors. However, depending on the size and location of the tumor, thoracoscopic wedge resection of trachea or bronchus can be performed with the assistance of a fiberoptic bronchoscope for some malignant and benign tumors (1, 2). It significantly reduces the difficulty and trauma of surgery while preserving the lung tissue and ensuring surgical results. It also reduces the incidence of postoperative complications (3–8). This article comprehensively demonstrates the advantages, disadvantages and indications of this technique through a detailed case study and previous literature.
Case description
A 46-year-old man was admitted with a left main bronchus tumor. He was in good health in the past. Physical examination, routine blood examination, biochemistry and tumor markers revealed no abnormalities. After admission, fiberoptic bronchoscopy (Figure 1) and chest computed tomography (CT) (Figure 1) were performed. At the bronchoscopy, a certain amount of tissue was taken for biopsy, although we found that the tumor was tough and difficult to clamp.
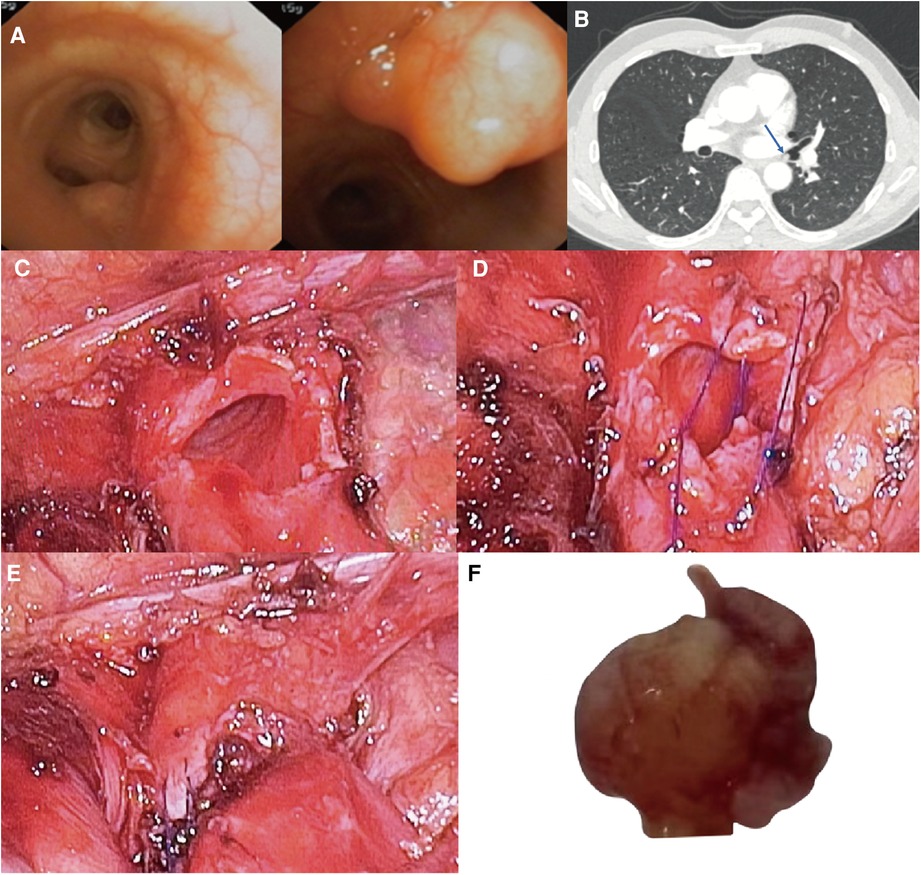
Figure 1. (A) neoplastic bulge was seen on the medial wall of left main bronchus terminal, 1 cm away from the opening of left lower lobe bronchus. (B) Bronchial tumor at the end of left main bronchus (arrow), the diameter of tumor base is 5 mm, and the diameter of bronchus is 8 mm. Surgical Technique: (C) The left main bronchus wall after wedge-shaped resection of the tumor with endoscopic scissors. (D) Continuous suture of the incision using 4–0 Prolene. (E) Left main bronchus wall after suture. (F) Left main bronchus tumor with a size of 7 × 5 × 5 mm.
Bronchoscopy results revealed a neoplastic bulge on the medial wall of left main bronchus terminal, 1 cm away from the left lower lobe bronchus opening. The surface mucosa was still smooth, and the surface blood vessels were visible. The histopathological results of the biopsy showed that there were very small pieces of proliferated spindle cells with background myxoid changes under microscope, and the cell heterogeneity was not obvious. Although there was no definite diagnosis, it also helped us to preliminarily rule out the diagnosis of malignant tumor. In this case, we first communicated with a respiratory endoscopist and were told that the tumor could not be safely removed by intraluminal bronchoscopic treatment due to the large basal area of the tumor, so we decided to perform a single incision video-assisted bronchial wedge resection first.
Surgical technique
We drilled a 3 cm left thoracic and axillary midline fifth intercostal hole into the patient’s chest. After loosening some adhesions and dividing the inferior pulmonary ligament, the lung tissue was pulled to expose the left main bronchus from the back. The left main bronchus was separated after the mediastinal pleura was opened. At this time, the visual fiberoptic bronchoscope was used to determine tumor location and margin by two methods: (1) We asked the anesthesiologist to place the bronchoscope lens under the tumor and turn the lens direction so that the light source of the lens was directed directly at the bronchial wall. And then we could clearly see the position of the lens under the thoracoscopy. (2) We pressed the bronchus gently with the instrument under the thoracoscopy, and the part we pressed could be clearly seen under the fiberoptic bronchoscope. By comparing these two noninvasive methods, we could determine the location of the tumor.
After the bronchial tumor was leaked, the left main bronchus was cut along the distal end of tumor with endoscopic scissors, and the tumor was wedge-shaped along the edge of tumor. When the left main bronchial hamartoma was confirmed from the frozen section of the removed tumor, the incision was sutured by 4 - 0 Prolene with a continuous suture, needle distance 5 mm, and margin 5 mm (Figures 1, 2). There was no apparent stenosis or distortion of the bronchi observed by thoracoscopy and fiberoptic bronchoscopy after suturing. The operation lasted 1.5 h, and there was no visible bleeding during surgery.
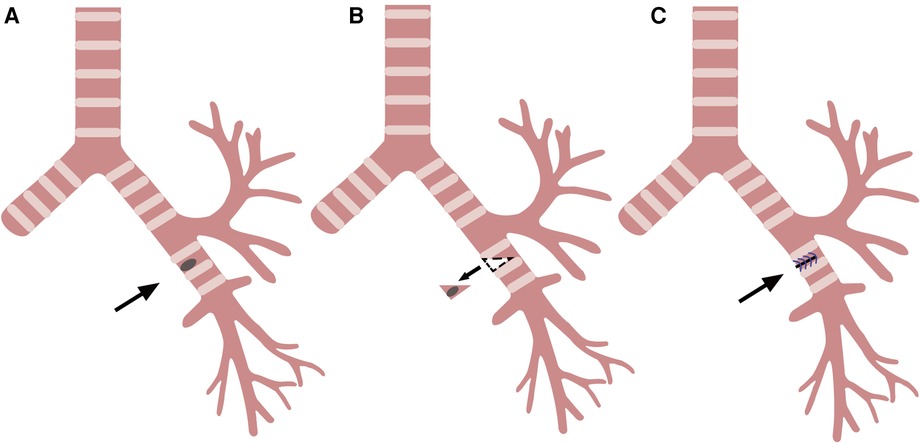
Figure 2. Wedge resection and reconstruction of the bronchi. (A) Left main bronchus tumor (arrow). (B) Resection of the tumor along the dotted line (arrow). (C) Left main bronchus wall after suture (arrow).
The chest drain was removed five days after the surgery, and the patient was discharged from the hospital six days after the surgery with no postoperative complications. Thoracoscopic wedge resection is significantly superior to sleeve resection in terms of recovery. There was no obvious discomfort during the 6-month postoperative follow-up, and the reexamination of fiberoptic bronchoscopy revealed no evident stenosis of the incision.
Discussion
Through the detailed case study and literature review, we believe that tracheal or bronchial wedge resection is feasible and excellent in treating some benign and malignant tumors of the trachea or bronchus. The indications for wedge resection include (1) Tumors with a base diameter smaller than the diameter of trachea or bronchus. (2) Tumors that are confined to the carina or bronchial corner. (3) Local tumor infiltration in the cranial and the caudal parts of adjoining main bronchus.
Indication 1
In the 6 cases in the Supplementary Table (9–13), the authors performed wedge resection of the trachea or bronchus to treat benign or low-grade bronchial tumors. Wedge resection significantly reduces the difficulty and risk of surgery while preserving the lung tissue and ensuring surgical results. Based on their experience with 83 cases of bronchial carcinoid tumors, Ismail Cüneyt Kurul et al. concluded that wedge resection could be considered if the diameter of the trachea or bronchial tumor base to be resected is smaller than the diameter of bronchus (2). In addition, Florian Augustin believe that the maximum distance between the upper and lower edge of the bronchus in wedge resection is preferably no longer than the transverse diameter of the bronchus (14), which can effectively avoid postoperative anastomotic stenosis. For benign and low-grade malignant tumors of the trachea or bronchus, the preferred surgical approach should be minimally invasive thoracoscopic wedge resection.
Indication 2
Dong Xie et al. performed wedge resection on a patient with a 1 cm squamous cell carcinoma confined to the tracheal carina, and a clear margin was confirmed during the surgery. The wedge-shaped excision and reconstruction of the carina under the original carina ensured no separation between the trachea and the main bronchus (Figure 3). The trachea and the bronchus remained continuous without creating longitudinal tension. The problem of longitudinal tension encountered by sleeve resection was skillfully circumvented with wedge resection, and the patient recovered well after surgery (15). Hiromasa Yamamoto et al. performed a bronchial wedge resection for a carcinoid tumor of the left upper bronchus near the upper and lower lobar bronchi bifurcation. The upper and healthy lower lobar bronchial corner was resected longitudinally, and the bronchial corner was reconstructed at a distance. At the five-month postoperative follow-up, there was no stenosis at the suture, indicating that this technique avoided anastomotic stenosis and longitudinal tension (16).
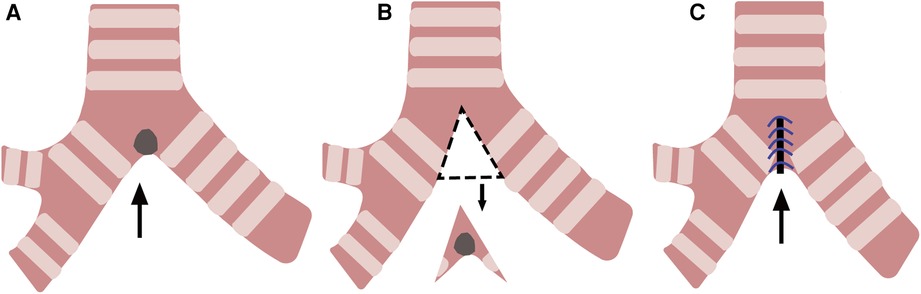
Figure 3. Wedge resection and reconstruction of the carina. (A) A tumor at the carina(arrow). (B) Resection of the tumor along the dotted line (arrow). (C) Reconstruction of the carina (arrow).
Daisuke Yuki et al. performed a deeper wedge resection and the reconstruction of right secondary carina for a 13 mm recurrent mucoepidermoid carcinoma located at the orifice of upper lobar bronchus with main bronchus involvement. The lung tissue was successfully preserved, and no recurrence occurred 18 months after surgery (17). Dong Xie, Hiromasa Yamamoto, and Daisuke Yuki performed wedge resection and reconstruction of the carina, bronchial corner, and the secondary carina, respectively, for resection of tracheal or bronchial malignancies. The technique avoided longitudinal tension and anastomosis stenosis and preserved the lung tissue intact.
Indication 3
Krishna Khargi et al. performed lobectomy with bronchial wedge resection in eight patients with lung malignancies involving the main bronchus, including four right upper lobectomies, two left upper lobectomies, and two left lower lobectomies. Postoperative histopathological results revealed seven cases of squamous cell carcinoma and one case of carcinoid. They believe it is feasible to remove one-third to one-half of the circumference of the main bronchus in the wedge resection (1). However, three patients experienced varying degrees of bronchial stenosis after the operation. Therefore, Florian Augustin et al. suggested that the maximum distance between the upper and lower edges of the bronchus in wedge resection should be less than the transverse diameter of the bronchus, which is more conducive to avoiding anastomotic stenosis (14).
In 16 patients with right lung malignancies, Christophoros Kotoulas et al. performed 12 right upper lobectomies and four right upper and middle lobectomies combined with main bronchial wedge resection. They dissected the inferior pulmonary ligament and released the hilum, allowing the trachea and main bronchi to move 1–2 cm (6). None of the 16 patients had anastomotic stenosis and distortion after surgery, and the long-term prognosis was satisfactory.
Although Krishna Khargi et al. thought that local tumor infiltration of the cranial and the caudal parts of adjoining main bronchus was the indication for wedge bronchoplasty (1), the use of wedge resection has many limitations. First, although the wedge bronchoplasty can be performed on either lobe, the right upper lobe is more suitable for anatomical reasons (6, 18) (Figure 4). In addition, the mobilization of the trachea and main bronchus, the limitation of resection range of main bronchus, and the determination of resection margin are all necessary to ensure the safety of wedge bronchoplasty.
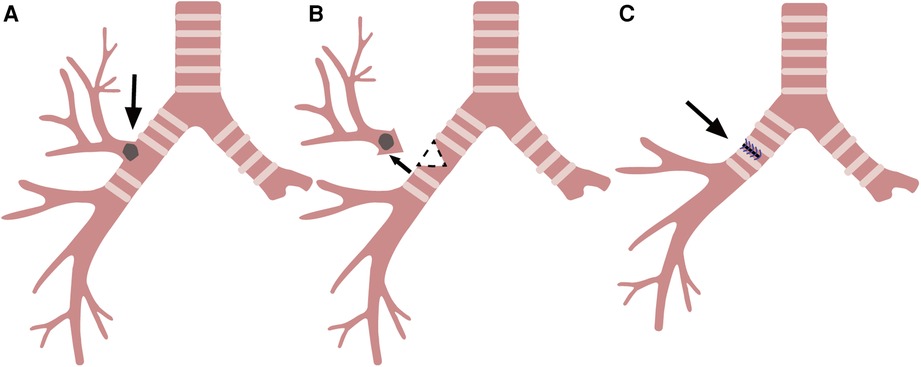
Figure 4. Right upper lobectomy with wedge resection and reconstruction of the bronchus. (A) Right upper lung malignancy involving the main bronchus (arrow). (B) Resection of the right superior lobar bronchus along the dotted line (arrow). (C) Right main bronchus wall after suture (arrow).
Similarly, for benign tracheal or bronchial tumors, we recommend lobectomy or segmentectomy with bronchial wedge resection rather than sleeve resection if the obstruction of the bronchus has resulted in irreversible destruction of the lung tissue and lobectomy or segmentectomy alone cannot resolve the problem. For example, Azevedo-Pereira AE (19), Galvez C (20), and Maeda M (21) used lobectomy or segmentectomy with wedge bronchoplasty for the treatment of bronchial glomus tumor, bronchial lipomas, and bronchial inflammatory pseudotumors, respectively.
These demonstrate that tracheal or bronchial wedge resection is a feasible and excellent technique when the indications for wedge resection are understood, particularly for some benign and malignant tumors with guaranteed margins. The indications for wedge resection include (1) Tumors with a base diameter smaller than the diameter of trachea or bronchus. (2) Tumors that are confined to the carina or bronchial corner. (3) Local tumor infiltration in the cranial and the caudal parts of adjoining main bronchus.
In addition, wedge resection requires the dissection of the inferior pulmonary ligament, the hilum release, the dissociation of intrapericardial pulmonary vein attachments, the mobilization of the trachea and main bronchus, the limitation of resection range, the determination of resection margin, and suturing from low tension area to high tension area. These are effective measures for preventing anastomotic stenosis and ensuring the safety of wedge resection.
Compared with sleeve resection, wedge resection preserves the continuity and blood supply of the trachea or bronchus (22, 23). It significantly reduces the difficulty and trauma of surgery and is easier to be performed under thoracoscopy without conversion to thoracotomy (22). It also reduces the incidence of postoperative complications such as bronchopleural fistula (24). For benign and low-grade malignant tumors of the trachea or bronchus, the preferred surgical approach should be video-assisted thoracoscopic wedge resection. For tumors such as non-small cell lung cancer, Park et al. found that wedge bronchoplastic lobectomy should be an appropriate alternative to sleeve lobectomy regardless of lymph node status (23).
However, we found that there is a debate about which technique is more likely to cause postoperative anastomotic complications. Although Krüger et al. believe that sleeve resection is more prone to result in anastomotic complications and pneumonia (24), many believe that wedge resection is more prone to result in various degrees of anastomotic stenosis (1, 2). Anastomotic stenosis can cause postoperative complications such as secretion retention, pneumonia, atelectasis, respiratory distress, and complete anastomotic obstruction (1–8, 14, 22, 23). It may result in the patient requiring bronchoscopic toileting or mechanical ventilation support after surgery (1, 23). When stricture is severe, a second operation is required to perform sleeve resection to relieve the anastomotic stenosis (6). However, according to our references, anastomotic stenosis after wedge resection is more of a technical problem. When the indications and precautions of wedge resection are strictly grasped, the probability of anastomotic stenosis after wedge resection is no greater than after sleeve resection.
In conclusion, we believe that tracheal or bronchial wedge resection is a significantly superior technique under the appropriate conditions. Video-assisted thoracoscopic wedge resection of trachea or bronchus should be a new and excellent development direction of minimally invasive bronchial surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZHJ drafted and edited this manuscript, assisted in the surgery, and analyzed patient data. JY and ZT performed the surgery, edited this manuscript, and analyzed patient data. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1122075/full#supplementary-material.
References
1. Khargi K, Duurkens VA, Versteegh MM, Huysmans HA, Quanjer PH, Verzijlbergen FF, et al. Pulmonary function and postoperative complications after wedge and flap reconstructions of the main bronchus. J Thorac Cardiovasc Surg. (1996) 112(1):117–23. doi: 10.1016/S0022-5223(96)70185-X
2. Toledo J, Roca R, Antón JA, Martin de Nicolás JL, Varela G, Yuste P. Surgery in bronchial carcinoids: experience with 83 patients. Eur J Cardiothorac Surg. (2002) 21(5):883–7. doi: 10.1016/S1010-7940(02)00089-1
3. Kurul IC, Topçu S, Taştepe I, Yazici U, Altinok T, Cetin G. Sleeve and wedge parenchyma-sparing bronchial resections in low-grade neoplasms of the bronchial airway. J Thorac Cardiovasc Surg. (2007) 134(2):373–7. doi: 10.1016/j.jtcvs.2007.03.020
4. Rahouma M, Kamel M, Narula N, Nasar A, Harrison S, Lee B, et al. Role of wedge resection in bronchial carcinoid (BC) tumors: SEER database analysis. J Thorac Dis. (2019) 11(4):1355–62. doi: 10.21037/jtd.2019.03.89
5. Stamatis G, Freitag L, Greschuchna D. Limited and radical resection for tracheal and bronchopulmonary carcinoid tumour. Report on 227 cases. Eur J Cardiothorac Surg. (1990) 4(10):527–32; discussion 533. doi: 10.1016/1010-7940(90)90140-U
6. Kotoulas C, Lazopoulos G, Foroulis C, Konstantinou M, Tomos P, Lioulias A. Wedge resection of the bronchus: an alternative bronchoplastic technique for preservation of lung tissue. Eur J Cardiothorac Surg. (2001) 20(4):679–83. doi: 10.1016/S1010-7940(01)00889-2
7. Jr HD, Feldman JM, Buchanan S, Young WG, Wolfe WG. Bronchial carcinoid tumors: a retrospective analysis of 126 patients. Ann Thorac Surg. (1992) 54(1):50–4; discussion 54–5. doi: 10.1016/0003-4975(92)91139-Z
8. Bueno R, Wain JC, Wright CD, Moncure AC, Grillo HC, Mathisen DJ. Bronchoplasty in the management of low-grade airway neoplasms and benign bronchial stenoses. Ann Thorac Surg. (1996) 62(3):824–8; discussion 828–9. doi: 10.1016/S0003-4975(96)00453-5
9. Al Kindi AH, Al Ibrahim MT, Al Marhoon MM, Al Kindi FA, Salem A, Al Ajmi R. Bronchial leiomyoma: importance of preoperative biopsy for lung preserving surgery. Oman Med J. (2022) 37(6):e445. doi: 10.5001/omj.2023.02
10. Yamada H, Katoh O, Yamaguchi T, Natsuaki M, Itoh T. Intrabronchial leiomyoma treated by localized resection via bronchotomy and bronchoplasty. Chest. (1987) 91(2):283–5. doi: 10.1378/chest.91.2.283
11. Tomos P, Karaiskos T, Lahanas E, Paulopoulos D, Papahristou D, Stauroulias A, et al. Transverse bronchoplasty of the membranous wall after resection of an endobronchial hamartoma. Ann Thorac Surg. (2007) 83(2):703–4. doi: 10.1016/j.athoracsur.2006.03.113
12. Kawano O, Yuki D, Fukai I, Tsubota N. Successful treatment of mucoepidermoid carcinoma in the left main bronchus. Surg Case Rep. (2015) 1:85. doi: 10.1186/s40792-015-0087-4
13. Kamiyoshihara M, Ibe T, Takeyoshi I. Pleomorphic adenoma of the main bronchus in an adult treated using a wedge bronchiectomy. Gen Thorac Cardiovasc Surg. (2009) 57(1):43–5. doi: 10.1007/s11748-008-0319-7
14. Augustin F, Maier H, Lucciarini P, Bodner J, Klotzner S, Schmid T. Extended minimally invasive lung resections: VATS bilobectomy, bronchoplasty, and pneumonectomy. Langenbecks Arch Surg. (2016) 401(3):341–8. doi: 10.1007/s00423-015-1345-4
15. Xie D, Ding J, Zhou X, Fei K, You X, Jiang G. Simplified carinal wedge resection and reconstruction. Ann Thorac Surg. (2014) 98(2):731–3. doi: 10.1016/j.athoracsur.2013.12.055
16. Yamamoto H, Toyooka S, Miyoshi S. Wedge resection of the bronchial corner at the bifurcation of the lobar bronchi for a low-grade bronchial tumor. Thorac Cardiovasc Surg. (2014) 62(2):181–3. doi: 10.1055/s-0032-1330924
17. Wang A, Chen X, Huang D, Wang S. Deeper wedge resection and parenchymal-sparing bronchoplasty of the secondary carina: an alternative surgical technique for removal of tumor located at the orifice of upper lobar bronchus. J Cardiothorac Surg. (2017) 12(1):51. doi: 10.1186/s13019-017-0610-8
18. Massard G, Kessler R, Gasser B, Ducrocq X, Elia S, Gouzou S, et al. Local control of disease and survival following bronchoplastic lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg. (1999) 16(3):276–82. doi: 10.1016/S1010-7940(99)00233-X
19. Azevedo-Pereira AE, Rigueiro MP, Abrão FC. Bronchial glomus tumor with right upper lobe atelectasis. J Bras Pneumol. (2010) 36(3):390–3. English, Portuguese. doi: 10.1590/S1806-37132010000300018
20. Galvez C, Sesma J, Bolufer S, Lirio F, Navarro-Martinez J, Galiana M, et al. Single-incision video-assisted anatomical segmentectomy with handsewn bronchial closure for endobronchial lipoma. Ann Transl Med. (2016) 4(15):284. doi: 10.21037/atm.2016.07.25
21. Maeda M, Matsuzaki Y, Edagawa M, Shimizu T, Onitsuka T, Kataoka H. Successful treatment of a bronchial inflammatory pseudotumor by bronchoplasty in an 8-year-old boy: report of a case. Surg Today. (2000) 30(5):465–8. doi: 10.1007/s005950050627
22. Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg. (2013) 44(4):616–23. doi: 10.1093/ejcts/ezt166
23. Park SY, Lee HS, Jang HJ, Joo J, Kim MS, Lee JM, et al. Wedge bronchoplastic lobectomy for non-small cell lung cancer as an alternative to sleeve lobectomy. J Thorac Cardiovasc Surg. (2012) 143(4):825–31.e3. doi: 10.1016/j.jtcvs.2011.10.057
Keywords: tracheal or bronchial tumor, tracheal or bronchial wedge resection, videoassisted thoracoscopic surgery, parenchymal sparing procedure, case report
Citation: Jiao Z, Tang Z and Yu J (2023) Tracheal or bronchial wedge resection: Case report. Front. Surg. 10:1122075. doi: 10.3389/fsurg.2023.1122075
Received: 12 December 2022; Accepted: 24 January 2023;
Published: 14 February 2023.
Edited by:
Yojiro Yutaka, Kyoto University, JapanReviewed by:
Jiaxi He, First Affiliated Hospital of Guangzhou Medical University, ChinaYasuto Sakaguchi, Osaka Red Cross Hospital, Japan
Tatsuya Imabayashi, National Cancer Centre (Japan), Japan
© 2023 Jiao, Tang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Yu MjcwMzg0NTY4QHFxLmNvbQ==
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Zhenhua Jiao
Zhenhua Jiao Zhe Tang
Zhe Tang