- 1Department of Anesthesiology, Shanxian Central Hospital, Heze, China
- 2Department of Anesthesiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
Objectives: To summarize the risk factors, onset time, and treatment of vasoplegic syndrome in patients undergoing heart transplantation.
Methods: The PubMed, OVID, CNKI, VIP, and WANFANG databases were searched using the terms “vasoplegic syndrome,” “vasoplegia,” “vasodilatory shock,” and “heart transplant*,” to identify eligible studies. Data on patient characteristics, vasoplegic syndrome manifestation, perioperative management, and clinical outcomes were extracted and analyzed.
Results: Nine studies enrolling 12 patients (aged from 7 to 69 years) were included. Nine (75%) patients had nonischemic cardiomyopathy, and three (25%) patients had ischemic cardiomyopathy. The onset time of vasoplegic syndrome varied from intraoperatively to 2 weeks postoperatively. Nine (75%) patients developed various complications. All patients were insensitive to vasoactive agents.
Conclusions: Vasoplegic syndrome can occur at any time during the perioperative period of heart tranplantation, especially after the discontinuation of bypass. Methylene blue, angiotensin II, ascorbic acid, and hydroxocobalamin have been used to treat refractory vasoplegic syndrome.
Introduction
Vasoplegic syndrome (VS) is a common life-threatening complication characterized by severe and persistent systemic arterial hypotension (mean arterial pressure, <50 mmHg), normal or slightly increased cardiac output (cardiac index, >2.5 L/min/m2), low systemic vascular resistance (SVR, <800 dyne/s/cm5), and insensitivity to appropriate fluid resuscitation and high-dose vasopressors (1). VS occurs in up to 34.8% of patients who undergo heart transplantation (HTX) (2). The incidence of VS is higher in patients who underwent HTX compared to other forms of cardiac surgery, e.g., off-pump coronary artery bypass graft (CABG) (2.8%) (3), on-pump CABG (6.9%–26%) (3, 4), and aortic valve replacement (AVR) (20%) (5). Earlier research (6) showed that the incidence of VS is as high as 45% in patients with a ventricular assist device (VAD) at the time of HTX. Chemmalakuzhy et al. (7) observed increased risk for early mortality among HTX recipients with VS, with a 30-day mortality rate of 33%. This study aimed to summarize the risk factors, onset time, and treatment of VS in patients undergoing HTX.
Materials and methods
Search strategy
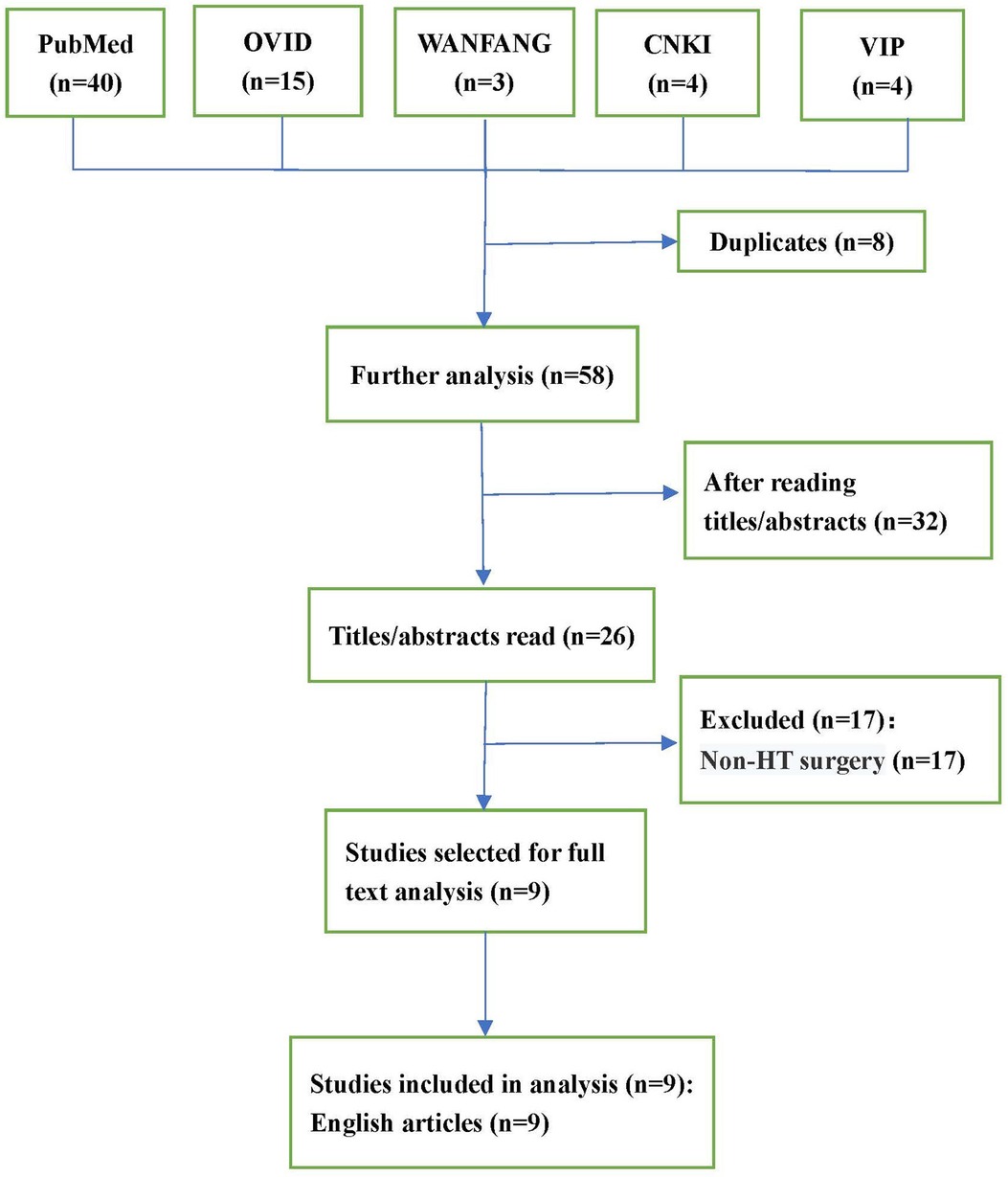
Relevant case reports were searched using the PubMed and OVID electronic databases from inception until January 14, 2022. Chinese literatures from the CNKI, VIP, and WANFANG databases were also searched. Different combinations of terms that included “vasoplegic syndrome,” “vasoplegia,” “vasodilatory shock,” and “heart transplant*” were used in the search strategy. All relevant case reports were included. The exclusion criteria were as follows: (a) non-English and non-Chinese studies; (b) studies based on animal models; and (c) duplicate publications. Each author independently read the titles and abstracts of all the identified reports for eligibility, excluding ineligible reports. The eligibility of the remaining reports for final inclusion was determined by examining the full-text versions of the publications.
Data abstraction
Data of interest from the included case reports were abstracted and tabulated by each author independently: (a) author, year, and journal of publication; (b) total number of patients, age, sex, medical history, number of thoracotomy surgeries, bleeding and coagulopathy or not, postoperative transesophageal echocardiography, treatment of ventricular dysfunction (VD), and complications; (c) onset time, clinical manifestation, and treatment of VS. Disagreements were resolved by discussion between both authors during the process of data abstraction.
Results
As depicted in the flowchart (Figure 1), the database search identified 26 potentially eligible studies. Nine case reports (8–16) describing 12 patients in total were deemed eligible and included. All case reports were written in English. A descriptive analysis of these cases is presented in Table 1. The 12 patients were aged 7–69 years, and included 9 males (75%) and 3 females (25%). Nine (75%) patients (8–11, 13–16) had nonischemic cardiomyopathy, and three (25%) patients (12, 13) had ischemic cardiomyopathy. Eight (66%) patients (10, 11, 13, 15, 16) had undergone preoperative thoracotomy, such as CABG, AVR, VAD, and Fontan operations. Five (63%) patients experienced intraoperative bleeding and coagulopathy intraoperatively due to the formation of dense adhesions between the mediastinum and pericardium. Six (50%) patients (8, 11–14, 16) used a variety of drugs before surgery, including angiotensin-converting enzyme inhibitors (ACEI), angiotensin II (ANG-II) receptor blockers (ARB), diuretics, β-blockers, and milrinone. After the discontinuation of bypass, eight (66%) patients (9, 12–14, 16) developed ventricular dysfunction, and were treated with milrinone, dobutamine, epinephrine, or norepinephrine. Other treatments include the inhalation of nitric oxide or epoprostenol, restarting cardiopulmonary bypass (CPB), and intra-aortic balloon counter-pulsation or extracorporeal membrane oxygenator.
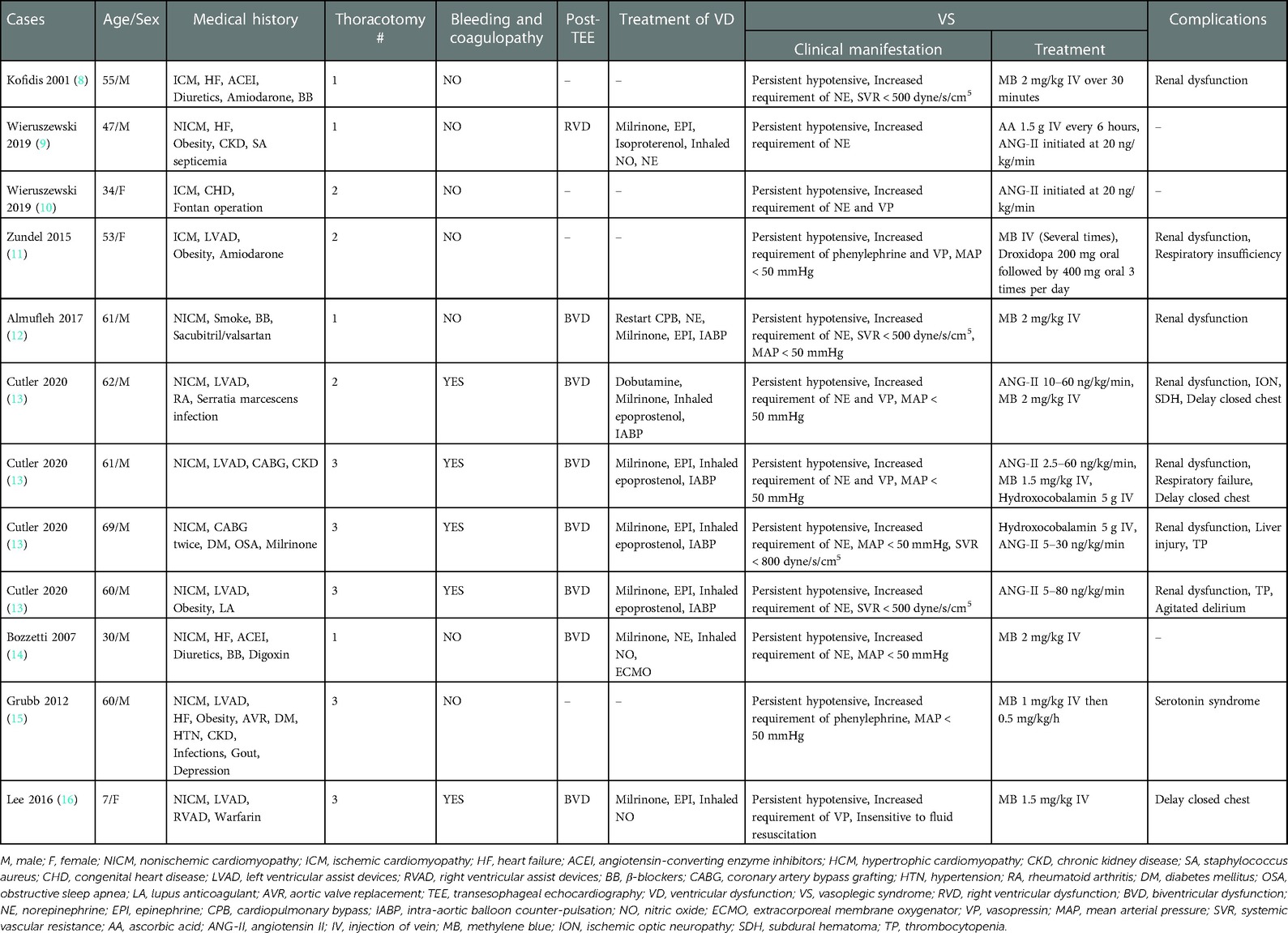
Table 1. General condition and characteristics of patients experienced vasoplegic syndrome undergoing heart transplantation.
Of the 12 patients, 9 (75%) developed various complications, with 7 (58%) patients having developed some degree of renal dysfunction, respiratory insufficiency, ischemic optic neuropathy, subdural hematoma, thrombocytopenia, liver injury, agitated delirium, serotonin syndrome, or delayed chest closure. Most patients were discharged, but one patient (11) died of multiple organ failure.
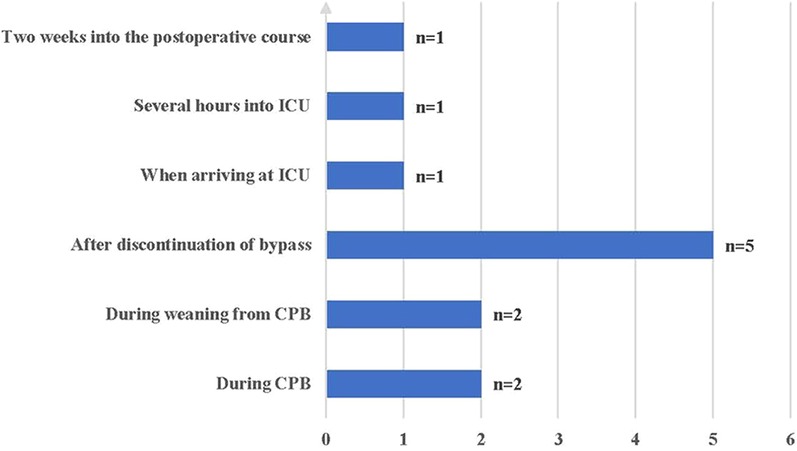
The time to onset of VS ranged from during CPB to 2 weeks postoperatively; nine (75%) patients experienced VS intraoperatively, and three (25%) patients experienced VS postoperatively (Figure 2). All patients were insensitive to vasoactive agents, developed persistent hypotension, and were subsequently administered methylene blue (MB), hydroxocobalamin, ascorbic acid (AA), and ANG-II.
Discussion
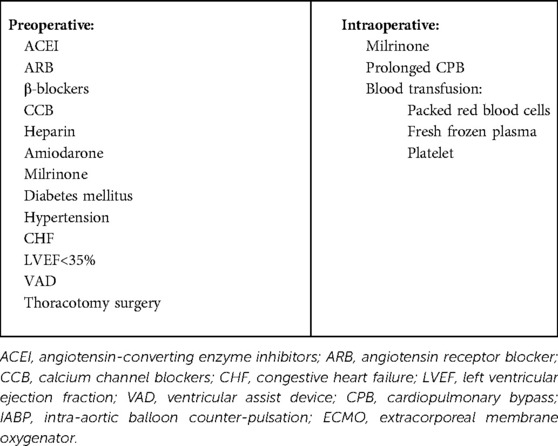
Several risk factors for VS have been identified, including ACEI, β-blockers, calcium channel blockers, heparin, amiodarone, diabetes mellitus, prolonged CPB, congestive heart failure, and left ventricular ejection fraction <35% (17, 18). The preoperative use of VAD in adults is an independent risk factor for VS (6). In this study, six (50%) (11, 13, 15, 16) patients had used LVAD before surgery. Of the 12 patients, 8 patients (10, 11, 13, 15, 16) had undergone previous thoracotomy. This easily led to dense adhesions between the mediastinum and pericardium, resulting in severe bleeding and coagulation disorders, requiring a large number of blood products and factor replacement. Administration of blood products activates pro-inflammatory mediators during surgery (18). Packed red blood cells, fresh frozen plasma, and platelet transfusion increase the prevalence of VS (19). In addition, packed red blood cell transfusion exhibited a dose-dependent increase in the development of VS with each packed red blood cell unit transfused (19).
Milrinone is a powerful inotropic agent commonly used for right ventricular dysfunction, and may exacerbate systemic vasoplegia (20). Of the 12 patients, 8 (9, 12–14, 16) used milrinone pre- or intraoperatively. A meta-analysis (21) revealed that 38% of patients with New York Heart Association class III heart failure symptoms and 42% of those with class IV symptoms experienced depression. Depression not only increases the incidence of hypertension, coronary heart disease, and diabetes, but also causes chronic inflammation (22, 23). The mechanism of VS is largely unknown, and study results suggest that VS is correlated with the release of cytokines, such as tumor necrosis factor (TNF) and interleukin-1, which increase nitric oxide (NO) production, resulting in marked relaxation of the vascular smooth muscles (24). Therefore, the chronic inflammatory state of patients before surgery may be a risk factor for VS. Other chronic inflammation diseases include obesity, obstructive sleep apnea, chronic kidney disease, and smoke (25–28). Eight (67%) patients (9, 11–13, 15) had at least one of these medical histories. The risk factors for VS in the patients undergoing HTX are summarized in Table 2.
Of the 12 patients, 9 (75%) experienced VS intraoperatively, including 4 patients before weaning from CPB and five after discontinuation of CPB. The other three patients had VS after arriving at the intensive care unit, and one developed VS 2 weeks post- operatively. Septic shock is considered more likely than VS 2 weeks after surgery. Therefore, the possibility of infection must be ruled out, especially infections of the chest, abdomen, genitourinary system, and bloodstream, which account for >80% of sepsis cases (29–31).
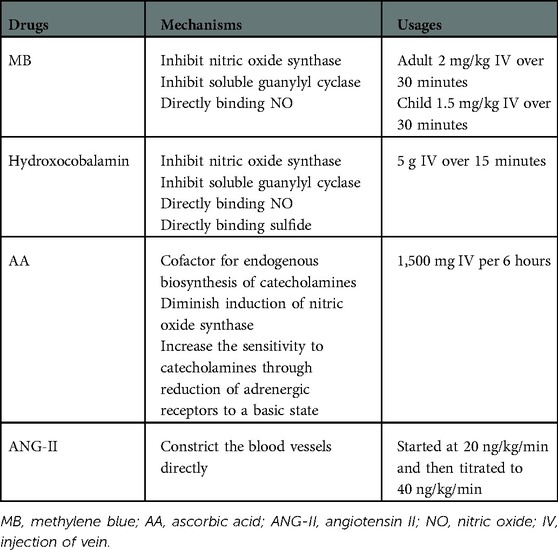
When VS occurs, catecholamines and vasopressin should be used at first. However, high-dose catecholamines may lead to tissue hypoperfusion and myocardial ischemia. Furthermore, prolonged hypotension may have adverse consequences, such as gradual deterioration of ventricular function and decreased urine output. At present, four drugs are used to treat refractory VS (Table 3). MB and hydroxocobalamin increase SVR by inhibiting NO synthase and reducing NO production, inhibiting the activation of soluble guanylyl cyclase, and binding to NO directly (32–35). Of the 12 patients, four were treated with at least two of these drugs. The combination of MB and hydroxocobalamin may be more beneficial than that of MB alone (36, 37). One study (38) found that MB reduced the duration of VS and mortality. However, a potentially lethal complication of MB is serotonin syndrome, especially in patients taking serotonergic antidepressants. Fentanyl is the most commonly used narcotic analgesics, which reduces serotonin reabsorption; therefore, it should be used cautiously when fentanyl was used during surgery. Hydroxocobalamin, an injectable form of vitamin B12, interferes with dialysis treatment owing to an alarm of blood leak, which can be overcome by continuous renal replacement therapy (39). AA is an essential cofactor for the endogenous biosynthesis of catecholamines, which cannot be synthesized by humans, and the concentration of AA in patients undergoing cardiac surgery after CPB is low (40–42). One study (43) found that the utilization of vasopressors was reduced when high-dose AA was administered for the treatment of VS after CPB. However, it should be noted that MB and AA cannot be used in patients with glucose-6-phosphate dehydrogenase deficiency to avoid hemolytic anemia. Prolonged exposure to CPB impairs the pulmonary capillary endothelium, thereby limiting the activity of angiotensin-converting enzyme (44). ANG- II acts directly on blood vessel walls, resulting in vasoconstriction, increased mean arterial pressure antidiuretic hormone secretion, adrenal cortex stimulation, and increased water reabsorption (44, 45). The adverse effects of ANG- II include thromboembolic events, hypoperfusion from vasoconstrictive actions, and increased pulmonary vascular resistance (46, 47). VS treatment of during the perioperative period of HTX is shown in Figure 3.
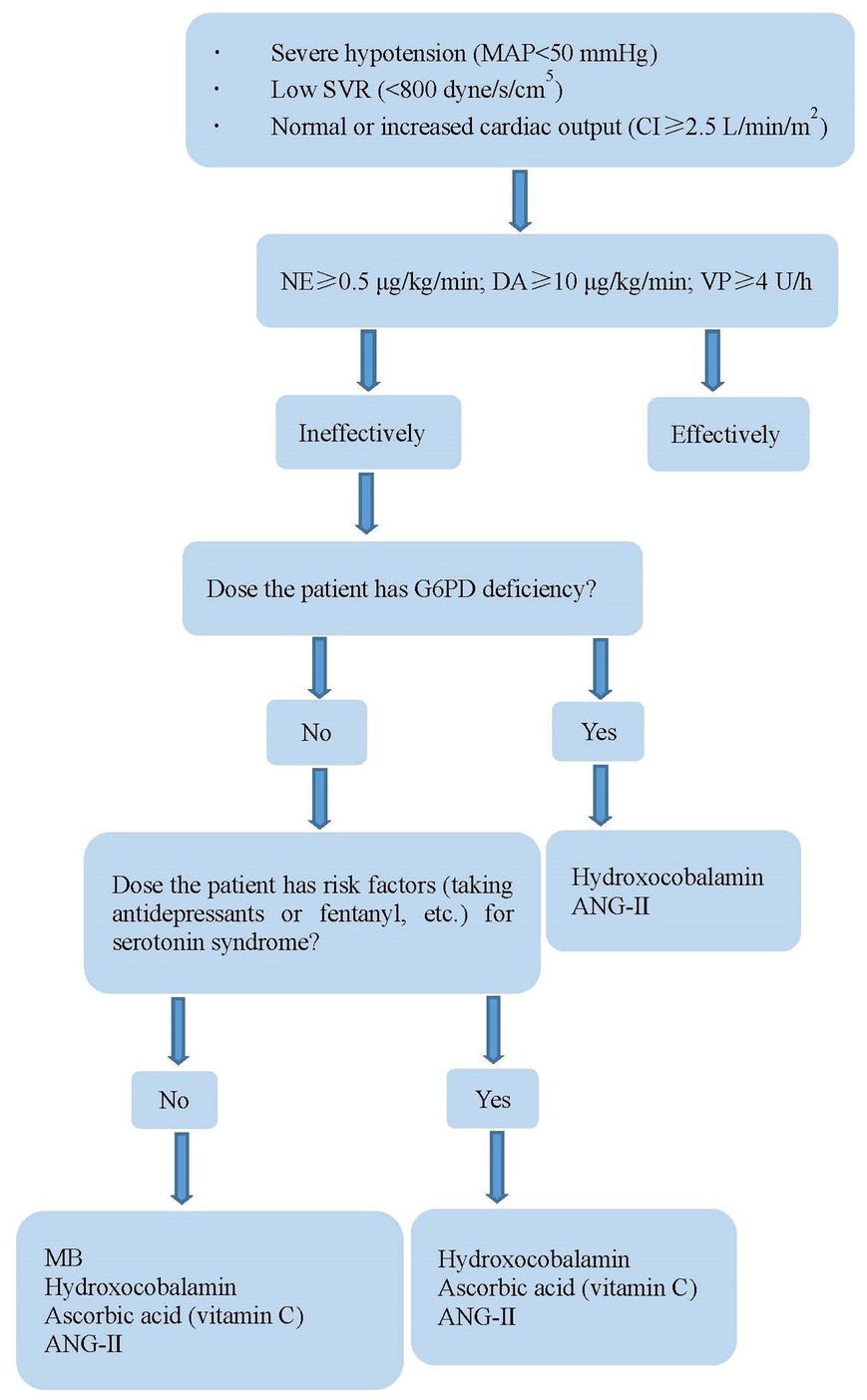
Figure 3. Treatment of vasoplegia during perioperative period of HTX. MAP, mean arterial pressure; NE, norepinephrine; DA, dopamine; VP, vasopressin; SVR, systemic vascular resistance; CI, cardiac index; G6PD, glucose-6-phosphate dehydrogenase; MB, methylene blue; AA, ascorbic acid; ANG-II, angiotensin II.
In addition to the abovementioned four drugs, induced mild hypothermia may be a useful treatment for VS. Earlier studies (48) showed that hypothermia decreases the release of cytokines. Furthermore, mild hypothermia effectively restored SVR and blood pressure within 4 h without adverse effects on pulmonary pressure (49), and improved the response to epinephrine (50) and norepinephrine (51). Therefore, it may be an excellent prevention and treatment method for VS by avoiding active rewarming after the operation and letting the patient gradually and spontaneously reach normothermia or maintain a 33°C–35°C corporeal temperature for the first 24 h after HTX. However, hypothermia can induce problems, such as cardiac arrhythmia and coagulopathy. Further research is necessary to determine the safety of mild hypothermia for the treatment of VS.
In-hospital mortality was more than 2.5-fold higher in patients with (25%) than in patients without VS (52). Therefore, the prevention of VS is crucial for patients undergoing HTX. Ozal et al. (4) reported that those who received preoperative MB had significantly higher postoperative SVR and MAP, and a significantly shorter mean length of stay in intensive care units. A randomized, double-blind, controlled trial showed that tranexamic acid attenuates the development of VS after CPB by blocking fibrinolysis (53). Further research should prioritize the mechanism and prevention measures for VS in patients undergoing HTX.
In summary, several risk factors for VS exist in patients undergoing HTX, including the chronic inflammatory exhibited by some patients before surgery. VS can occur at any time during the perioperative period in patients who underwent HTX, especially after the discontinuation of bypass. MB, ANG- II, hydroxocobalamin, and AA have been used to treat refractory VS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Both authors were involved in the analysis and interpretation of the data. YtY designed the research study. TxQ wrote the initial draft of the manuscript. Both authors revised the manuscript and approved the final version.
Funding
This work was supported by the Youth Teacher Training Program of Peking Union Medical College (2014zlgc07) and CAMS Innovation Fund for Medical Sciences (CIFMS)-2021-I2M-C&T-B-038.
Acknowledgments
The authors are grateful to the editor and reviewers for their suggestion and help. And we would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fischer GW, Levin MA. Vasoplegia during cardiac surgery: current concepts and management. Semin Thorac Cardiovasc Surg. (2010) 22:140–4. doi: 10.1053/j.semtcvs.2010.09.007
2. Chan JL, Kobashigawa JA, Aintablian TL, Dimbil SJ, Perry PA, Patel JK, et al. Characterizing predictors and severity of vasoplegia syndrome after heart transplantation. Ann Thorac Surg. (2018) 105:770–7. doi: 10.1016/j.athoracsur.2017.09.039
3. Sun X, Zhang L, Hill PC, Lowery R, Lee AT, Molyneaux RE, et al. Is incidence of postoperative vasoplegic syndrome different between off-pump and on-pump coronary artery bypass grafting surgery? Eur J Cardiothorac Surg. (2008) 34:820–5. doi: 10.1016/j.ejcts.2008.07.012
4. Ozal E, Kuralay E, Yildirim V, Kilic S, Bolcal C, Kücükarslan N, et al. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg. (2005) 79:1615–9. doi: 10.1016/j.athoracsur.2004.10.038
5. Kerbaul F, Collart F, Giorgi R, Ibrahim Z, Guillen JC, Gil JM, et al. Role of endogenous adenosine as a predictive marker of vasoplegia during cardiopulmonary bypass and postoperative severe systemic inflammatory response. Crit Care Med. (2006) 34:640–5. doi: 10.1097/01.CCM.0000201005.34203.50
6. Patarroyo M, Simbaqueba C, Shrestha K, Starling RC, Smedira N, Tang WH, et al. Preoperative risk factors and clinical outcomes associated with vasoplegia in recipients of orthotopic heart transplantation in the contemporary era. J Heart Lung Transplant. (2012) 31:282–7. doi: 10.1016/j.healun.2011.10.010
7. Chemmalakuzhy J, Costanzo MR, Meyer P, Piccione W, Kao W, Winkel E, et al. Hypotension, acidosis, and vasodilatation syndrome post-heart transplant: prognostic variables and outcomes. J Heart Lung Transplant. (2001) 20:1075–83. doi: 10.1016/s1053-2498(01)00299-6
8. Kofidis T, Strüber M, Wilhelmi M, Anssar M, Simon A, Harringer W, et al. Reversal of severe vasoplegia with single-dose methylene blue after heart transplantation. J Thorac Cardiovasc Surg. (2001) 122:823–4. doi: 10.1067/mtc.2001.115153
9. Wieruszewski PM, Radosevich MA, Kashani KB, Daly RC, Wittwer ED. Synthetic human angiotensin II for postcardiopulmonary bypass vasoplegic shock. J Cardiothorac Vasc Anesth. (2019) 33:3080–4. doi: 10.1053/j.jvca.2019.03.004
10. Wieruszewski PM, Sims CR, Daly RC, Taner T, Wittwer ED. Use of angiotensin II for vasoplegic shock in a combined heart and liver transplant recipient with systolic anterior motion physiology. J Cardiothorac Vasc Anesth. (2019) 33:2366–7. doi: 10.1053/j.jvca.2019.03.054
11. Zundel MT, Boettcher BT, Feih JT, Gaglianello N, Pagel PS. Use of oral droxidopa to improve arterial pressure and reduce vasoactive drug requirements during persistent vasoplegic syndrome after cardiac transplantation. J Cardiothorac Vasc Anesth. (2016) 30:1624–6. doi: 10.1053/j.jvca.2015.12.013
12. Almufleh A, Mielniczuk LM, Zinoviev R, Moeller A, Davies RA, Stadnick E, et al. Profound vasoplegia during sacubitril/valsartan treatment after heart transplantation. Can J Cardiol. (2018) 34:5–7. doi: 10.1016/j.cjca.2017.12.004
13. Cutler NS, Rasmussen BM, Bredeck JF, Lata AL, Khanna AK. Angiotensin II for critically ill patients with shock after heart transplant. J Cardiothorac Vasc Anesth. (2021) 35:2756–62. doi: 10.1053/j.jvca.2020.07.087
14. Bozzetti G, Ranucci M, Grillone G. Concomitant pulmonary hypertension and vasoplegia syndrome after heart transplant: a challenging picture. J Cardiothorac Vasc Anesth. (2008) 22:868–71. doi: 10.1053/j.jvca.2007.11.003
15. Grubb KJ, Kennedy JL, Bergin JD, Groves DS, Kern JA. The role of methylene blue in serotonin syndrome following cardiac transplantation: a case report and review of the literature. J Thorac Cardiovasc Surg. (2012) 144:113–6. doi: 10.1016/j.jtcvs.2012.07.030
16. Lee JK, Ing C. Prothrombin complex concentrate and methylene blue for treatment of coagulopathy and vasoplegia in a pediatric heart transplant patient. A Case Rep. (2016) 6:127–9. doi: 10.1213/XAA.0000000000000271
17. Hosseinian L, Weiner M, Levin MA, Fischer GW. Methylene blue: magic bullet for vasoplegia? Anesth Analg. (2016) 122:194–201. doi: 10.1213/ANE.0000000000001045
18. Omar S, Zedan A, Nugent K. Cardiac vasoplegia syndrome: pathophysiology, risk factors and treatment. Am J Med Sci. (2015) 349:80–8. doi: 10.1097/MAJ.0000000000000341
19. Alfirevic A, Xu M, Johnston D, Figueroa P, Koch CG. Transfusion increases the risk for vasoplegia after cardiac operations. Ann Thorac Surg. (2011) 92:812–9. doi: 10.1016/j.athoracsur.2011.04.020
20. Fischer LG, Aken HV, Bürkle H. Management of pulmonary hypertension: physiological and pharmacological considerations for anesthesiologists. Anesth Analg. (2003) 96:1603–16. doi: 10.1213/01.ANE.0000062523.67426.0B
21. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. (2006) 48:1527–37. doi: 10.1016/j.jacc.2006.06.055
22. Zhang Y, Chen Y, Ma L. Depression and cardiovascular disease in elderly: current understanding. J Clin Neurosci. (2018) 47:1–5. doi: 10.1016/j.jocn.2017.09.022
23. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
24. LinksLehot JJ, Villard J, Piriz H, Philbin DM, Carry PY, Gauquelin G, et al. Hemodynamic and hormonal responses to hypothermic and normothermic cardiopulmonary bypass. J Cardiothorac Vasc Anesth. (1992) 6:132–9. doi: 10.1016/1053-0770(92)90186-b
25. Lauterbach MA, Wunderlich FT. Macrophage function in obesity-induced inflammation and insulin resistance. Pflugers Arch. (2017) 469:385–96. doi: 10.1007/s00424-017-1955-5
26. Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. (2013) 9:1003–12. doi: 10.5664/jcsm.3070
27. Mazzaferro S, De Martini N, Rotondi S, Tartaglione L, Ureña-Torres P, Bover J, et al. Bone, inflammation and chronic kidney disease. Clin Chim Acta. (2020) 506:236–40. doi: 10.1016/j.cca.2020.03.040
28. Glynos C, Bibli SI, Katsaounou P, Pavlidou A, Magkou C, Karavana V, et al. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am J Physiol Lung Cell Mol Physiol. (2018) 315:662–72. doi: 10.1152/ajplung.00389.2017
29. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. (2003) 348:1546–54. doi: 10.1056/NEJMoa022139
30. Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-rea network. Am J Respir Crit Care Med. (2003) 1687:165–72. doi: 10.1164/rccm.2201087
31. Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. (2002) 28:108–21. doi: 10.1007/s00134-001-1143-z
32. Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of methylene blue in the nervous system. Med Res Rev. (2011) 31:93–117. doi: 10.1002/med.20177
33. Lenglet S, Mach F, Montecucco F. Methylene blue: potential use of an antique molecule in vasoplegic syndrome during cardiac surgery. Expert Rev Cardiovasc Ther. (2011) 9:1519–25. doi: 10.1586/erc.11.160
34. Gerth K, Ehring T, Braendle M, Schelling P. Nitric oxide scavenging by hydroxocobalamin may account for its hemodynamic profile. Clin Toxicol. (2006) 44:29–36. doi: 10.1080/15563650600811805
35. Weinberg JB, Chen Y, Jiang N, Beasley BE, Salerno JC, et al. Inhibition of nitric oxide synthase by cobalamins and cobinamides. Free Radic Biol Med. (2009) 46:1626–32. doi: 10.1016/j.freeradbiomed.2009.03.017
36. Shah PR, Reynolds PS, Pal N, Tang D, McCarthy H, Spiess BD. Hydroxocobalamin for the treatment of cardiac surgery-associated vasoplegia: a case series. Can J Anaesth. (2018) 65:560–8. doi: 10.1007/s12630-017-1029-3
37. Feih J, Rinka J, Zundel M. Methylene blue monotherapy compared with combination therapy with hydroxocobalamin for the treatment of refractory vasoplegic syndrome: a retrospective cohort study. J Cardiothorac Vasc Anesth. (2019) 33:1301–7. doi: 10.1053/j.jvca.2018.11.020
38. Levin RL, Degrange MA, Bruno GF, Del Mazo CD, Taborda DJ, Griotti JJ, et al. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg. (2004) 77:496–9. doi: 10.1016/S0003-4975(03)01510-8
39. Cheungpasitporn W, Hui J, Kashani KB, Wittwer ED, Albright RC Jr, Dillon JJ. High-dose hydroxocobalamin for vasoplegic syndrome causing false blood leak alarm. Clin Kidney J. (2017) 10:357–62. doi: 10.1093/ckj/sfx004
40. Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbatedependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care. (2015) 19:418. doi: 10.1186/s13054-015-1131-2
41. Berger MM, Oudemans-van Straaten HM. Vitamin C supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care. (2015) 18:193–201. doi: 10.1097/MCO.0000000000000148
42. Rodemeister S, Duquesne M, Adolph M, Nohr D, Biesalski HK, Unertl K. Massive and long-lasting decrease in vitamin C plasma levels as a consequence of extracorporeal circulation. Nutrition. (2014) 30:673–8. doi: 10.1016/j.nut.2013.10.026
43. Wieruszewski PM, Nei SD, Maltais S, Schaff HV, Wittwer ED. Vitamin C for vasoplegia after cardiopulmonary bypass: a case series. A Pract. (2018) 11:96–9. doi: 10.1213/XAA.0000000000000752
44. Hall A, Busse LW, Ostermann M. Angiotensin in critical care. Crit Care. (2018) 22:69. doi: 10.1186/s13054-018-1995-z
45. Bissell BD, Browder K, McKenzie M, Flannery AH. A blast from the past: revival of angiotensin II for vasodilatory shock. Ann Pharmacother. (2018) 52:920–7. doi: 10.1177/1060028018767899
46. Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. (2017) 377:419–30. doi: 10.1056/NEJMoa1704154
47. Bauer SR, Sacha GL, Lam SW. Safe use of vasopressin and angiotensin II for patients with circulatory shock. Pharmacotherapy. (2018) 38:851–61. doi: 10.1002/phar.2147
48. Menasché P, Haydar S, Peynet J, Du Buit C, Merval R, Bloch G, et al. A potential mechanism of vasodilation after warm heart surgery: the temperature-dependent release of cytokines. J Thorac Cardiovasc Surg. (1994) 107:293–9. PMID: 8283900. doi: 10.1016/S0022-5223(94)70484-8
49. Tripathi M, Singh PK, Kumar N, Pant KC. Induced mild hypothermia in post-cardiopulmonary bypass vasoplegia syndrome. Ann Card Anaesth. (2009) 12:49–52. doi: 10.4103/0971-9784.45013
50. Kondratiev TV, Tveita T. Effects of sympathetic stimulation during cooling on hypothermic as well as posthypothermic hemodynamic function. Can J Physiol Pharmacol. (2006) 84:985–91. doi: 10.1139/y06-051
51. Weiss SJ, Muniz A, Ernst AA, Lippton HL. The physiological response to norepinephrine during hypothermia and rewarming. Resuscitation. (1998) 39:189–95. doi: 10.1016/s0300-9572(98)00137-3
52. Bryne JG, Leacche M, Paul S, Mihaljevic T, Rawn JD, Shernan SK, et al. Risk factors and outcomes for ‘vasoplegia syndrome’ following cardiac transplantation. Eur J Cardiothorac Surg. (2004) 25:327–32. doi: 10.1016/j.ejcts.2003.11.032
53. Jimenez JJ, Iribarren JL, Lorente L, Rodriguez JM, Hernandez D, Nassar I, et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. (2007) 11:117. doi: 10.1186/cc6173
Keywords: vasoplegic syndrome, heart transplant, risk factor, onset time, treatment
Citation: Qin T and Yao Y (2023) Vasoplegic syndrome in patients undergoing heart transplantation. Front. Surg. 10:1114438. doi: 10.3389/fsurg.2023.1114438
Received: 2 December 2022; Accepted: 11 January 2023;
Published: 13 February 2023.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, Switzerland© 2023 Qin, Yao and the Evidence in Cardiovascular Anesthesia(EICA) Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin T-x, Yao Y-t eXVudGFpeWFvQDEyNi5jb20=
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
 Tong-xin Qin
Tong-xin Qin Yun-tai Yao
Yun-tai Yao the Evidence in Cardiovascular Anesthesia(EICA) Group
the Evidence in Cardiovascular Anesthesia(EICA) Group