
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 17 March 2023
Sec. Thoracic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1108699
Background: The aim of this study was to assess the impact of preoperative chemotherapy on long-term survival (≥1 month) in patients with thymic epithelial tumors (TETs) and conditions suitable for chemotherapy using data from surveillance, epidemiology, and end-result databases.
Methods: This retrospective study controlled for confounding factors by propensity score matching (PSM), analyzed overall survival (OS) and cancer-specific survival (CSS) by Kaplan-Meier methods, and analyzed factors affecting the prognosis of patients undergoing surgery for thymic epithelial tumors by univariate and multifactorial Cox regression.
Results: A total of 2,451 patients who underwent surgery for TETs were identified from the Surveillance, Epidemiology, and End Results database. Preoperative chemotherapy significantly improved OS and CSS in patients with stage III/IV TETs compared to patients without preoperative chemotherapy. Subgroup analysis showed that patients younger than 60 years of age with TETs, patients with thymic carcinoma, and patients with TETs with multiple cancers were more likely to benefit from preoperative chemotherapy.
Conclusion: This study found that preoperative chemotherapy is a viable option for advanced thymoma with favorable overall and cancer-specific survival rates, but patient history and physical condition should be fully considered in conjunction with diagnostic imaging findings to assess patient tolerance to chemotherapy.
Thymic epithelial tumors (TETs) represent a group of heterogeneous, rare neoplasms arising from thymic epithelial cells and are the most common tumors of the anterior mediastinum (1). With disease progression, neoplastic cells invade mediastinal and thoracic organs, such as the lungs, heart, great vessels, surrounding nerves and lymph nodes, and may damage those organs (2). Thymic epithelial tumors include thymomas (Ts), thymic carcinomas (TCs) and thymic neuroendocrine tumors (NETs) (3, 4). Ts are classified into five types (A, AB, B1, B2, B3) in accordance with the shape and atypia of their epithelial cells as well as the abundance of lymphocytes (5).
The rarity of TETs, with an overall incidence of 0.13–0.32 per 100,000 people per year, has somewhat limited prospective studies, and optimal treatment options remain an unresolved issue (6). Studies on the effects of preoperative chemotherapy in patients with TETs are still inadequate, and the prognostic impact of chemotherapy on patients with TETs is still controversial and requires further study (7–11).
In this study, we aimed to assess the prognostic value of preoperative chemotherapy for TETs, its safety and its optimal conditions of application.
The Surveillance, Epidemiology, and End Results (SEER) database is one of the largest publicly available databases, with approximately 28% of the U.S. population covered (12). In this study, all cases were obtained from the SEER program (www.seer.cancer.gov), and patients from the SEER 17 registry (2007–2019; dataset submitted in November 2019) maintained by the National Cancer Institute were analyzed to extract patients with TETs using SEER*Stat software (version 8.4.0.1). Patients enrolled in this study were those with the ICD-O-3 site code C37.9 (thymus) and the ICD-O-3 histology codes thymoma (8580–8585), thymic carcinoma (8000, 8010, 8020, 8070, 8071, 8072, 8074, 8082, 8083, 8123, 8140, 8200, 8260, 8310, 8430, 8480, 8481, 8560, 8586, 8589) and thymic neuroendocrine tumor (8012, 8013, 8041, 8044, 8240, 8246, 8249 and 8574) (13–15).
We identified 3,555 cases according to the following admission criteria: (1) year of diagnosis from 2007 to 2019; (2) ICD-O-3 site code C37.9 (thymus); (3) pathologically confirmed TETs, not diagnosed by autopsy or death certificate; (4) patients with complete data on age at diagnosis, sex, stage, treatment, histology, vital status, or months of survival; (5) considering that surgery may lead to immediate death in the short term (16) and the lack of information on postoperative complications in the SEER database, patients surviving <1 month were excluded from this study, which focused on the impact of the treatment approach on long-term survival (≥1 month) in patients with thymic epithelial tumors.
For further study, patients who met the following criteria were excluded: (1) no/unknown cancer-directed surgery of primary site performed; (2) unknown sequence of surgery and radiotherapy; (3) the systemic treatment received was not chemotherapy; (4) age at diagnosis less than 18; (4) ethnicity information unknown Figure 1 demonstrates the flowchart of case inclusion and exclusion in detail.
This study used previously collected anonymized and de-identified data from the SEER database. Therefore, this study was exempted from ethical review by the Institutional Review Board of the Fifth Affiliated Hospital of Zhengzhou University.
The variables involved in our study included basic demographic information (age at diagnosis, sex, marital status, and race), neoplasm-related information: tumor size, WHO classification (A, AB, B1, B2, B3, TCs and NETs), Masaoka–Koga Stage (I/IIA, IIB, III/IV), Number of tumors (One primary tumor only, With other malignant tumors), and therapeutic information: Lymph node biopsy (Negative, Not performed, Positive); Radiotherapy Information (no_Radiotherapy, Radiotherapy after surgery, Radiotherapy prior to surgery);Chemotherapy (no_systematic treatment, Preoperative systemic treatment, Postoperative systemic treatment), survival information: survival months (from diagnosis to death or last follow-up), vital status (Live, Dead). Overall survival (OS) and cancer-specific survival (CSS) were the primary study endpoints. OS was defined as the time from diagnosis of TETs to death or loss to follow-up for any reason; CSS was defined as the time from the date of diagnosis to direct or indirect death from thymic epithelial tumors.
For two groups of continuity indicators (age and tumor size) X-tile software was used to select the best cut-off point in the survival data and to group the age and tumor size. The optimal groupings for age at diagnosis in this study were the <60 years group and the ≥60 years group; the optimal groupings for tumor size were the <80 mm group, the ≥80 mm group and the unknown size group. For marital status, patients were divided into a married group, a single (never married) group, and an other group. For race, patients were grouped into white group, black group, and Asian/Other ethnic group (including Pacific Islander, Alaska Native, etc.). The sequence of surgical vs. systemic treatment is recorded in the SEER database, in which systemic treatment mainly refers to chemotherapy, but also includes hormonal treatment, BRM treatment and transplant/endocrine cases. In this study, we included only cases where the type of systemic treatment was chemotherapy. The staging in the SEER database was divided into local, regional, and distal disease, and we reclassified the included patients with TETs into three groups according to the corresponding Masaoka–Koga staging as follows (the exact correspondence is indicated in Table 1): stage-I/IIA (Localized only), stage-IIB (Regional), and stage-III/IV (Distant site(s)/node(s) involved) (11, 17).

Table 1. Definition of Masaoka–Koga staging compared to staging groupings assigned using tumor data from SEER data.
This study was analyzed using R statistical software (www.r-project.org). Among patients who underwent surgery for TETs, Pearson χ2 tests or Fisher's exact tests were performed for patients who received different treatment modalities (no preoperative chemotherapy and preoperative chemotherapy) and treatment-related factors. Univariate and multifactorial Cox regression models were performed using “tableone”, “dplyr”, and “skimr” in R software to estimate hazard ratios (HR) and 95% confidence intervals (CI) to analyze independent prognostic factors associated with overall survival (OS) and cancer-specific survival (CSS) for patients undergoing TETs. Kaplan-Meier curves were plotted using the “Survival” package and “ggsurvplot” in R software to estimate OS and CSS for each group of patients, and P values were determined using the log-rank method. A 1 : 1 optimal nearest neighbor propensity score matching (PSM) was performed using the “MatchIt” package in R software to balance the baseline characteristics of patients in the study and control groups with a caliper value of 0.1. P < 0.05 was considered a statistically significant difference.
Our study enrolled 2,451 eligible patients who underwent surgery for TETs between 2007 and 2019. Among them, 1,686 were white, 346 were black, and 419 were patients of other ethnicities, including Pacific Islanders and Asians; The study included 1,747 patients with Ts, 576 patients with TCs, and 128 patients with NETs; Slightly more men than women were included in this study, with 53.7% of male patients compared to 46.3% of female patients.
Data retrievable in the study included age at diagnosis, race, sex, marital status, WHO classification, Masaoka–Koga stage, lymph node biopsy, sequence of radiation/chemotherapy vs. surgery, tumor size, and the presence of other tumors. The clinicopathological characteristics of patients with thymic epithelial tumor surgery before and after propensity score matching are presented in Table 2. Of the 2,451 patients with TETs, 348 received preoperative chemotherapy and 2,103 underwent direct surgery without preoperative chemotherapy. χ2 tests showed significant differences in the proportion of patients between the preoperative chemotherapy group and the no preoperative chemotherapy group at different levels of exposure variables (including Masaoka–Koga stage, tumor size, etc.). After PSM, the P-values for all covariates were above 0.1, indicating that all patient- and treatment-related factors were well balanced between the study and control groups. The results of the descriptive statistical analysis for the entire cohort and the matched cohorts are presented in Table 3.

Table 2. Clinicopathological characteristics of patients with thymic epithelial tumor surgery before and after propensity score matching.
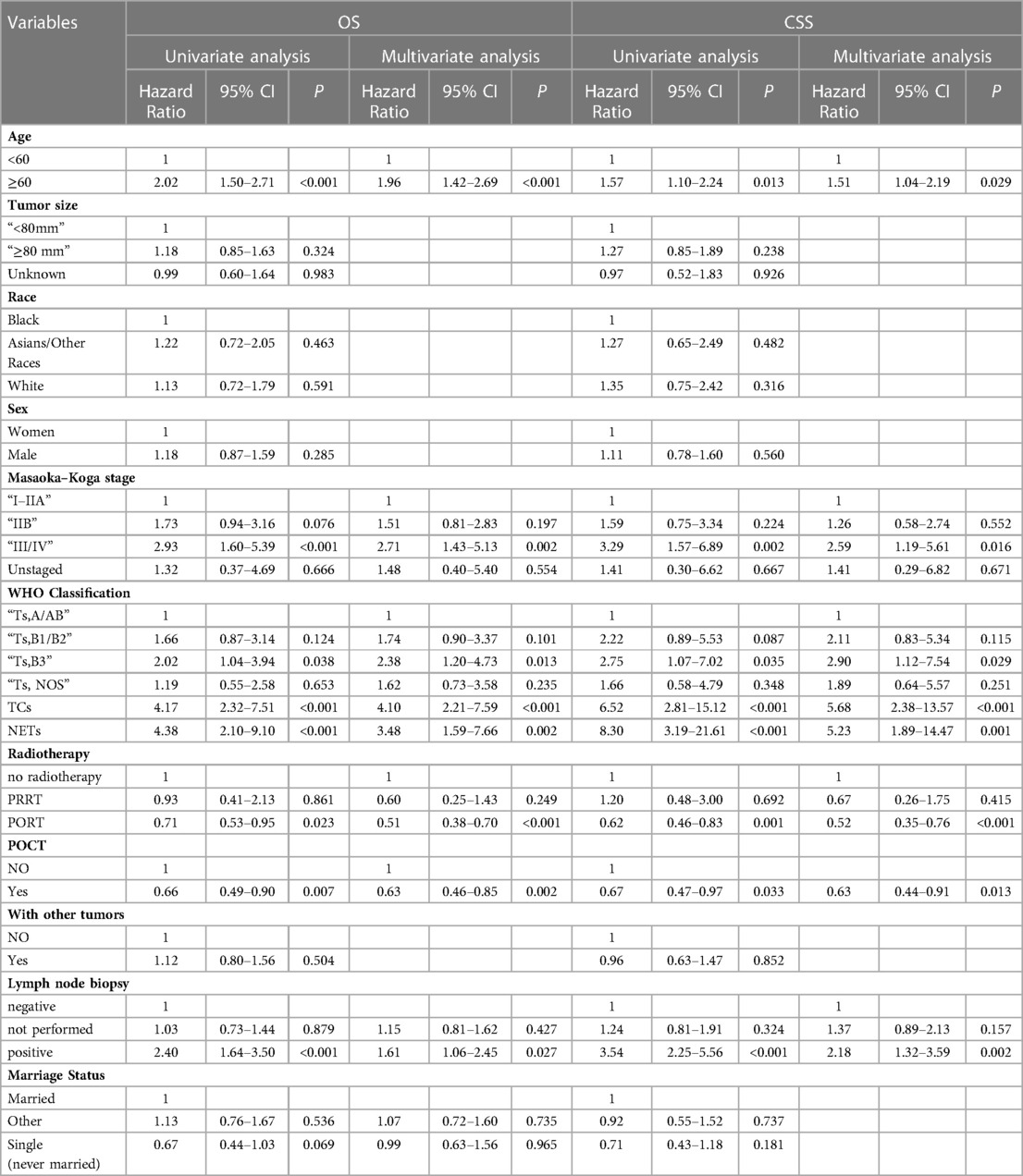
Table 3. Univariate and multifactorial analyses of propensity score matching affecting overall survival in patients undergoing surgery for thymic epithelial tumors.
Table 4 lists the 11 variables included in the univariate Cox regression model to analyze the factors associated with overall survival or cancer-specific survival in patients undergoing surgery for TETs. Variables with univariate analysis P < 0.1 were enrolled in multivariate Cox Regression models. Multivariate Cox regression analysis demonstrated that preoperative chemotherapy was an independent prognostic factor for OS (P = 0.002) and CSS (P = 0.013) in patients undergoing surgery for TETs. In addition, age at diagnosis, Masaoka–Koga staging, WHO classification, radiotherapy and lymph node biopsy findings were all independent prognostic factors for both OS and CSS.
In the entire cohort before propensity score matching, Figures 2, 3 illustrate the prognosis of patients with TETs with different Masaoka–Koga staging who received preoperative chemotherapy vs. those who did not. Preoperative chemotherapy significantly improved OS (Median survival times in months, 102.72 vs. 79.67, P = 0.0035) in patients with stage III/IV and did not significantly improve OS or CSS in patients with stage I/IIA or IIB TETs (P > 0.05). Similarly, hazard ratios and 95% CIs for OS and CSS in patients with TETs of different Masaoka–Koga staging are shown in Figure 4, with preoperative chemotherapy being a favorable factor for OS in patients with III/IV.
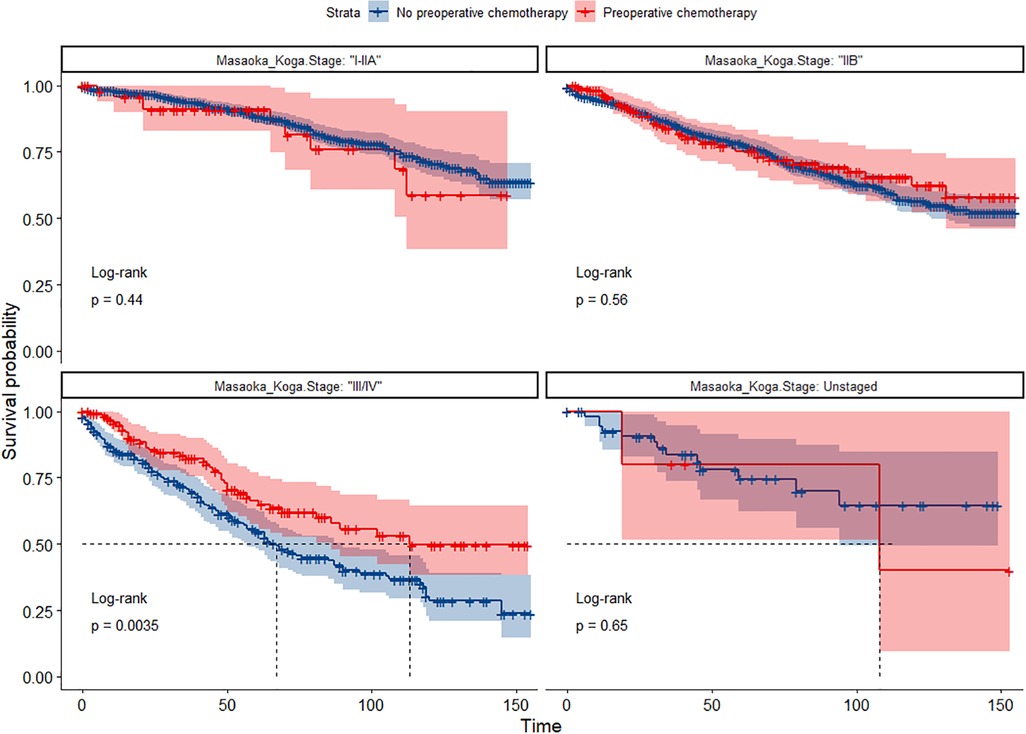
Figure 2. Survival curves of OS in the POCT group and no-POCT group in patients undergoing surgery for TETs at different stages before propensity matching.
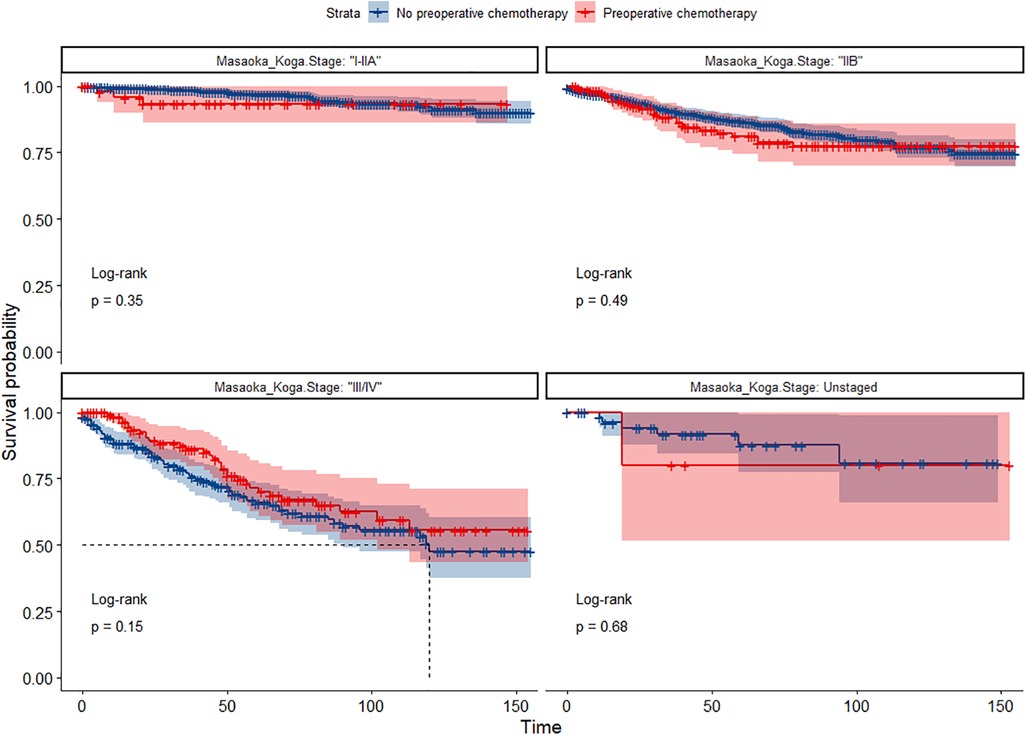
Figure 3. Survival curves of CSS in the POCT group and no-POCT group in patients undergoing surgery for TETs at different stages before propensity matching.
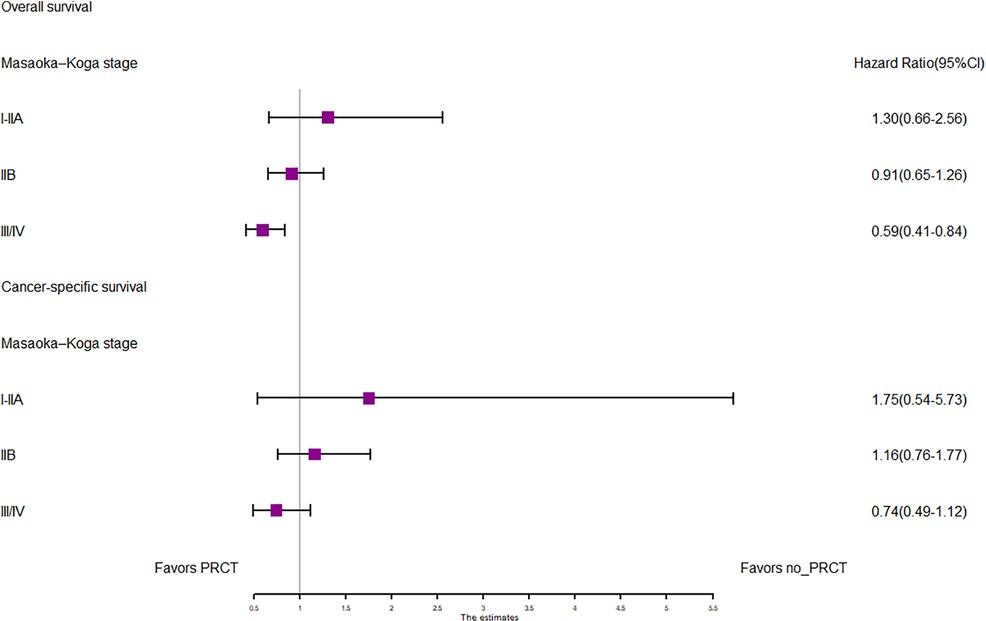
Figure 4. Forest plots of OS and CSS in the POCT and no POCT groups in patients undergoing surgery for TETs at different stages before propensity matching.
In the propensity score-matched cohort, the overall survival and cancer-specific survival curves for the preoperative chemotherapy and no-preoperative chemotherapy groups are shown in Figures 5, 6. Preoperative chemotherapy significantly improves OS (Median survival times in months, 114.85 vs. 100.26, P = 0.0067) and CSS (Median survival times in months, 126.51 vs. 115.76, P = 0.031) in patients with TETs. In the subgroup analysis based on Masaoka–Koga staging, hazard ratios and 95% CIs for OS and CSS are shown in Figure 7, Overall survival and cancer-specific survival curves for the preoperative chemotherapy and no-preoperative chemotherapy groups are shown in Figures 8A,B. We observed that preoperative chemotherapy was a favorable factor for OS (HR: 0.45, 95%CI: 0.28–0.71) and CSS (HR: 0.49, 95%CI: 0.29–0.82) in patients with stage III/IV TETs, and preoperative chemotherapy significantly improved OS (Median survival times in months, 107.92 vs. 73.69, P = 0.00039) and CSS (Median survival times in months, 114.86 vs. 89.21, P = 0.0059) in stage III/IV patients, but the efficacy in stage I/IIA or IIB patients was not significant. In addition, subgroup analysis based on age, WHO staging, and presence of other cancers in Figure 7 showed that in terms of age, preoperative chemotherapy was a protective factor for OS (HR: 0.53, 95%CI: 0.33–0.84) and CSS (HR: 0.51, 95%CI: 0.30–0.87) in patients younger than 60 years of age with TETs, significantly improving OS (median survival times in months, 128.34 vs. 109.19, P = 0.0059, Figure 9) and CSS (median survival times in months, 134.69 vs. 118.00, P = 0.011, Figure 10) in this group of patients, but not in older patients; In terms of WHO-classification, preoperative chemotherapy was a protective factor for OS (HR: 0.58, 95%CI: 0.37–0.91) and CSS (HR: 0.58, 95%CI: 0.34–0.98) in patients with thymic carcinoma and significantly improved OS (Median survival times in months, 95.67 vs. 69.86, P = 0.017, Figure 11) and CSS (median survival times in months, 109.45 vs. 88.19, P = 0.038, Figure 12) in these patients; And in terms of the presence of other tumors, preoperative chemotherapy was a protective factor for OS (HR: 0.42, 95%CI: 0.23–0.79) and CSS (HR: 0.31, 95%CI: 0.13–0.73) in patients with TETs with multiple cancers and significantly improved OS (Median survival times in months, 121.13 vs. 89.00, P = 0.005, Figure 13) and CSS (median survival times in months, 137.76 vs. 106.32, P = 0.0046, Figure 14) in these patients.
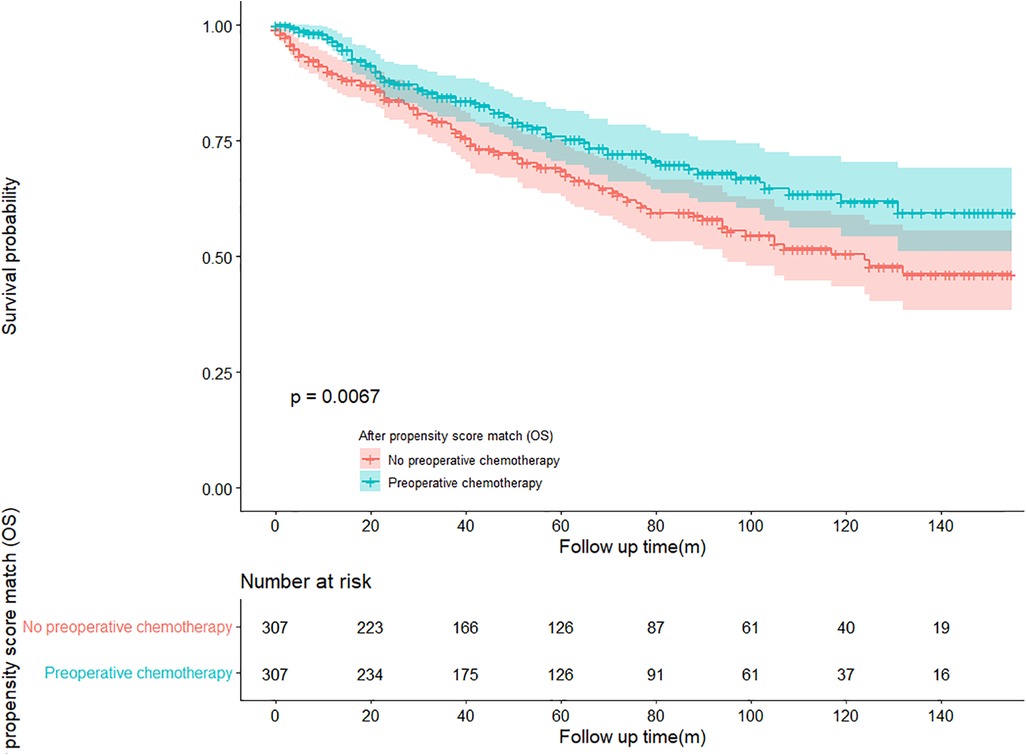
Figure 5. Survival curves of OS in the POCT group and no-POCT group in patients undergoing surgery for TETs after propensity matching.
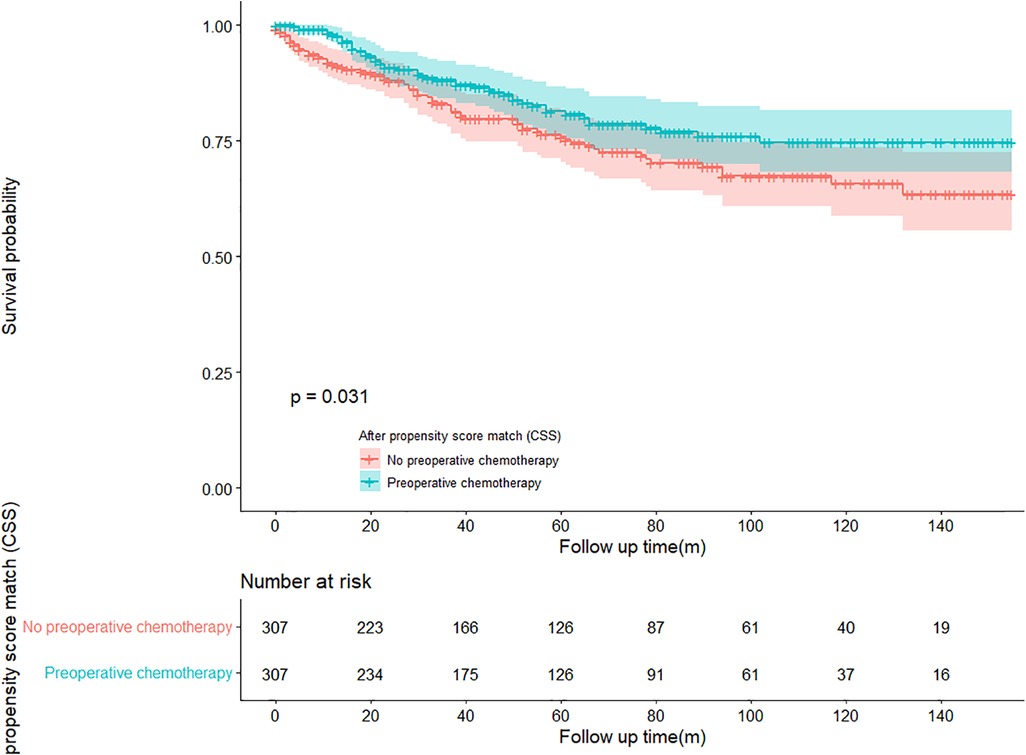
Figure 6. Survival curves of CSS in the POCT group and no-POCT group in patients undergoing surgery for TETs after propensity matching.
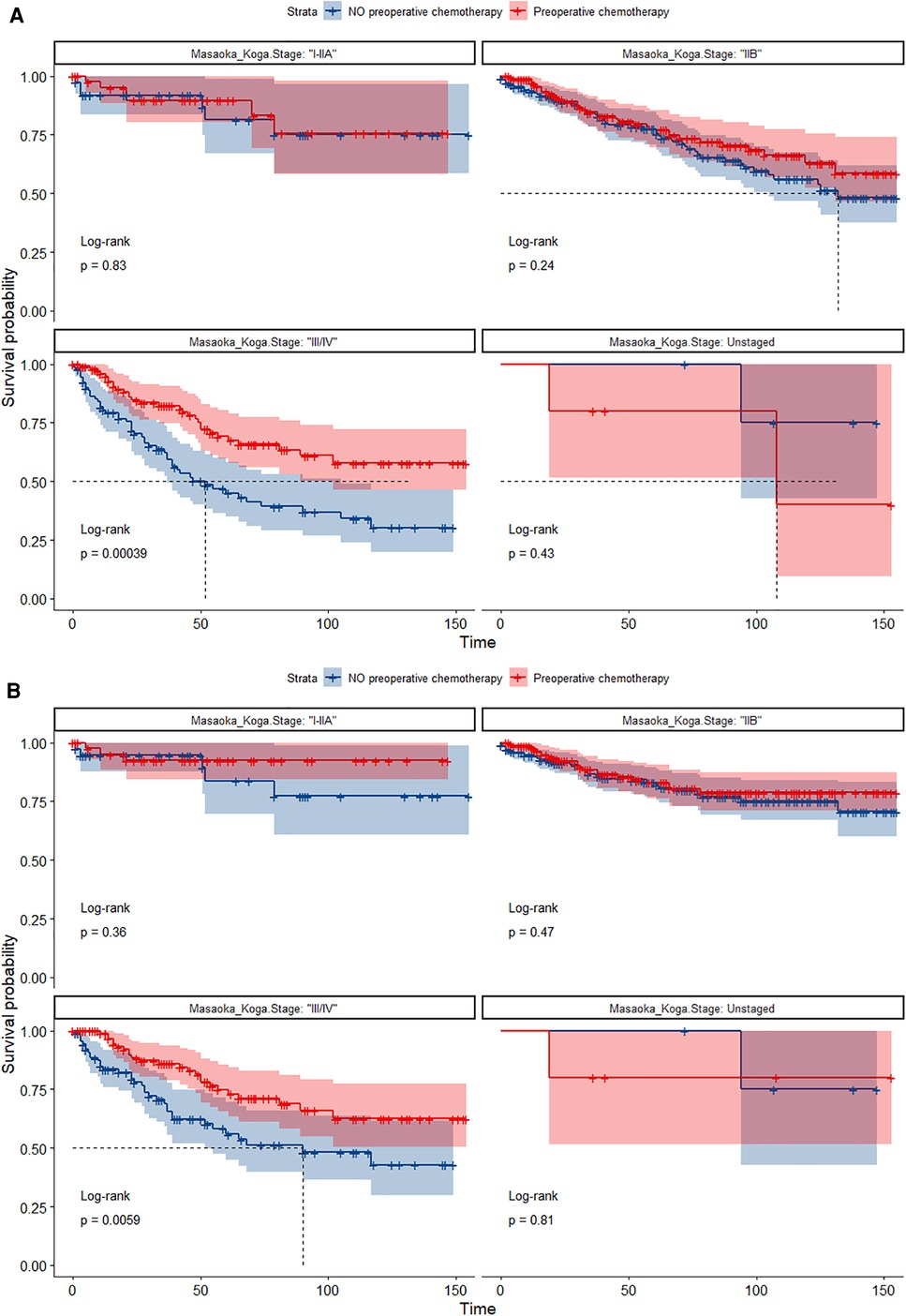
Figure 8. (A) Survival curves of OS in the POCT group and no-POCT group in patients undergoing surgery for TETs at different stages after propensity matching. (B) Survival curves of CSS in the POCT group and no-POCT group in patients undergoing surgery for TETs at different stages after propensity matching.
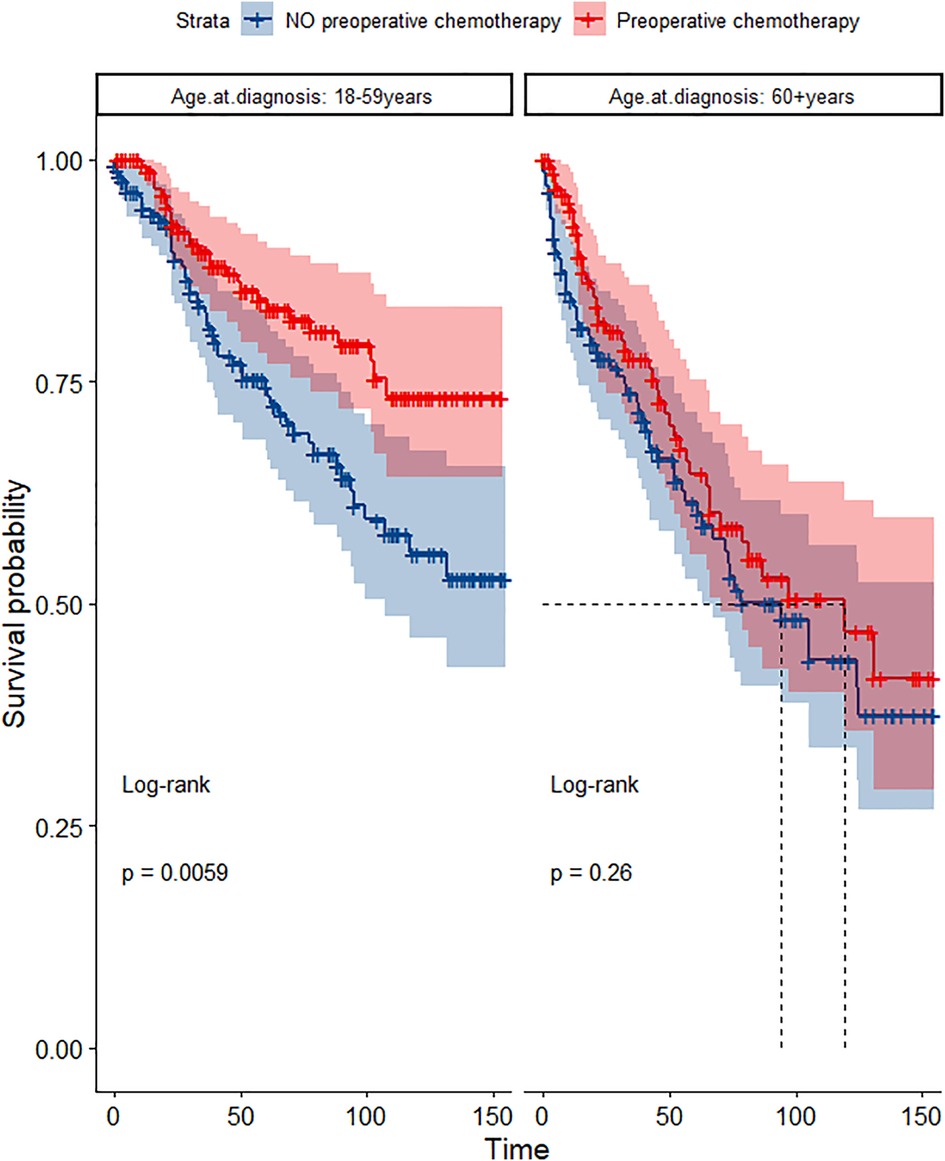
Figure 9. Survival curves of OS in the POCT and no POCT groups in patients undergoing surgery for TETs in different age groups after propensity matching.
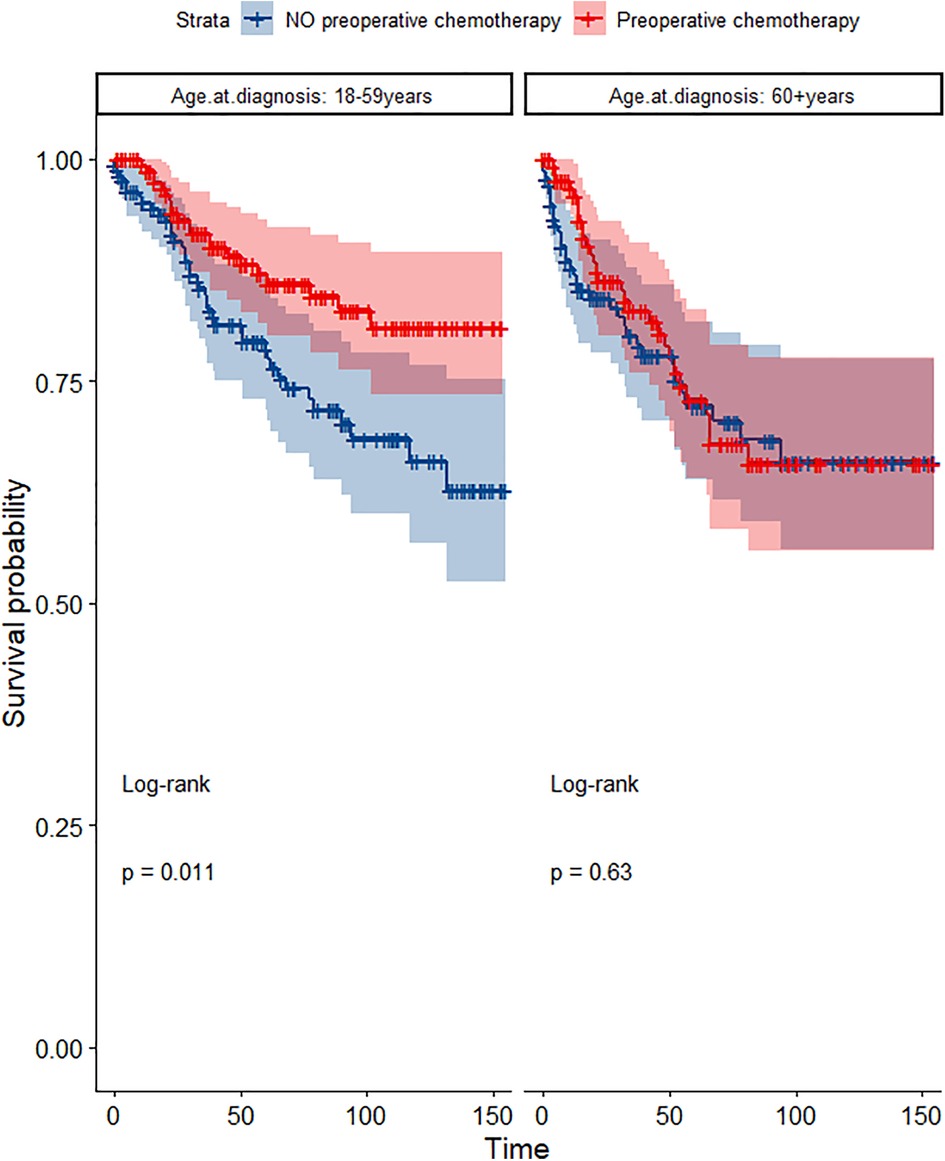
Figure 10. Survival curves of CSS in the POCT and no POCT groups in patients undergoing surgery for TETs in different age groups after propensity matching.
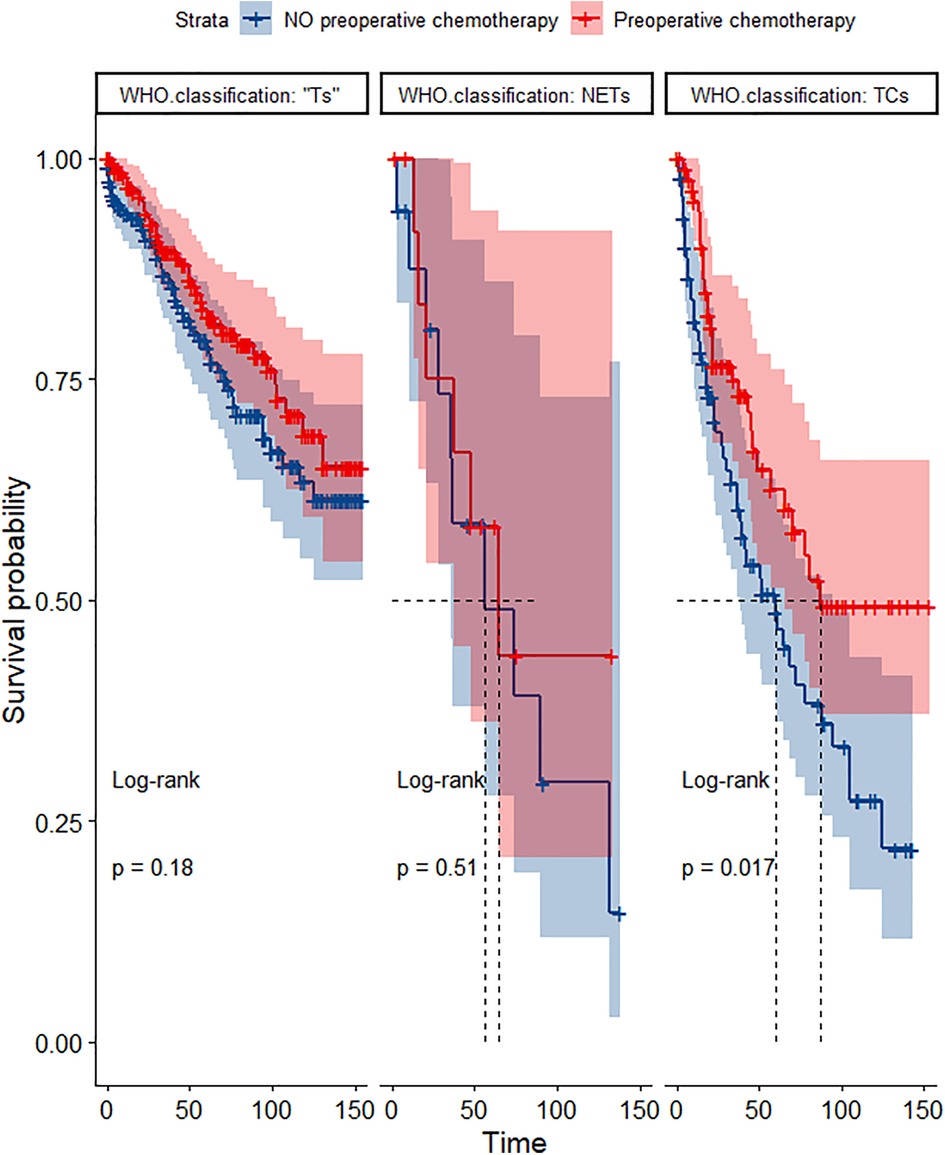
Figure 11. Survival curves of OS in the POCT and no POCT groups in patients undergoing surgery for TETs in different wHO classification groups after propensity matching.
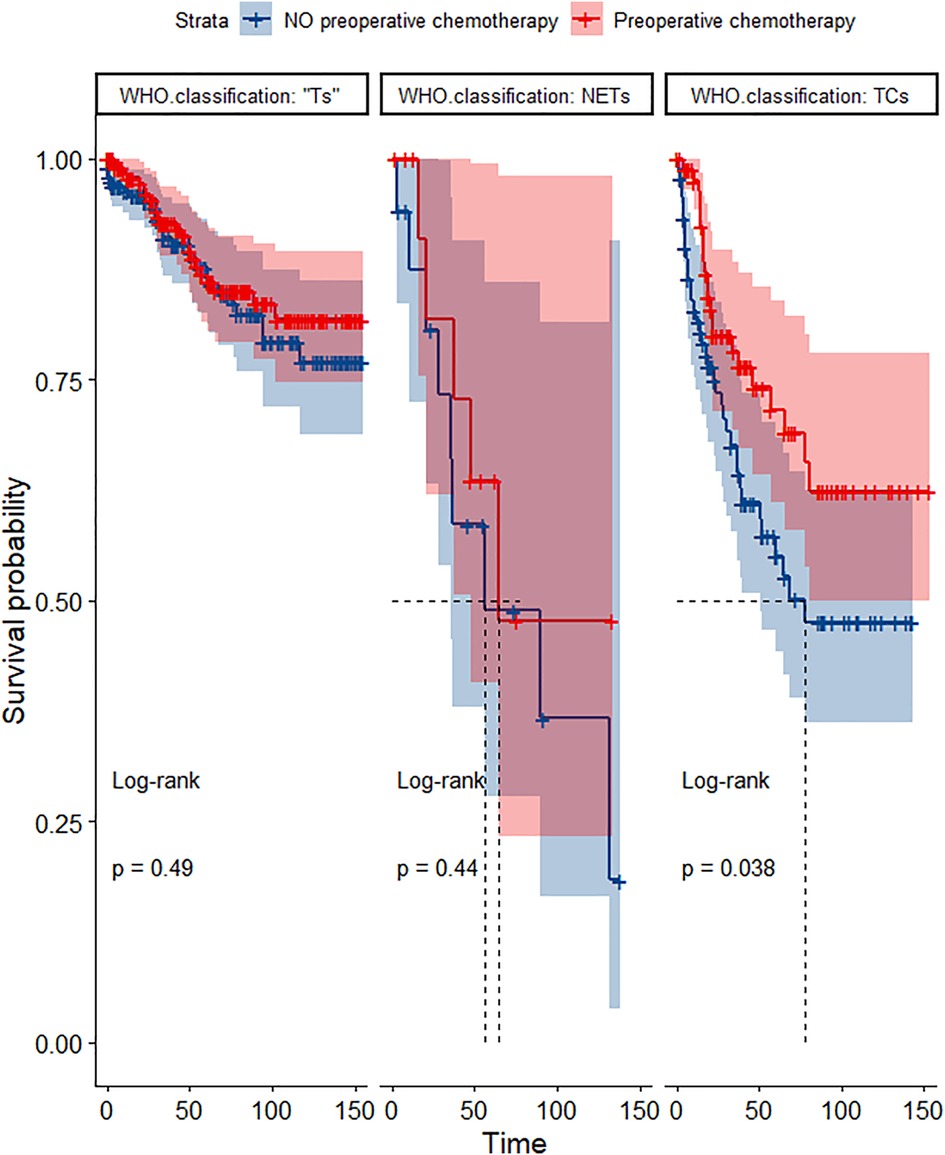
Figure 12. Survival curves of CSS in the POCT and no POCT groups in patients undergoing surgery for TETs in different WHO classification groups after propensity matching.
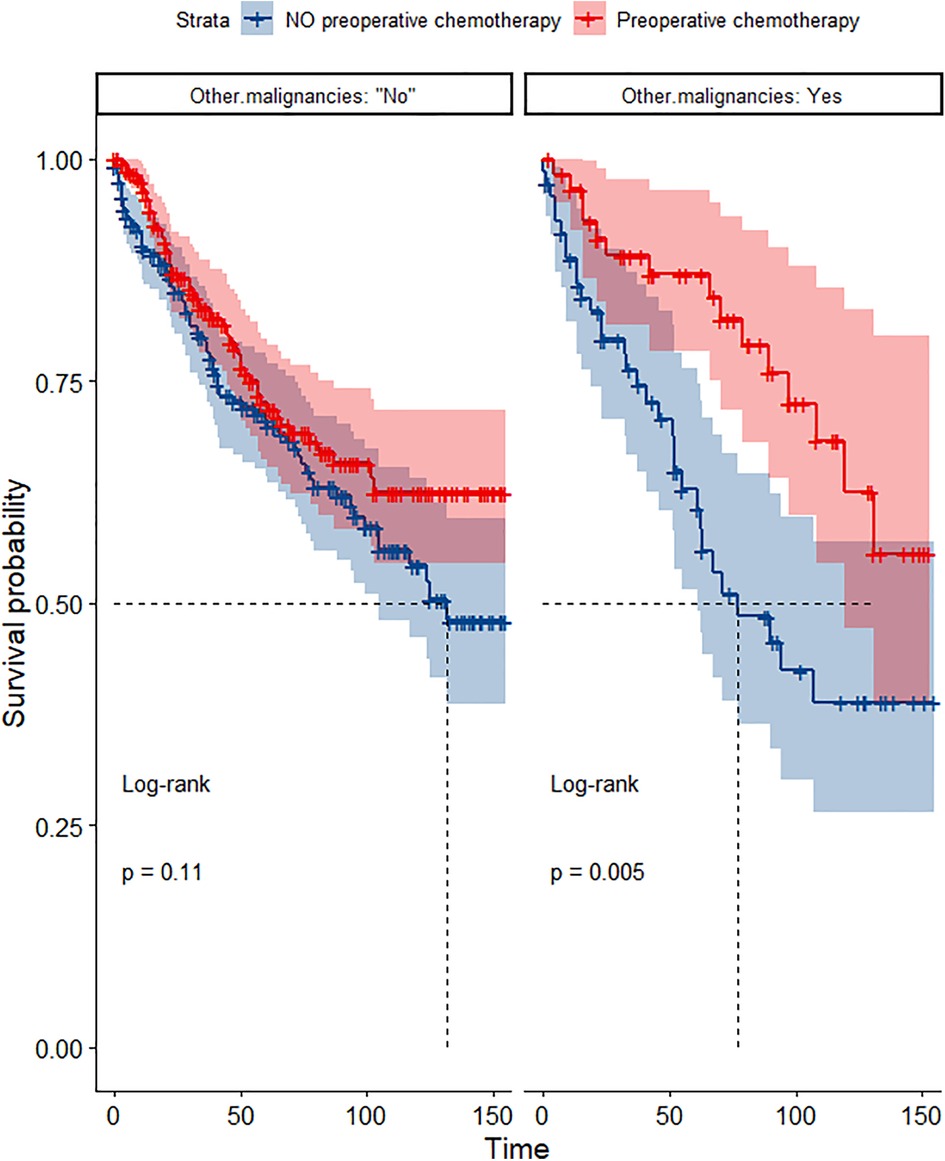
Figure 13. Survival curves of OS in the POCT and no POCT groups in patients with or without other malignancies undergoing surgery for TETs after propensity matching.

Figure 14. Survival curves of CSS in the POCT and no POCT groups in patients with or without other malignancies undergoing surgery for TETs after propensity matching.
In this population-based study, we use data from the SEER database to evaluate the survival outcomes of 2,451 patients with TETs over the past 10-plus years. Compared to patients without preoperative chemotherapy, preoperative chemotherapy significantly improved OS in patients with stage III/IV TETs and did not significantly improve OS and CSS in stage I/IIA or IIB patients with TETs, both before and after propensity score matching. As a systemic treatment with some toxicities, preoperative chemotherapy significantly improved OS and CSS in younger patients or patients with multiple cancers with TETs. Therefore, we prefer to apply preoperative chemotherapy to advanced TETs detected by imaging, and patient history and physical condition should be carefully considered when applying chemotherapy.
Whether chemotherapy can improve the prognosis of patients undergoing surgery for TETs has been controversial. A multicenter analysis in Japan reveals that chemotherapy did not provide any survival advantage for patients with completely resected stage III and IV thymoma and thymic carcinoma (19), but chemotherapy in the study was limited to postoperative chemotherapy. Wei et al. reported a higher 5-year OS rate in patients with thymoma or thymic carcinoma who underwent direct surgery compared with those who received preoperative chemotherapy (20). In contrast, a study by Lucchi M et al. noted that patients who underwent surgery after neoadjuvant chemotherapy had better OS compared with patients who underwent primary surgery (21). Studies by Venuta F et al. and Macchiarini P et al. also reported a significant survival advantage after preoperative chemotherapy (22, 23).
In our study population, the median tumor size of 94.01 mm in patients who opted for preoperative chemotherapy was greater than the median of 66.19 mm in the group without preoperative chemotherapy, implying that preoperative chemotherapy was more frequently administered in patients with larger tumors. Early case reports and some small review studies have also shown that chemo can help to reduce tumor size and relieve symptoms (24). In the study by Federico Venuta et al. it was also suggested that preoperative chemotherapy had a down-staging effect (22). In the study by Macchiarini et al. in which preoperative chemotherapy was administered to seven patients with invasive thymoma, all patients had at least a 50% reduction in tumor size (23). Almost all research has concluded that R0 resection is a determinant factor associated with thymic tumor survival (19, 25, 26), but advanced thymic epithelial tumors often invade the lung parenchyma, heart and large vessel tumors making R0 resection difficult. The high chemosensitivity of thymic epithelial tumors (27) makes it possible to use use preoperative chemotherapy to improve R0 resection rates resulting in better OS and CSS. A phase II study by Kim et al. demonstrated that preoperative induction chemotherapy optimized surgical resectability of TETs, which, along with surgery and postoperative radiotherapy, comprised multidisciplinary treatment, which prolonged life (28).
As first-line neoadjuvant therapy, the most popular chemotherapy regimens are platinum derivatives, mainly cisplatin with anthracyclines and/or etoposide, and they show good activity against both thymoma and thymic carcinoma, often with response rates above 50% (29). Carboplatin-paclitaxel is mainly recommended for thymic carcinoma. The main side effects reported for chemotherapy tend to be nausea and vomiting, bone marrow suppression, and cardiotoxicity of anthracyclines (28, 30). Elderly patients have more complications and poor physical condition (10), which might decrease the tolerance of chemotherapy, so whether and when to use chemotherapy should be carefully chosen based on the patient's medical condition. A retrospective study by Samina Park et al. (31) suggested that the surgical group after neoadjuvant chemotherapy showed significantly higher transfusion rates (P = 0.003) and longer operative times (P < 0.001), but there was no evidence that neoadjuvant chemotherapy reduced long-term survival in patients with thymic epithelial tumors. The study by Cameron D et al. also concluded that chemotherapy induction followed by surgical treatment followed by radiotherapy is safe and probably the best sequence of treatment for carefully screened patients with advanced thymoma (32).
In addition, in our study, although the proportion of thymic carcinomas was smaller compared to thymomas, it appeared that thymic carcinomas responded more to induction chemotherapy, which is consistent with the findings of Robert et al. (27). Preoperative chemotherapy had several advantages over postoperative chemotherapy. For example, to prevent local tumor spread during surgery, to reduce tumor staging and thus improve surgical resection rates (33). Compared to postoperative chemotherapy, preoperative chemotherapy is better tolerated and most patients are able to reach surgery in good health, which also helps to improve the clinical status of the patient and to relieve symptoms (including myasthenia gravis remission) (24).
Previously, several SEER-based studies investigated the prognostic value of different treatment regimens for patients with TETs. However, we differ from previous studies by (1) using the SEER database for the first time to investigate the efficacy of preoperative chemotherapy in patients with thymic epithelial tumors, (2) performing propensity score matching to increase comparability and reduce bias at baseline, and (3) performing subgroup analysis by factors such as Masaoka–Koga staging to find the characteristics of patients with TETs suitable for preoperative chemotherapy.
The present study, like other SEER-based studies, has several limitations. First, although there is a wealth of data in the SEER database, it is not comprehensive and it lacks information on several important demographics, clinically relevant variables, and treatment modalities. For example, details of preoperative chemotherapy (including total dose of chemotherapy, daily fractions, and type of chemotherapeutic agent), whether the surgical margins were positive, and the patient's medical history and comorbidities. The lack of these variables leads to an incomplete clinical picture and potential bias, which may limit our assessment of preoperative chemotherapy. For this reason, we used the available data to focus on the impact of preoperative chemotherapy on long-term survival (≥1 month) in patients with thymic epithelial tumors. Second, although systemic treatment variables report the sequence of surgery and chemotherapy, they do not take into account the timing of events. Thus, it is possible that chemotherapy was administered more than 6 months prior to surgery or only 6 days prior to surgery. Chemotherapy administered prior to surgery may not have had sufficient time to anticipate the associated tumor response, which may underestimate the effect of preoperative chemotherapy. Although the SEER database is a representative national cancer registry with outstanding reliability and reproducibility of data collection and reporting procedures, and we used propensity score matching to minimize selection bias in preoperative chemotherapy, we could not completely exclude unmeasured or unpredictable confounding factors.
This study found that preoperative chemotherapy is a viable option for advanced thymoma with favorable overall and cancer-specific survival rates, but patient history and physical condition should be fully considered in conjunction with diagnostic imaging findings to assess patient tolerance to chemotherapy. Patients with thymic or multiple cancers may benefit from preoperative chemotherapy.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
XG designed this work. YF and TC integrated and analyzed the data. YF wrote this manuscript. XG edited and revised the manuscript. SW proposed practical measures and new solutions to the issues raised by the reviewers, controlled the language of the article, and corrected typos. All authors approved this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1108699/full#supplementary-material.
1. Prays J, Ortiz-Villalon C. Molecular landscape of thymic epithelial tumors. Semin Diagn Pathol. (2022) 39(2):131–6. doi: 10.1053/j.semdp.2021.06.011
2. Tateishi Y, Horita N, Namkoong H, Enomoto T, Takeda A, Kaneko T. Postoperative radiotherapy for completely resected Masaoka/Masaoka–Koga stage II/III thymoma improves overall survival: an updated meta-analysis of 4746 patients. J Thorac Oncol. (2021) 16(4):677–85. doi: 10.1016/j.jtho.2020.12.023
3. Remon J, Bernabe R, Diz P, Felip E, Gonzalez-Larriba JL, Lazaro M, et al. SEOM-GECP-GETTHI clinical guidelines for the treatment of patients with thymic epithelial tumours (2021). Clin Transl Oncol. (2022) 24(4):635–45. doi: 10.1007/s12094-022-02788-w
4. Jia R, Sulentic P, Xu JM, Grossman AB. Thymic neuroendocrine neoplasms: biological behaviour and therapy. Neuroendocrinology. (2017) 105(2):105–14. doi: 10.1159/000472255
5. Okumura M, Shiono H, Minami M, Inoue M, Utsumi T, Kadota Y, et al. Clinical and pathological aspects of thymic epithelial tumors. Gen Thorac Cardiovasc Surg. (2008) 56(1):10–6. doi: 10.1007/s11748-007-0177-8
6. Conforti F, Marino M, Vitolo V, Spaggiari L, Mantegazza R, Zucali P, et al. Clinical management of patients with thymic epithelial tumors: the recommendations endorsed by the Italian association of medical oncology (AIOM). ESMO Open. (2021) 6(4):100188. doi: 10.1016/j.esmoop.2021.100188
7. <clinical significance of age at diagnosis among patients with thymic epithelial tumors] (1).pdf>.
8. Fu H, Gu ZT, Fang WT, Fu JH, Shen Y, Han YT, et al. Long-Term survival after surgical treatment of thymic carcinoma: a retrospective analysis from the Chinese alliance for research of thymoma database. Ann Surg Oncol. (2016) 23(2):619–25. doi: 10.1245/s10434-015-4825-4
9. Benítez JC, Bluthgen MV, Boucher M, Dansin E, Kerjouan M, Bigay-Game L, et al. MA04.01 Multimodality treatment and outcome in stage III thymic epithelial tumors (TETs): a retrospective analysis from the French RYTHMIC network. J Thorac Oncol. (2021) 16(10):S895–896. doi: 10.1016/j.jtho.2021.08.123
10. Zhang T, Liu L, Qiu B. Development of a competing risk nomogram for the prediction of cause-specific mortality in patients with thymoma: a population-based analysis. J Thorac Dis. (2021) 13(12):6838–47. doi: 10.21037/jtd-21-931
11. Zhao M, Yin J, Yang X, Jiang T, Lu T, Huang Y, et al. Nomogram to predict thymoma prognosis: a population-based study of 1312 cases. Thorac Cancer. (2019) 10(5):1167–75. doi: 10.1111/1759-7714.13059
12. Lim YJ, Kim HJ, Wu HG. Role of postoperative radiotherapy in nonlocalized thymoma: propensity-matched analysis of surveillance, epidemiology, and End results database. J Thorac Oncol. (2015) 10(9):1357–63. doi: 10.1097/JTO.0000000000000619
13. Shin DW, Cho JH, Ha J, Jung KW. Trends in incidence and survival of patients with thymic epithelial tumor in a high-incidence Asian country: analysis of the Korean central cancer registry 1999 to 2017. J Thorac Oncol. (2022) 17(6): 827–37. doi: 10.1016/j.jtho.2022.02.001
14. Marx A, Chan JKC, Chalabreysse L, Dacic S, Detterbeck F, French CA, et al. The 2021 WHO classification of tumors of the Thymus and mediastinum: what is new in thymic epithelial, germ cell, and mesenchymal tumors? J Thorac Oncol. (2022) 17(2):200–13. doi: 10.1016/j.jtho.2021.10.010
15. Marx A, Chan JK, Coindre JM, Detterbeck F, Girard N, Harris NL, et al. The 2015 world health organization classification of tumors of the Thymus: continuity and changes. J Thorac Oncol. (2015) 10(10):1383–95. doi: 10.1097/JTO.0000000000000654
16. Mahal BA, Inverso G, Aizer AA, Ziehr DR, Hyatt AS, Choueiri TK, et al. Incidence and determinants of 1-month mortality after cancer-directed surgery. Ann Oncol. (2015) 26(2):399–406. doi: 10.1093/annonc/mdu534
17. Kim E, Thomas CR Jr.. Conditional survival of malignant thymoma using national population-based surveillance, epidemiology, and end results (SEER) registry (1973-2011). J Thorac Oncol. (2015) 10(4):701–7. doi: 10.1097/JTO.0000000000000472
18. Gadalla SM, Rajan A, Pfeiffer R, Kristinsson SY, Bjorkholm M, Landgren O, et al. A population-based assessment of mortality and morbidity patterns among patients with thymoma. Int J Cancer. (2011) 128(11):2688–94. doi: 10.1002/ijc.25583
19. Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. (2003) 76(3):878–84. doi: 10.1016/S0003-4975(03)00555-1
20. Wei Y, Gu Z, Shen Y, Fu J, Tan L, Zhang P, et al.; members of the Chinese alliance for research in, T., preoperative induction therapy for locally advanced thymic tumors: a retrospective analysis using the ChART database. J Thorac Dis. (2016) 8(4):665–72. doi: 10.21037/jtd.2016.03.02
21. Lucchi M, Ambrogi MC, Duranti L, Basolo F, Fontanini G, Angeletti CA, et al. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg. (2005) 79(6):1840–4. doi: 10.1016/j.athoracsur.2004.12.047
22. Venuta F, Rendina EA, Longo F, De Giacomo T, Anile M, Mercadante E, et al. Long-term outcome after multimodality treatment for stage III thymic tumors. Ann Thorac Surg. (2003) 76(6):1866–72. doi: 10.1016/S0003-4975(03)01020-8
23. Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg. (2010) 251(6):1117–21. doi: 10.1097/SLA.0b013e3181dd4ec4
24. Venuta F, Rendina EA, Anile M, de Giacomo T, Vitolo D, Coloni GF. Thymoma and thymic carcinoma. Gen Thorac Cardiovasc Surg. (2012) 60(1):1–12. doi: 10.1007/s11748-011-0814-0
25. Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg. (2004) 77(5):1860–9. doi: 10.1016/j.athoracsur.2003.10.001
26. Zhai Y, Hui Z, Ji W, Wang X, Liang J, Mao Y, et al. A single-center analysis of the treatment and prognosis of patients with thymic carcinoma. Ann Thorac Surg. (2017) 104(5):1718–24. doi: 10.1016/j.athoracsur.2017.06.025
27. Zhai Y, Chen D, Gao Y, Hui Z, Xue L, Zhou Z, et al. Role of modern neoadjuvant chemoradiotherapy in locally advanced thymic epithelial neoplasms. Tumori. (2021) 107(5):407–15. doi: 10.1177/0300891620967980
28. Kim ES, Putnam JB, Komaki R, Walsh GL, Ro JY, Shin HJ, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer. (2004) 44(3):369–79. doi: 10.1016/j.lungcan.2003.12.010
29. Berghmans T, Durieux V, Holbrechts S, Jungels C, Lafitte JJ, Meert AP, et al. Systemic treatments for thymoma and thymic carcinoma: a systematic review. Lung Cancer. (2018) 126:25–31. doi: 10.1016/j.lungcan.2018.10.018
30. Scorsetti M, Leo F, Trama A, D'Angelillo R, Serpico D, Macerelli M, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol. (2016) 99:332–50. doi: 10.1016/j.critrevonc.2016.01.012
31. Kang CH. The change of therapeutic trends in the thymic epithelial tumor. J Thorac Dis. (2019) 11(12):5652–4. doi: 10.21037/jtd.2019.11.15
32. Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVA thymoma. Ann Thorac Surg. (2006) 82(4):1234–9. doi: 10.1016/j.athoracsur.2006.05.028
Keywords: preoperative chemotherapy, chemotherapy, surveillance, epidemiology, end results program
Citation: Fan Y, Cui T, Wei S and Gao X (2023) Prognostic value of preoperative chemotherapy for thymic epithelial tumors: A propensity-matched analysis based on the SEER database. Front. Surg. 10:1108699. doi: 10.3389/fsurg.2023.1108699
Received: 26 November 2022; Accepted: 27 February 2023;
Published: 17 March 2023.
Edited by:
Mohamed Rahouma, Weill Cornell Medical Center, NewYork-Presbyterian, United StatesReviewed by:
Mehmet Ali Bedirhan, Yedikule Teaching Hospital, Türkiye© 2023 Fan, Cui, Wei and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
†These authors have contributed equally to this work
*Correspondence: Xingcai Gao Z3hjNTc1NzU3QDE2My5jb20=
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
Abbreviations TETs, thymic epithelial tumors; POCT, preoperative chemotherapy; OS, overall survival; CSS, cancer-specific survival;SEER: Surveillance, Epidemiology, and End Results; Ts, thymoma, TCs, thymic carcinomas; NENTs, thymic neuroendocrine neoplasms; K–M, Kaplan–Meier.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.