
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 01 March 2023
Sec. Otorhinolaryngology - Head and Neck Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1098704
This article is part of the Research TopicNo time to die: Or if tomorrow never comes? - What can we draw from the pandemic and general instability? An opportunity for surgeons to graspView all 6 articles
Purpose: Nuclear protein in testis (NUT) carcinoma is a rare, aggressive tumor defined by the presence of NUT gene rearrangement. The aim of this study was to describe the clinical, radiologic, and biological features of sinonasal NUT carcinoma.
Methods: We retrospectively investigated NUT expression with clinicopathologic features in 145 cases with sinonasal malignancies diagnosed from January 2017 to December 2021 and reviewed the reported cases.
Results: Three (3/145, 2.07%) cases showed strong nuclear expression for NUT immunohistochemical, including one male and two females with ages from 37 to 57 years (mean, 45.33 years). All three cases involved the nasal cavity and sinuses; one of them involved the orbit and intracranial area. Histologically, all subjects showed poorly differentiated, small round cell morphology with distinct nuclei. All patients received surgery and chemoradiotherapy. One patient died of the disease 13 months after diagnosis, and two survived 12 and 15 months, respectively, without evidence of tumor recurrence. 51 cases of sinonasal NUT carcinoma (mean age 40.96 years) have been described to date. Among them, 28 are male, and 23 are female. Most cases expressed p63, AE1/AE3, as well as p40.
Conclusion: NUT carcinoma is a rare and aggressive disease with a poor prognosis. It is crucial to perform NUT rearrangement-related tests for differential diagnosis of poorly differentiated/undifferentiated tumors in the nasal cavity and sinuses.
NUT (nuclear protein in testis) carcinoma is a type of poorly differentiated or undifferentiated malignancy defined by the rearrangement of the nuclear protein in testis (NUT) gene (also known as NUTM1) (1, 2). The first case with chromosomal translocation t(15;19) involving the thymus was reported in 1991 (3). Since most cases were found in the midline of the body, such as the thorax or head and neck, it was first called “NUT midline carcinoma.” Afterward, many cases have been diagnosed arising outside the midline (4, 5).
Sinonasal NUT carcinoma is relatively rare, and the actual incidence is unknown due to the lack of comprehensive analysis of a large number of tumors as well as the underdiagnosed (6, 7). For instance, Lee et al. analyzed 362 cases of poorly differentiated or undifferentiated carcinoma of the head and neck, four (1.1%) of which were sinonasal NUT carcinoma (8). And of 151 cases of primary sinonasal carcinoma diagnosed at Johns Hopkins Hospital, only three were NUT positive (9).
In 2003, French et al. identified the fusion gene BRD-NUT in NUT carcinoma, which can encode a chimeric protein blocking differentiation and maintain cells in a highly proliferative, poorly differentiated state (10–12). Most NUT carcinoma cases harbor a reciprocal translocation between the NUT gene on chromosome 15q14 and bromodomain and extraterminal motif (BET) family genes bromodomain 4 (BRD4) on chr19p131 (10). In addition to BRD4, NUT can also be fused to BRD3, NSD3, ZNF532, and ZNF592 (5, 11, 13).
The prognosis for this tumor is comparatively poor, with median overall survival (OS) ranging from 6.5 to 9.7 months, according to different studies (4, 7, 14, 15). Most patients with NUT carcinoma will die from rapid disease progression because of early metastasis to local and distant sites (7). However, in some cohort studies, patients with head and neck NUT carcinoma had a slightly better prognosis than patients with thoracic NUT carcinoma (4). NUT carcinoma affects males and females equally, and though it can affect people of any age (range 0.1–80 years), the median age is in teens and young adults (median age 16–23.6 years) (4, 15).
At present, there are no treatment guidelines for NUT carcinoma. For head and neck NUT carcinoma, aggressive primary surgical resection (with or without postoperative chemoradiation or radiation therapy) is associated with significantly improved survival. Chemotherapy or radiotherapy alone is often not sufficient (5, 7). Several promising classes of drugs, including BET inhibitors (BETi) and histone deacetylase inhibitors (HDACi), have emerged as candidates for treatment (1, 13). Therefore, making an accurate diagnosis is essential for the choice of treatment.
Morphologically, NUT carcinomas present nested and sheet-like monomorphic, undifferentiated round oval cells with a small to moderate amount of cytoplasm and frequent cell division with necrosis. The chromatin is typically vesicular. Occasionally, it appears abrupt differentiation of squamous cells or keratinization. Although infiltrating lymphocytes are occasionally seen, a more common finding is the presence of infiltrating neutrophils (5, 9). In the sinonasal tract, the appearance of NUT carcinoma overlaps with those of other poorly differentiated neoplasms or small round blue cell tumors, including sinonasal undifferentiated carcinoma (SNUC), Ewing sarcoma/primitive neuroectodermal tumors (PNET), Epstein-Barr virus (EBV)-associated lymphoepithelial carcinoma, lymphoma/leukemia, olfactory neuroblastoma, small cell neuroendocrine carcinoma, melanomas, rhabdomyosarcoma and the recently described SMARCB1(INI1)-deficient sinonasal carcinoma (5, 16–19). The accurate diagnosis of sinonasal NUT carcinoma is difficult without ancillary tests.
The application of NUT rabbit monoclonal antibody (clone C52B1, Cell Signaling Technology) has greatly improved the diagnosis rate in recent years (5, 20). In addition to immunohistochemistry (IHC), fluorescence in situ hybridization (FISH) using NUT split-apart probes is a sensitive method for detecting NUT rearrangements (5).
In 2017, NUT carcinoma was added to the 4th edition of the World Health Organization (WHO) classification of sinonasal tumors for the first time (21). However, the lack of reliable morphologic features, its rarity, and the lack of awareness contribute to the underdiagnosis of NUT carcinoma (5). In this study, we retrospectively reported the clinical characteristic, histological appearance, treatment, and outcome of patients with sinonasal NUT carcinoma in order to raise clinicians’ awareness of this disease.
A total of 145 patients with sinonasal malignancies treated at Peking Union Medical College Hospital from January 2017 to December 2021 were reviewed retrospectively. Three of them showed strong positive for NUT IHC, and one was weakly positive. Further clinical histological and immunohistochemical reviews and FISH were performed on all NUT IHC positive cases. All pathological diagnoses were confirmed by experienced pathologists. Criteria for analysis included the description of the population, initial clinical and radiologic presentation, pathological features, treatment administered, and outcome. Tumor staging was performed using the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system. This study was approved by the Ethics Committee of Peking Union Medical College Hospital, and the requirement of informed consent was waived.
Hematoxylin-eosin (HE) stained sections were assessed for cell morphology, growth pattern, presence or absence of squamous differentiation, and necrosis. IHC for NUT was performed on formalin-fixed paraffin-embedded tumor sections, using the rabbit monoclonal primary antibody against NUT (Cell Signaling Technologies, 3625) in a dilution of 1:50. Cases with diffuse (>50%) strong, speckled nuclear staining were considered as positive. IHC for p63 (Abcam, ab124762, 1:5,000 dilution) and PD-L1 (Proteintech, 66248, 1:5,000 dilution) were performed according to standard procedures. IHC slides were observed using a microscope (Leica DM6 B, Wetzlar, Germany).
FISH analysis of NUT IHC positive cases was performed using NUT break-apart probes (Anbiping, Guangzhou, China). FISH slides were observed using a microscope (Leica DM6 B, Wetzlar, Germany) under a ×100 objective. Red fluorescence (R) labels the 5'NUT (15q14) probe, and green fluorescence (G) labels the 3'NUT probe. The normal signal pattern is shown as two red-green fluorescence fusions (2F), and the typical positive signal pattern is 1G1R1F. A total of 200 tumor cells were counted. If more than 15% contained NUT splitting signals, they were considered positive for FISH.
The 145 cases of sinonasal malignancies we retrieved exhibited a wide variety of pathological types (e.g., olfactory neuroblastoma, adenoid cystic carcinoma, sarcoma, etc.). Of these, 5 were undifferentiated and 30 were poorly differentiated. A total of three cases (3/145, 2.07%) were strongly positive for NUT IHC, and one was weakly positive (eventually diagnosed as sinonasal poorly differentiated squamous carcinoma). The age at diagnosis ranged from 37 to 57 years, with a mean age of 45.33 years. The male-to-female ratio was 1–2. All three cases involved the nasal cavity and sinuses, one involved the orbit and intracranial region, and two had cervical lymph node metastases. They were treated with radical surgical resection and all obtained negative margins at the initial surgery. In addition, all three underwent postoperative radiotherapy. Two of them received chemotherapy. Prognostically, the patient with T4bN2M0 recurred 9 months after initial surgery and died 13 months after diagnosis due to intracranial recurrence/metastasis. The other two patients showed no signs of recurrence at the end of follow-up, which was 15 and 12 months, respectively. The clinical data of NUT carcinoma are shown in Table 1.
Case 1: A 37-year-old woman visited Ophthalmology due to a bulge above her left eye with vision loss for a month. Four years ago, she began to develop a nasal obstruction on the left with nasal discharge stained with blood. Computed tomography (CT) and magnetic resonance imaging (MRI) showed a mass in the left frontal sinus, involving the left orbit and ethmoid sinus, and the lesion extended to the cranium (Figure 1A). Follow-up positron emission tomography (PET)-CT suggested that cervical lymph node metastasis was possible. She had a history of smoking and no history of drinking. The patient received open surgery, followed by two cycles of chemotherapy (vincristine + ifosfamide + epirubicin) and radiotherapy (dose unknown). The tumor recurred 9 months after surgery. She underwent surgery again and 1.5 months later developed intracranial recurrence or metastasis. The patient died 13 months after the initial diagnosis.
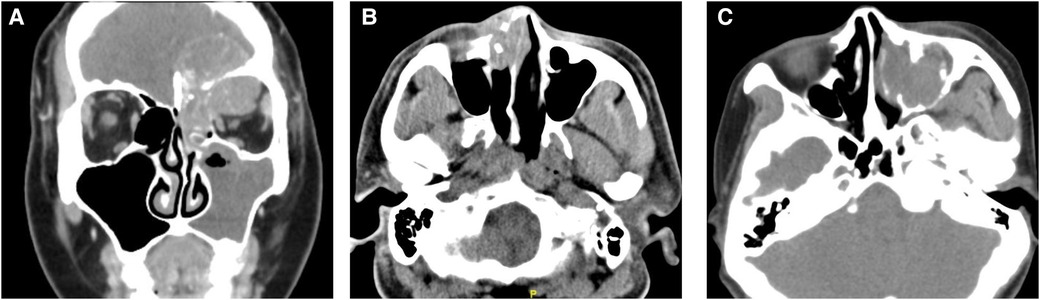
Figure 1. Computed tomography (CT) images of NUT carcinoma cases. (A) Coronal enhanced CT of case 1: the mass involved the left orbit, ethmoid sinus, and cranium. (B) Axial CT showed the tumor of case 2 located in the nasal cavity and destroyed the surrounding bone. (C) CT suggested a mass in the left maxillary sinus of case 3.
Case 2: A 42-year-old male presented with right-sided nasal obstruction and rhinorrhea for 1 month. He also presented with ipsilateral dorsum nasi swelling and epiphora. The patient smoked and occasionally drank alcohol. CT (Figure 1B) and PET-CT suggested right maxillary sinus, ethmoid sinus, and nasal cavity masses with possible cervical lymph node metastasis. He underwent open surgery and radiotherapy (dose not known). No signs of tumor recurrence were seen 15 months after surgery, after which the patient was lost to follow-up.
Case 3: A 57-year-old woman visited our hospital for “left maxillofacial pain with nasal obstruction and rhinorrhea for 1 month”. CT (Figure 1C) suggested a mass in the left maxillary sinus, nasal cavity, and ethmoid sinus with multiple bone destruction. The patient had no history of alcohol or tobacco use. She underwent endoscopic surgery. Afterward, she received chemoradiotherapy at another hospital (protocol unknown). Postoperatively, she has been followed up for 12 months to date, and no tumor recurrence has been observed.
Histologically, all three cases presented with poorly differentiation. The tumor consisted of relatively homogeneous, small to medium-sized cells with sparse cytoplasm and deep-stained nuclei with prominent nucleoli. No abrupt squamous differentiation or keratinization was evident in the three cases we collected. Neutrophil infiltration was seen in all but case 3 (Figures 2A,B). NUT IHC was performed, showing speckled nuclear staining with NUT fusion characteristics (Figures 2D,E). Furthermore, IHC results showed that all tumor cells expressed p63 (Figure 2C) but not PD-L1 (Figure 2F). The results of other immunohistochemical parameters are detailed in Table 2.
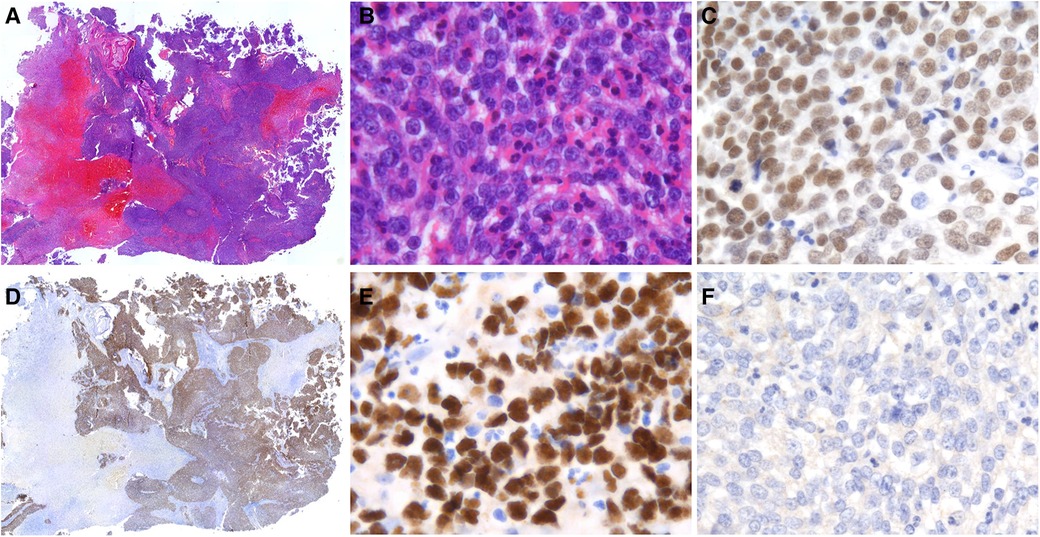
Figure 2. Histological and IHC findings of sinonasal NUT carcinoma. (A) HE staining showed that NUT carcinoma grew as sheets and nested of cells (Case 1, original magnification ×50). (B) The tumor was predominantly composed of small to middle-sized cells with scant cytoplasm. Marked infiltration of neutrophils was seen (Case 2, original magnification ×400). (C) Diffuse expression of p63 was observed in all cases (Case 1, original magnification ×400). (D,E) Positive nuclear NUT immunostaining was present (Case 1, original magnification ×50, ×400). (F) PD-L1 was negative in all cases (Case 1, original magnification ×400).
The three sinonasal NUT carcinoma cases exhibited different FISH results. Typical NUT break-apart was observed in 62.0% of the tumor cells in case 1 (Figure 3A). However, the percentage of typical splitting signals was meager (1G1R1F 1.5%) in case 2 (Figure 3B). Notably, case 3 lacked the typical NUT break signal. Nevertheless, the additional green signal was present in most tumor cells, demonstrating an atypical abnormality of the NUT gene (Figure 3C).
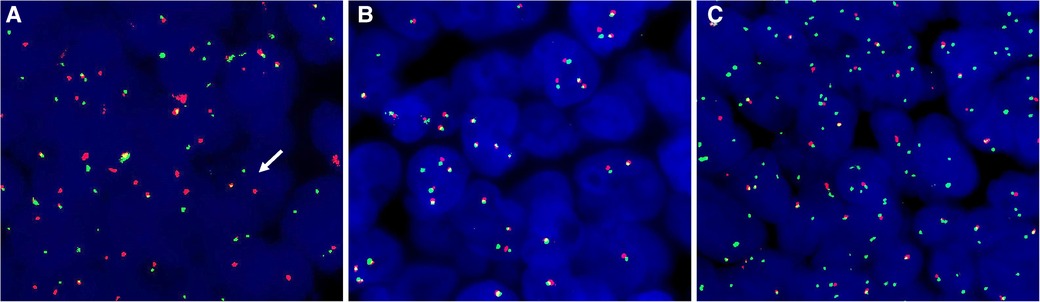
Figure 3. FISH with NUT break-apart probes. (A) Case 1 exhibited typical split signals. (B) Case 2 showed no splitting of NUT signals. (C) Additional 3'NUT signals were present in case 3.
Morphologically, this case was very similar to the sinonasal NUT carcinoma: tumor also consisted of monomorphic cells with prominent nuclei, even with neutrophil infiltration. In addition, the tumor cells expressed p63 positively but not PD-L1. The apparent difference compared to NUT carcinoma was that the NUT-positive signal was present in the cytoplasm rather than the nucleus. Meanwhile, the FISH test was negative (Figure 4). Combined with other immunohistochemical tests, the case was finally diagnosed as poorly differentiated squamous carcinoma.
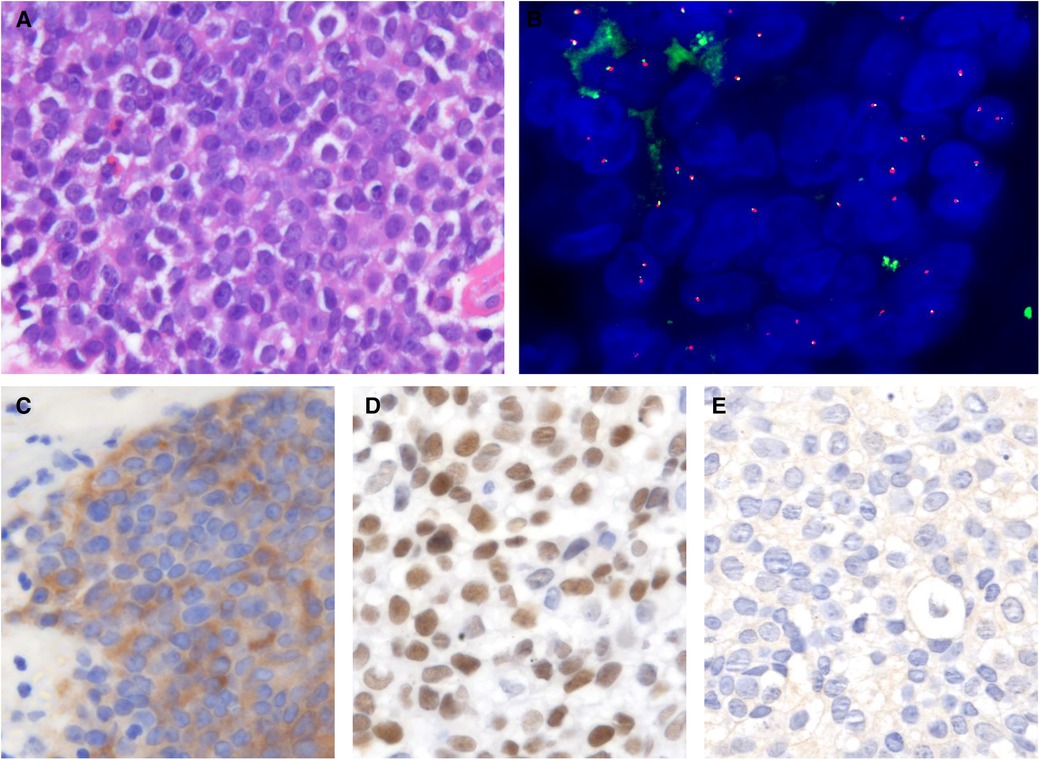
Figure 4. Histological and IHC features of the case with weakly NUT positive. (A) Tumor cell nuclei were large and round. (B) FISH assay was negative. (C) NUT positivity was seen in the cytoplasm but not the nucleus. (D) Diffuse p63 positivity is present. (E) Negative staining for PD-L1 (HE and IHC, original magnification ×400).
Approximately 51 cases of sinonasal NUT carcinoma have been described to date. It is important to note that some cases may have the potential to be reported repeatedly. For example, French et al. summarized the cases of NUT carcinoma at different years or from different perspectives in the study units (4, 7, 19). The age at diagnosis for these 51 cases ranged from 9 months to 67 years, with a mean of 40.96 years. Among them, 28 are male, and 23 are female, with a sex ratio of 1.22. Most cases expressed p63, AE1/AE3, as well as p40. In contrast, all relevant tests for EBV were negative. The details of each case are summarized in Table 3.
The overall incidence of NUT carcinoma is very low. To better summarize and study the disease, in 2010, French et al. established the International NUT Midline Carcinoma Registry (INMCR) to perform analyses of clinical and pathologic data for natural history, therapeutic intervention, and outcome (7). From 1993 to 2014, 107 patients were collected in the INMCR, of which 48 (45%) were head and neck NUT carcinoma, with 57% originating in the nasal cavity. BRD4-NUT gene fusion was present in 86% of cases (7). Although sinonasal NUT carcinoma is relatively frequent (8), the number of reported cases is still rare, making it difficult to summarize the epidemiological features, optimal treatment options, and prognoses. Here we reported three cases from a single institution and summarized the previously reported 51 cases to improve the knowledge about the clinical, radiologic, and pathologic characteristics of this disease.
Histologically, NUT carcinoma is an undifferentiated or poorly differentiated cancer marked by the persistent expression of epithelial markers, such as whole pancytokeratins (AE1/AE3), CAM5.2, and EMA on IHC (14, 16). Besides, NUT carcinoma of the sinonasal tract can be positive for p63, p40, and CD34 (9, 16).
Interestingly, the case with weakly positive for NUT IHC in our study expressed Myc. Myc is expressed in a variety of tumors, including adenocarcinoma and lymphoma. Although Myc is not a specific marker for NUT carcinoma, evidence suggests that this oncogene plays a vital role in the disease (12, 16). NKX2.2 is a new sensitive marker to differentiate Ewing's sarcoma and olfactory neuroblastoma from other small round cell tumors (16). No NKX2.2-positive sinonasal NUT carcinoma cases have been reported to date. Previous studies have shown that NUT carcinoma lack expression of checkpoint immunotherapy markers (39). Similarly, none of our three cases expressed PD-L1 in tumor tissue.
There is no evidence that smoking or virus infection is associated with NUT carcinoma (5, 6). Consistent with previous reports, our cases were also negative for EBER-ISH. However, there were some sinonasal NUT carcinoma cases positive for p16 IHC in other studies (Table 3).
In addition to IHC, various assays can be used to identify NUT rearrangements, including FISH, reverse transcriptase polymerase chain reaction (RT-PCR), cytogenetics, and next-generation sequencing (NGS) (5). The three cases we reported exhibited different FISH results. However, FISH is not completely specific for diagnosing NUT carcinoma, and a negative result cannot be used as a definitive exclusion. Some unexpected cases of “cryptic” BRD4-NUT rearrangements strongly positive for NUT IHC were negative for standard FISH (20). For example, McLean-Holden et al. reported a case with negative FISH result diagnosed by IHC and RNA sequencing. The reason for false-negative FISH results in some NUT carcinoma cases is not entirely clear. However, it may be due to the fact that many NUT translocations are caused by chromosomal abnormalities, in which up to 30 rearrangements arise from a single catastrophic event resulting in a single oncogenic fusion (40). For the NUT carcinoma diagnosis, the sensitivity of FISH is 93%, and as a standard, IHC has a sensitivity of 87% and a specificity of 100% (20). More than 50% positive staining is considered diagnostic as NUT carcinoma according to the WHO tumor classification. Germ cell tumors such as seminoma, dysgerminoma, and embryonal carcinoma, or rare poorly differentiated carcinoma may also stain, but only focally (<10%) (5).
For poorly differentiated/undifferentiated malignant with relatively homogeneous morphology, it is necessary to perform NUT IHC assays for differential diagnosis (16). Accurate diagnosis is vital, not only because of the tumor's aggressiveness but also for detecting potential molecular targeted therapies. NUT cancer is unique in that epithelial cancers are usually characterized by multiple sequential mutations that can progress to carcinogenesis through a multistep pathway. Translocation-associated fusion oncoproteins are commonly found in hematopoietic and mesenchymal malignancies (11).
NUT is a protein with largely unknown functions, shuttling between the nucleus and cytoplasm (1). Under normal conditions, the NUT promoter is active only in adult testis and ciliary ganglia. Thus, only one type of the fusion genes is expressed, such as BRD-NUT (where the BRD4 promoter and bromodomains drive aberrant NUT expression and chromatin binding), but not NUT-BRD (18, 41). The BET family of proteins consists of two tandem bromodomains (BD) and an extra-terminal structural domain (ET) (5). BRD2, BRD3, BRD4, and BRDT, these BETs are highly homologous (5). Normally, the function of BRD4 is to facilitate transcriptional elongation through the recruitment of CDK9/Cyclin T1 heterodimer (P-TEFb) (42). NUT is trapped in the nucleus when fused to BRD4 or BRD3. This is due to the acetylated lysine residues bound to and localized on the histone by the bromodomain protein. When NUT protein binds to histone acetyltransferase p300, p300 is isolated to the site of the BRD4/3-NUT complex, leading to local hyperacetylation of the histone (1). In vitro studies have shown that NUT fusion proteins drive tumor growth and block differentiation through aberrant histone acetylation depending on the targeting of Myc and TP63 genes by BRD bromodomains (12). In addition to NUT carcinoma, other types of tumors may also have NUT gene rearrangements, such as sarcoma (1).
BET inhibitor drugs are acetylated histone analogs that competitively inhibit the binding of fusion products such as BRD4-NUT, and clinical trials have demonstrated efficacy in the treatment of NUT carcinoma. HDACi can promote overall histone acetylation and facilitate differentiation to the squamous phenotype, of which clinical results have also been seen (40). Nevertheless, approximately 1/3 of NUT rearranged tumors are so-called “NUT variants,” defined as cases in which NUT is fused to non-BRD genes, some of which do not encode or interact with bromodomain-containing proteins. This increases the likelihood that some cases will not respond to BETi therapy (1). Moreover, since BRD4 is expressed in most tissues, toxicity (most commonly thrombocytopenia) also limits the efficacy of BETi in the treatment (5).
Despite the availability of targeted drugs, the overall prognosis of NUT carcinoma remains poor. In a recent review of NUT carcinoma, Chau et al. reviewed 141 cases reported by the INMCR. Of these patients, only 16 survived at least 3 years, 6 survived at least 5 years, and only 1 survived at least 10 years (15). A few exceptional cases had more prolonged survival after diagnosis, ranging from 35 to 144 months (40).
In the present study, we sought to explore the pathological features and clinical manifestations of NUT carcinoma in the sinonasal tract. For this purpose, we retrospectively analyzed all of the sinonasal tumors in our hospital. Out of 145 cases of sinonasal tumors, a total of three cases were diagnosed as NUT carcinoma. It is important to note that the proportion of adolescent patients is lower than adult in our hospital. This could be the reason for the higher mean age of disease.
Sinonasal NUT carcinoma is a rare disease with aggressive behavior and a poor prognosis. Tests for NUT rearrangement should be performed in all suspicious cases, especially in the paranasal sinuses and nasal cavity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethics Committee of Peking Union Medical College Hospital, and the requirement of informed consent was waived. All the authors have followed the applicable ethical standards to maintain the research integrity without any duplication, fraud, or plagiarism issues.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
National Natural Science Foundation of China (grant number 82071027 and 82101200); Natural Science Foundation of Beijing (grant number 7202162); National High Level Hospital Clinical Research Funding (grant number 2022-PUMCH-B-096 and 2022-PUMCH-A-030).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Stevens TM, Morlote D, Xiu J, Swensen J, Brandwein-Weber M, Miettinen MM, et al. NUTM1-rearranged neoplasia: a multi-institution experience yields novel fusion partners and expands the histologic spectrum. Mod Pathol. (2019) 32(6):764–73. doi: 10.1038/s41379-019-0206-z
2. French CA, Kutok JL, Faquin WC, Toretsky JA, Antonescu CR, Griffin CA, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. (2004) 22(20):4135–9. doi: 10.1200/JCO.2004.02.107
3. Kubonishi I, Takehara N, Iwata J, Sonobe H, Ohtsuki Y, Abe T, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. (1991) 51(12):3327–8. doi: 10.1002/1097-0142(19910615)67:12%3C3165::AID-CNCR2820671238%3E3.0.CO
4. Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Luer SC, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. (2012) 18(20):5773–9. doi: 10.1158/1078-0432.CCR-12-1153
5. French CA. NUT carcinoma: clinicopathologic features, pathogenesis, and treatment. Pathol Int. (2018) 68(11):583–95. doi: 10.1111/pin.12727
6. Salles P, Moura RD, Menezes L, Bacchi CE. Expression of P16 in NUT carcinomas with no association with human papillomavirus (HPV). Appl Immunohistochem Mol Morphol. (2014) 22(4):262–5. doi: 10.1097/PAI.0b013e3182a4ef2e
7. Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. (2016) 122(23):3632–40. doi: 10.1002/cncr.30242
8. Lee T, Cho J, Baek CH, Son YI, Jeong HS, Chung MK, et al. Prevalence of NUT carcinoma in head and neck: analysis of 362 cases with literature review. Head Neck. (2020) 42(5):924–38. doi: 10.1002/hed.26067
9. Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. (2012) 36(8):1216–21. doi: 10.1097/PAS.0b013e318254ce54
10. French C, Miyoshi I, Kubonishi I, Grier H, Perez-Atayde A, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. (2003) 63(2):304–7.
11. French CA, Rahman S, Walsh EM, Kuhnle S, Grayson AR, Lemieux ME, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov. (2014) 4(8):928–41. doi: 10.1158/2159-8290.CD-14-0014
12. Grayson AR, Walsh EM, Cameron MJ, Godec J, Ashworth T, Ambrose JM, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene. (2014) 33(13):1736–42. doi: 10.1038/onc.2013.126
13. Chen M, Zhao S, Liang Z, Wang W, Zhou P, Jiang L. NUT carcinoma of the parotid gland: report of two cases, one with a rare ZNF532-NUTM1 fusion. Virchows Arch. (2022) 480(4):887–97. doi: 10.1007/s00428-021-03253-9
14. Lemelle L, Pierron G, Freneaux P, Huybrechts S, Spiegel A, Plantaz D, et al. NUT carcinoma in children and adults: a multicenter retrospective study. Pediatr Blood Cancer. (2017) 64(12). doi: 10.1002/pbc.26693
15. Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R, et al. An anatomical site and genetic-based prognostic model for patients with Nuclear Protein in Testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr. (2019) 4(2):pkz094. doi: 10.1093/jncics/pkz094
16. Minato H, Kobayashi E, Nakada S, Kurose N, Tanaka M, Tanaka Y, et al. Sinonasal NUT carcinoma: clinicopathological and cytogenetic analysis with autopsy findings. Hum Pathol. (2018) 71:157–65. doi: 10.1016/j.humpath.2017.10.011
17. Hsieh MS, French CA, Liang CW, Hsiao CH. NUT midline carcinoma: case report and review of the literature. Int J Surg Pathol. (2011) 19(6):808–12. doi: 10.1177/1066896909353600
18. Suzuki S, Kurabe N, Minato H, Ohkubo A, Ohnishi I, Tanioka F, et al. A rare Japanese case with a NUT midline carcinoma in the nasal cavity: a case report with immunohistochemical and genetic analyses. Pathol Res Pract. (2014) 210(6):383–8. doi: 10.1016/j.prp.2014.01.013
19. French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol. (2010) 63(6):492–6. doi: 10.1136/jcp.2007.052902
20. Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. (2009) 33(7):984–91. doi: 10.1097/PAS.0b013e318198d666
21. Thompson LDR, Franchi A. New tumor entities in the 4th edition of the world health organization classification of head and neck tumors: nasal cavity, paranasal sinuses and skull base. Virchows Arch. (2018) 472(3):315–30. doi: 10.1007/s00428-017-2116-0
22. Davis BN, Karabakhtsian RG, Pettigrew AL, Arnold SM, French CA, Brill YM. Nuclear protein in testis midline carcinomas: a lethal and underrecognized entity. Arch Pathol Lab Med. (2011) 135(11):1494–8. doi: 10.5858/arpa.2010-0389-CR
23. Stelow E, Bellizzi A, Taneja K, Mills S, Legallo R, Kutok J, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. (2008) 32(6):828–34. doi: 10.1097/PAS.0b013e31815a3900
24. Solomon LW, Magliocca KR, Cohen C, Muller S. Retrospective analysis of nuclear protein in testis (NUT) midline carcinoma in the upper aerodigestive tract and mediastinum. Oral Surg Oral Med Oral Pathol Oral Radiol. (2015) 119(2):213–20. doi: 10.1016/j.oooo.2014.09.031
25. Stirnweiss A, McCarthy K, Oommen J, Crook ML, Hardy K, Kees UR, et al. A novel BRD4-NUT fusion in an undifferentiated sinonasal tumor highlights alternative splicing as a contributing oncogenic factor in NUT midline carcinoma. Oncogenesis. (2015) 4:e174. doi: 10.1038/oncsis.2015.33
26. Kakkar A, Antony VM, Irugu DVK, Adhikari N, Jain D. NUT midline carcinoma: a series of five cases, including one with unusual clinical course. Head Neck Pathol. (2018) 12(2):230–6. doi: 10.1007/s12105-017-0858-2
27. Klijanienko J, Le Tourneau C, Rodriguez J, Caly M, Theocharis S. Cytological features of NUT midline carcinoma arising in sino-nasal tract and parotid gland: report of two new cases and review of the literature. Diagn Cytopathol. (2016) 44(9):753–6. doi: 10.1002/dc.23506
28. Edgar M, Caruso AM, Kim E, Foss RD. NUT midline carcinoma of the nasal cavity. Head Neck Pathol. (2017) 11(3):389–92. doi: 10.1007/s12105-016-0763-0
29. Arimizu K, Hirano G, Makiyama C, Matsuo M, Sasaguri T, Makiyama A. NUT carcinoma of the nasal cavity that responded to a chemotherapy regimen for Ewing's sarcoma family of tumors: a case report. BMC Cancer. (2018) 18(1):1134. doi: 10.1186/s12885-018-5087-x
30. Crocetta FM, Botti C, Fornaciari M, Castellucci A, Murri D, Santandrea G, et al. Sinonasal NUT carcinoma: delayed diagnosis due to the COVID-19 pandemic and a review of the literature. Head Neck Pathol. (2021) 15(4):1409–14. doi: 10.1007/s12105-021-01311-x
31. Vakani P, Maheshwari J, Maheshwari M, Shah B. Sinonasal NUT midline carcinoma: a new histological entity. Indian J Pathol Microbiol. (2020) 63(1):103–5. doi: 10.4103/IJPM.IJPM_373_19
32. Chan W, Bullock MJ, Samad AF, Archibald CW, Heathcote JG. NUT carcinoma of the sinonasal tract infiltrating the orbit in a man with birdshot chorioretinitis. Saudi J Ophthalmol. (2018) 32(1):62–5. doi: 10.1016/j.sjopt.2018.02.018
33. Albrecht T, Harms A, Roessler S, Goeppert B. NUT carcinoma in a nutshell: a diagnosis to be considered more frequently. Pathol Res Pract. (2019) 215(6):152347. doi: 10.1016/j.prp.2019.01.043
34. Patel SA, Singer B, Shen C, Zanation AM, Yarbrough WG, Weiss J. A case of metastatic NUT carcinoma with prolonged response on gemcitabine and nab-paclitaxel. Clin Case Rep. (2021) 9(8):e04616. doi: 10.1002/ccr3.4616
35. Shaikh F, Pagedar N, Awan O, McNeely P. Sinonasal NUT-midline carcinoma - A multimodality approach to diagnosis, staging and post-surgical restaging. Cureus. (2015) 7(7):e288. doi: 10.7759/cureus.288
36. Laco J, Kovarikova H, Chmelarova M, Vosmikova H, Sieglova K, Bubancova I, et al. Analysis of DNA methylation and microRNA expression in NUT (nuclear protein in testis) midline carcinoma of the sinonasal tract: a clinicopathological, immunohistochemical and molecular genetic study. Neoplasma. (2018) 65(1):113–23. doi: 10.4149/neo_2018_161122N581
37. Wei X, Teng X, Zhang Y, Cheng M, Chen G. Case report: NUT carcinoma in an elderly woman with unique morphology and immunophenotype highlights a diagnostic pitfall. Transl Cancer Res. (2022) 11(6):1850–60. doi: 10.21037/tcr-22-364
38. Fang W, French C, Cameron M, Han Y, Liu H. Clinicopathological significance of NUT rearrangements in poorly differentiated malignant tumors of the upper respiratory tract. Int J Surg Pathol. (2013) 21(2):102–10. doi: 10.1177/1066896912451651
39. He M, Chernock R, Zhou S, Gondim M, Dehner L, Pfeifer JD, et al. Tumor mutation burden and checkpoint immunotherapy markers in NUT midline carcinoma. Appl Immunohistochem Mol Morphol. (2020) 28(7):495–500. doi: 10.1097/PAI.0000000000000781
40. McLean-Holden AC, Moore SA, Gagan J, French CA, Sher D, Truelson JM, et al. NUT carcinoma in a patient with unusually long survival and false negative FISH results. Head Neck Pathol. (2021) 15(2):698–703. doi: 10.1007/s12105-020-01220-5
41. French CA. NUT midline carcinoma. Cancer Genet Cytogenet. (2010) 203(1):16–20. doi: 10.1016/j.cancergencyto.2010.06.007
Keywords: NUTM1 protein human, paranasal sinuses, nasal cavity, prognosis, molecular targeted therapy
Citation: Wang L, Zhu Z, Wang W, Zha Y, Wang X, Surita A, Liu Y and Lv W (2023) Sinonasal NUT carcinoma: A retrospective case series from a single institution. Front. Surg. 10:1098704. doi: 10.3389/fsurg.2023.1098704
Received: 15 November 2022; Accepted: 9 February 2023;
Published: 1 March 2023.
Edited by:
John Charles Rotondo, University of Ferrara, ItalyReviewed by:
AB Zulkiflee, University Malaya Medical Centre, Malaysia© 2023 Wang, Zhu, Wang, Zha, Wang, Surita, Liu and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Lv bGlsaTIwMDIwNjE1QHNpbmEuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Otorhinolaryngology - Head and Neck Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.