- 1Department of Hepatobiliary Surgery, Wuhan No.1 Hospital (Wuhan Hospital of Traditional Chinese and Western Medicine), Wuhan, China
- 2Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: An increased risk of gallstones has been observed in patients undergoing hepatectomy. This study attempted to analyze the risk factors for gallstones after hepatectomy.
Methods: From January 2013 to December 2016, clinical data of 1,452 eligible patients who underwent hepatectomy were consecutively reviewed. According to the imaging, including gallbladder ultrasound, computerized tomography, and magnetic resonance imaging, all patients were divided into the gallstone group and the nongallstone group. Univariate and multivariate logistic regression analyses were performed to select indicators associated with gallstone formation among patients after hepatectomy.
Results: In the total sample of included patients, there were 341 patients with gallstones and 1,147 patients without gallstones. The incidence of gallstones was 23.5% (341/1,452). The incidence of gallstones in the primary liver cancer group was higher than that in the benign liver tumor group (25.7% vs. 18.9%, P = 0.004). Univariate and multivariate logistic regression analyses showed that female gender, high body mass index, tumor located in S5, and severe postoperative complication were factors related to gallstones in patients with benign liver tumors after hepatectomy. In addition, Child–Pugh B, low albumin, liver cirrhosis, and transcatheter arterial chemoembolization (TACE) after recurrence were factors related to gallstones in patients with primary liver cancer after hepatectomy.
Conclusions: Hepatectomy increased the risk of gallstones in benign or malignant liver tumors, especially when the tumor was located in S5. TACE further increased the risk of gallstones in patients with postoperative recurrence.
Introduction
The incidence of gallstones in adults is 10%–20% (1–3). The morbidity of gallstones in patients with chronic liver disease (CLD) ranges from 3.6% to 46%, with a 1.2- to 5-fold increase compared with the general population (1, 3–5). Clinically, we have found that the incidence of gallstones is significantly increased in those patients who have undergone liver resection or other therapies such as transcatheter arterial chemoembolization (TACE). Previous studies have shown that any factors involving changes in the composition of hepatic bile and gallbladder hypomotility will facilitate gallstone formation (6–8). The anatomy of the intrahepatic bile duct is complex and closely related to liver tissue. It is inevitable that hepatectomy or TACE treatment will lead to iatrogenic biliary tract injury including the nourishing blood vessels of the biliary tract or the function of the gallbladder's vagus. In addition, whether postoperative complications such as abdominal adhesion or abdominal infection can lead to impaired function of contraction of the gallbladder still needs to be further verified. Therefore, we retrospectively analyzed the risk factors for gallstone formation after hepatectomy.
Materials and methods
Patients and definitions
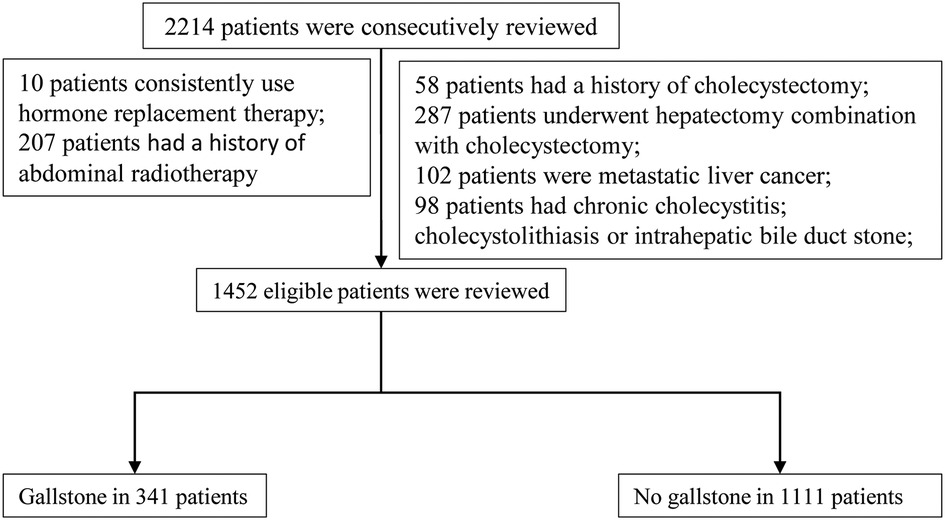
This was a retrospective case–control study. We consecutively reviewed 1,452 eligible patients who underwent hepatectomy between January 2013 and December 2016 in the Hepatic Surgery Center of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. According to the imaging, including gallbladder ultrasound, computerized tomography (CT), and magnetic resonance imaging (MRI), all patients were divided into the gallstone group and the nongallstone group. The flow chart of patients is displayed in Figure 1.
The inclusion criteria are as follows: (1) Child–Pugh A or B; (2) primary liver cancer Barcelona Clinic Liver Cancer (BCLC) A/B stages; and (3) benign tumor of liver including hepatic adenoma, hepatic hemangioma, and hepatic focal nodular hyperplasia (FNH). The exclusion criteria are as follows: (1) metastatic liver cancer; (2) history of cholecystectomy; (3) already existing cholecystitis or gallstones; (4) intrahepatic bile duct stones; (5) consistent use of hormone replacement therapy; and (6) abdominal radiotherapy.
The gallbladder is attached to S5 of the liver. In general, anatomical hepatectomy usually combines with cholecystectomy when a tumor is located in S5 of the liver. When the tumor is small and nonanatomic hepatectomy is performed, gallbladder preservation is feasible. Thus, there is a considerable number of patients with tumors located in S5 of the liver in our study who underwent hepatectomy with gallbladder preservation.
Postoperative complications are common after hepatectomy. The severity of complications was classified according to the Clavien–Dindo classification system (9). Grade I complications included mild pleural, peritoneal effusion, electrolyte disturbance, and other deviations from the normal postoperative course. Grade II complications included blood transfusion, plasma infusion, or albumin infusion. Grade III covered severe pleural and peritoneal effusion requiring percutaneous aspiration or drainage. Grade IV complications covered severe lung infections, respiratory failure, or kidney failure, requiring intubation or dialysis treatment.
Surgical methods
Hepatectomy was accomplished in a hepatic surgery center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. All patients were given general anesthesia. The methods of hepatectomy included open and laparoscopic hepatectomy. Partial resection or anatomic resection of the liver was performed based on the tumor location. Complete removal of at least one Couinaud segment containing the focus and the portal vein in the drainage area of the lesion was defined as an anatomic resection. A complete tumor plus a rim of non-neoplastic liver parenchyma was considered a nonanatomic resection. Partial resection makes preservation of the gallbladder possible when the surgeon removes the small lesions located at S5.
The study was approved by the Ethical Committee of Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology. All procedures performed in this study abided by the Declaration of Helsinki.
Statistical analysis
Continuous variables were presented as the median and interquartile range (IQR), and categorical data as were presented numbers and percentages. A χ2 test and the Mann–Whitney U test were used to compare groups where appropriate. Univariate and multivariate logistic regression analyses were performed to evaluate risk factors for gallstone development. P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 19.0 for Windows (SPSS, Chicago, IL, United States).
Results
Clinical characteristics of patients
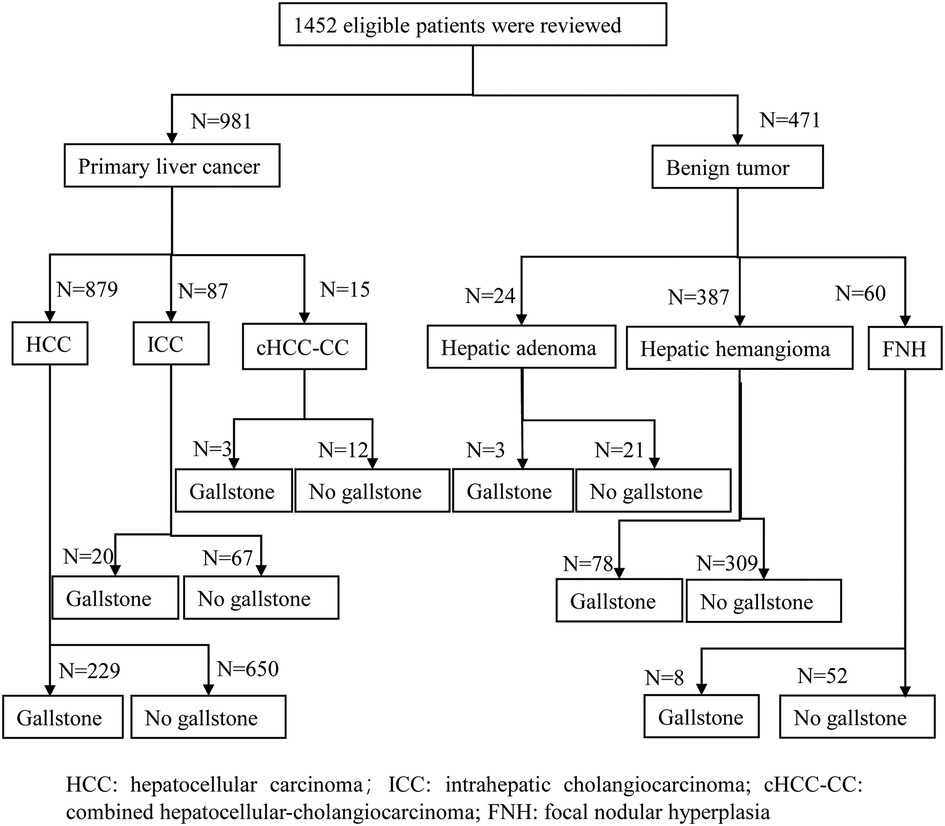
Figure 2 shows that 1,452 eligible patients were stratified according to the malignant or benign tumor. In the total sample of included patients, there were 981 patients with primary liver cancer and 471 patients with benign tumors, including 24 patients with hepatic adenoma, 387 patients with hepatic hemangioma, and 60 patients with FNH.
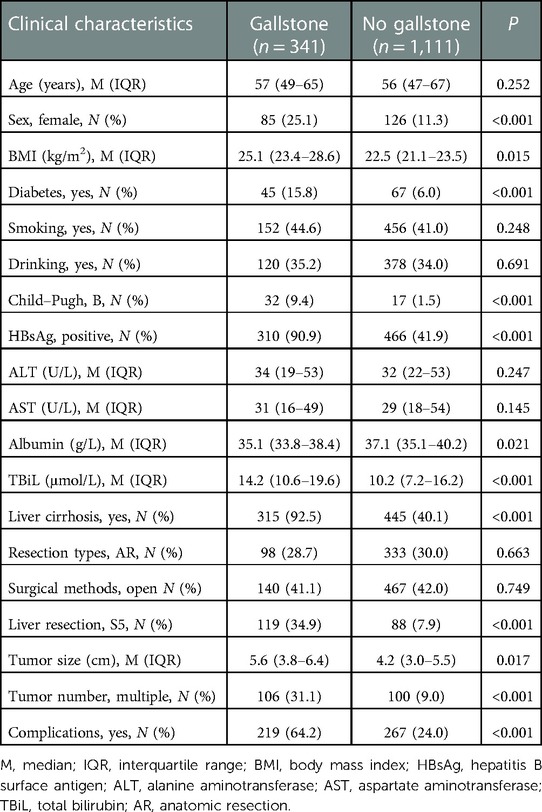
The median age was 56 years, and 85.5% (1,241/1,452) of patients were men. In total, 96.6% (1,403/1,452) of patients were Child–Pugh A. The etiology of patients was 776 (53.4%) positive for hepatitis B virus (HBV), 18 (1.2%) positive for hepatitis C virus (HCV), 41 (2.8%) for other causes, and 617 (42.6%) with no hepatitis background. A higher body mass index (BMI), total bilirubin, tumor size, and lower albumin were observed in the gallstone group. The proportion of the female gender, diabetes, Child–Pugh B, hepatitis B surface antigen (HBsAg)-positive, liver cirrhosis, S5 liver resection, multiple tumor, and postoperative complications was higher in the gallstone group (Table 1).
The overall gallstone incidence was 23.5% (341/1,452), and the asymptomatic gallstone incidence was 83.6% (285/341). The incidence of gallstones in the primary liver cancer group was 25.7% (252/981) higher than that in the benign tumor group [18.9% (89/471), P = 0.004]. Asymptomatic gallstones accounted for 81.7% (206/252) of all gallstones in the primary liver cancer group and 89.2% (79/89) of all gallstones in the benign tumor group (P = 0.11).
In 4 years, 41 out of 341 patients (12.0%) with gallstones underwent cholecystectomy. All patients with cholecystectomy had significant right upper abdominal pain, and their Murphy sign was positive. In addition, CT indicated thickening of the gallbladder wall and chronic cholecystitis. No cholecystectomy was performed for all asymptomatic and some symptomatic gallstones. Moreover, we had not observed any serious disease caused by gallstones, including acute pancreatitis and gallbladder cancer.
Factors associated with a gallstone in patients with benign liver tumors
Univariate and multivariate logistic regression analyses showed that female gender (OR: 1.547, 95% CI: 1.358–2.356, P = 0.018), BMI (OR: 3.255, 95% CI: 2.789–4.589, P = 0.009), liver resection at S5 (OR: 3.687, 95% CI: 2.987–4.698, P = 0.012), and postoperative complication (OR: 3.684, 95% CI: 3.489–4.398, P < 0.001) were factors related to gallstone in patients with benign liver tumors after hepatectomy (Table 2).

Table 2. Univariate and multivariate logistic regression analyses on factors associated with gallstones for patients with benign liver tumors.
Factors associated with gallstones in patients with primary liver cancer
Univariate and multivariate logistic regression analyses showed that female gender (OR: 2.314, 95% CI: 2.021–4.529, P = 0.037), BMI (OR: 2.125, 95% CI: 1.546–3.356, P = 0.011), diabetes (OR: 1.846, 95% CI: 1.259–2.136, P = 0.019), Child–Pugh B (OR: 2.472, 95% CI: 1.261–4.025, P = 0.008), albumin (OR: 2.851, 95% CI: 2.622–3.914, P < 0.001), liver cirrhosis (OR: 4.258, 95% CI: 3.258–6.398, P < 0.001), liver resection at S5 (OR: 4.589, 95% CI: 3.514–6.215, P < 0.001), postoperative complication (OR: 6.256, 95% CI: 5.021–7.654, P < 0.001), and TACE after recurrence (OR: 2.691, 95% CI: 2.102–3.028, P = 0.019) were factors related to gallstones in patients with primary liver cancer after hepatectomy (Table 3).

Table 3. Univariate and multivariate logistic regression analyses on factors associated with gallstones for patients with primary liver cancer.
Typical cases of gallstone stone formation
Figure 3 shows three typical cases of gallstone formation after hepatectomy or recurrent patients receiving TACE. Case 1 displayed that the tumor was located in S5 (white triangle) and there was no gallstone in the gallbladder before surgery (Figures 3A,B). The gallstone developed in the gallbladder (arrow), and the gallbladder tightly adhered to the area of abdominal adhesions (Figure 3C). Case 2 showed that the surgical area was located in S4 and partial S5, and the surgical area presented with effusion and adhesions (arrow) (Figures 3D,E). The gallstone developed in the gallbladder ultimately (arrow) (Figure 3F). Case 3 showed that a recurrent patient after surgery whose lesion was located in S5 received TACE treatment; most areas of the lesion are necrotic (white triangle). The gallstone developed in the gallbladder ultimately and the wall of the gallbladder was significantly thickened (arrow) (Figures 3G,H).

Figure 3. Three typical cases of gallstone formation after hepatectomy or recurrent patients receiving TACE. (A) Tumor was located in S5 (white triangle); (B) there was no gallstone in the gallbladder before surgery; (C) gallstone developed in the gallbladder (arrow), and the gallbladder tightly adhered to the area of abdominal adhesions; (D,E) surgical area was located in S4 and partial S5 and the surgical area presented with effusion and adhesions (arrow); (F) gallstone developed in the gallbladder ultimately (arrow); (G) recurrent patient after surgery whose lesion was located in S5 received TACE treatment; most areas of the lesion are necrotic (white triangle); (H) gallstone developed in the gallbladder ultimately and the wall of the gallbladder was significantly thickened (arrow).
Discussion
The traditional risk factors for gallstone disease are the four “F's: female, fat, forty, and fertile,” with many studies supporting the known risk factors for gallstone disease (10, 11). In our study, female gender and BMI are risks factor for the formation of gallstones in patients with benign or malignant tumors after hepatectomy. BMI, which positively correlates with blood lipids, is also a well-known risk factor for gallstones (12, 13). A cohort has confirmed a close association (in both genders, but especially pronounced in women) between increasing BMI and increased risk of symptomatic gallstone disease (14). In addition, it was reported that insulin resistance is closely related to gallstone formation (15–17). In the study, diabetes was observed to be associated with gallstone formation in malignant tumor patients but not in patients with benign tumors.
Interestingly, in our study, the incidence of gallstones after hepatectomy was significantly higher in patients with primary liver cancer than that in patients with benign liver tumors. Generally speaking, most patients with primary liver cancer are accompanied by chronic liver disease including cirrhosis or hypoalbuminemia. It has been reported that liver cirrhosis has been identified as another risk factor for the formation of gallstones, and more severe cirrhosis and longer cirrhosis duration result in a higher incidence of gallstones (18–20). Previous studies have shown that a thickened gallbladder wall and impaired contractility have been observed when patients present with liver cirrhosis, hepatic failure, and portal hypertension (21–24). It has been reported that hypoalbuminemia will increase tissue edema, including the gallstone, which leads to impaired gallstone motility and contractility and provides a potential pathophysiologic basis for gallstone formation (24). In addition, patients with more severe chronic liver disease are more likely to have postoperative complications. Severe surgical complication grade increased the duration of parenteral nutrition and prolonged fasting, resulting in bile concentration in the gallstone and impaired gallstone motility. Peritoneal effusion, peritoneal cavity infection, and bile leakage often indicated the existence of serious complications contributing to a severer inflammatory response in the abdominal cavity and severer abdominal organ adhesions, leading to swelling and exudation of local tissues and impairing gallstone motility.
In addition, we observed an increased risk of gallstone formation when the tumor is located in S5. Gallstone function was extremely affected when the behavior of liver resection was related to the gallbladder. On the one hand, peritoneal effusion or abdominal adhesion will inevitably occur near the gallbladder, which will impair its motility. On the other hand, when hepatectomy is performed near the gallbladder, it may be disturbed (for instance, it may be torn or squeezed), resulting in gallbladder motility and contractility.
In our study, we found that patients receiving TACE for recurrence after hepatectomy had a higher incidence of gallstones. On the one hand, as we know, the gallbladder artery arises from the right hepatic artery. When TACE is performed, the embolization agent or chemotherapy drug may reflux into the gallbladder artery, leading to a decreased blood supply to the gallbladder and mucosal necrosis of the gallstone; this is more likely when TACE is performed via the right hepatic artery. Therefore, decreased blood supply to the gallbladder leads to gallstone hypomotility and eventually to gallbladder atrophy. On the other hand, chemotherapy drugs stimulate the gallbladder mucosa for a long period, such that the gallbladder mucosa thickens and chronic cholecystitis develops.
Conclusion
In conclusion, the retrospective case–control study suggests an association between the incidence of gallstones and the location of tumors, especially in S5. In addition, for patients with hepatocellular carcinoma (HCC), TACE further increases the risk of gallstones in patients with postoperative recurrence. Thus, the increased risk of gallstone development after hepatectomy deserves to be concerned.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found here: 10.6084/m9.figshare.21505629.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ and LC analyzed data and wrote the manuscript. ZZ designed the research route. BW modified the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Chang Shu (Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology) for his assistance with statistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CLD, chronic liver disease; TBiL, total bilirubin; HBV, hepatitis B virus; HCV, hepatitis C virus; HBsAg, hepatitis B surface antigen; TACE, transcatheter arterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer; CT, computerized tomography; MRI, magnetic resonance imaging; IQR, interquartile range; FNH, focal nodular hyperplasia.
References
1. Godrey PJ, Bates T, Harrison M, King MB, Padley NR. Gall stones and mortality: a study of all gall stone related deaths in a single health district. Gut. (1984) 25:1029–33. doi: 10.1136/gut.25.10.1029
3. Barker DJ, Gardner MJ, Power C, Hutt MS. Prevalence of gall stones at necropsy in nine British towns: a collaborative study. Br Med J. (1979) 2:1389–92. doi: 10.1136/bmj.2.6202.1389
4. Bouchier IA. Postmortem study of the frequency of gallstones in patients with cirrhosis of the liver. Gut. (1969) 10:705–10. doi: 10.1136/gut.10.9.705
5. Conte D, Barisani D, Mandelli C, Bodini P, Bianchi PA. Cholelithiasis in cirrhosis: analysis of 500 cases. Am J Gastroenterol. (1991) 86:1629–32.
7. Sheen IS, Liaw YF. The prevalence and incidence of cholecystolithiasis in patients with chronic liver diseases: a prospective study. Hepatology. (1989) 9:538–40. doi: 10.1002/hep.1840090405
8. Acalovschi M. Gallstones in patients with liver cirrhosis: incidence, etiology, clinical and therapeutical aspects. World J Gastroenterol. (2014) 20:7277–85. doi: 10.3748/wjg.v20.i23.7277
9. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
10. Acalovschi M. Cholesterol gallstones: from epidemiology to prevention. Postgrad Med J. (2001) 77:221–9. doi: 10.1136/pmj.77.906.221
11. Shabanzadeh DM. Incidence of gallstone disease and complications. Curr Opin Gastroenterol. (2018) 34:81–9. doi: 10.1097/mog.0000000000000418
12. Kimura Y, Takada T, Kawarada Y, Nimura Y, Hirata K, Sekimoto M, et al. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. (2007) 14:15–26. doi: 10.1007/s00534-006-1152-y
13. Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. (2006) 368:230–9. doi: 10.1016/s0140-6736(06)69044-2
14. Stender S, Nordestgaard BG, Tybjaerg-Hansen A. Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatology. (2013) 58:2133–41. doi: 10.1002/hep.26563
15. Ruhl CE, Everhart JE. Association of diabetes, serum insulin, and C-peptide with gallbladder disease. Hepatology. (2000) 31:299–303. doi: 10.1002/hep.510310206
16. Shabanzadeh DM, Skaaby T, Sørensen LT, Eugen-Olsen J, Jørgensen T. Metabolic biomarkers and gallstone disease—a population-based study. Scand J Gastroenterol. (2017) 52:1270–7. doi: 10.1080/00365521.2017.1365166
17. Shabanzadeh DM, Sørensen LT, Jørgensen T. Determinants for gallstone formation—a new data cohort study and a systematic review with meta-analysis. Scand J Gastroenterol. (2016) 51:1239–48. doi: 10.1080/00365521.2016.1182583
18. Fornari F, Imberti D, Squillante MM, Squassante L, Civardi G, Buscarini E, et al. Incidence of gallstones in a population of patients with cirrhosis. J Hepatol. (1994) 20:797–801. doi: 10.1016/s0168-8278(05)80152-7
19. Conte D, Fraquelli M, Fornari F, Lodi L, Bodini P, Buscarini L. Close relation between cirrhosis and gallstones: cross-sectional and longitudinal survey. Arch Intern Med. (1999) 159:49–52. doi: 10.1001/archinte.159.1.49
20. Benvegnù L, Noventa F, Chemello L, Fattovich G, Alberti A. Prevalence and incidence of cholecystolithiasis in cirrhosis and relation to the etiology of liver disease. Digestion. (1997) 58:293–8. doi: 10.1159/000201457
21. Li CP, Hwang SJ, Lee FY, Chang FY, Lin HC, Lu RH, et al. Evaluation of gallbladder motility in patients with liver cirrhosis: relationship to gallstone formation. Dig Dis Sci. (2000) 45:1109–14. doi: 10.1023/a:1005537632665
22. Acalovschi M, Dumitraşcu DL, Csakany I. Gastric and gall bladder emptying of a mixed meal are not coordinated in liver cirrhosis—a simultaneous sonographic study. Gut. (1997) 40:412–7. doi: 10.1136/gut.40.3.412
23. Saverymuttu SH, Grammatopoulos A, Meanock CI, Maxwell JD, Joseph AE. Gallbladder wall thickening (congestive cholecystopathy) in chronic liver disease: a sign of portal hypertension. Br J Radiol. (1990) 63:922–5. doi: 10.1259/0007-1285-63-756-922
Keywords: primary liver cancer, benign tumor, hepatectomy, gallstone, retrospective case–control study
Citation: Zhou J, Chen L, Zhang Z and Wu B (2023) Analysis of risk factors for the increased incidence of gallstone caused by hepatectomy: A retrospective case–control study. Front. Surg. 10:1097327. doi: 10.3389/fsurg.2023.1097327
Received: 14 November 2022; Accepted: 24 January 2023;
Published: 1 March 2023.
Edited by:
Suk Kyun Hong, Seoul National University, Republic of KoreaReviewed by:
Adianto Nugroho, Rumah Sakit Umum Pusat Fatmawati, IndonesiaJinsoo Rhu, Sungkyunkwan University, Republic of Korea
© 2023 Zhou, Chen, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiwei Zhang emhpd2Vpemhhbmd0amhAMTYzLmNvbQ== Biao Wu d3VoYW55eXdiQDE2My5jb20=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Jiangmin Zhou
Jiangmin Zhou Lin Chen
Lin Chen Zhiwei Zhang
Zhiwei Zhang Biao Wu
Biao Wu