- Department of Gastrointestinal Surgery, The People's Hospital of Deyang, Deyang, China
Background: Limited data are available about superior rectal artery (SRA) preservation in laparoscopic resection for sigmoid colon cancer (SCC). This study aimed to evaluate the short-term and long-term efficacies of SRA preservation in laparoscopic radical resection for SCC.
Methods: We retrospectively analyzed 207 patients with SCC who underwent laparoscopic radical resection for SCC from January 2017 to June 2021. A total of 84 patients received lymph node clearance around the inferior mesenteric artery (IMA) root (D3 lymph node dissection) with preservation of SRA (SRA preservation group), and 123 patients received high ligation of the IMA (control group). The clinicopathological data of the two groups were compared, and Kaplan–Meier method was performed to estimate patient survival.
Results: Compared with the control group, the operation time of the SRA preservation group was longer (p < 0.001), but the postoperative exhaust and defecation times were significantly shorter (p = 0.003, p < 0.001). Two cases of postoperative ileus and four cases of anastomotic leakage were observed in the control group, whereas the SRA preservation group had none. However, no statistical difference was observed between the groups (p = 0.652, p = 0.248). The overall survival also showed no significant difference in (p = 0.436).
Conclusion: Preservation of SRA plus dissection of lymph nodes around IMA did not increase postoperative morbidity and mortality nor affect the prognosis of patients but increased the bowel blood supply, which may have a significant positive effect on the recovery of postoperative intestinal function and reduction of anastomotic leakage.
Introduction
For rectal cancer and sigmoid colon cancer (SCC), high ligation of inferior mesenteric artery (IMA) is performed for further lymph node dissection (1). High ligation of IMA with D3 lymph node dissection can improve the survival rate of patients (2, 3). However, insufficient perfusion of stumps after high ligation of IMA may lead to anastomotic ischemia (4–6). The blood supply of the proximal and distal stumps of anastomosis is an important factor affecting its healing. Preservation of superior rectal artery (SRA) theoretically increases the blood supply of the distal rectum and can reduce anastomotic leakage (AL) (7). However, reports on SRA preservation and its long-term efficacy are limited. In the present study, we investigated the short-term and long-term efficacies of SRA preservation to evaluate the clinical value of SRA preservation in SCC surgery.
Methods
Patient selection and data collection
In this retrospective cohort study, data were collected from patients who underwent laparoscopic radical resection of SCC at Department of Gastrointestinal Surgery, People’s Hospital of Deyang from January 2017 to June 2021.
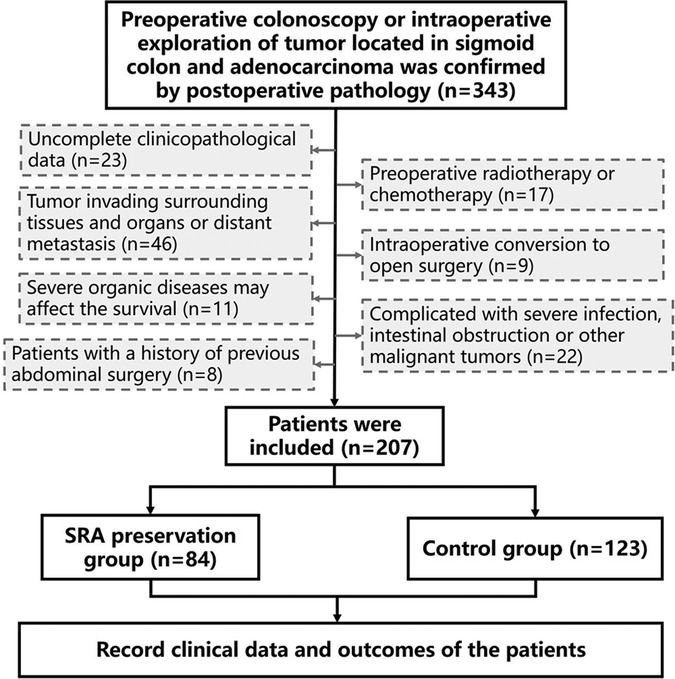
The inclusion criteria were as follows: (1) complete clinicopathological data, (2) preoperative colonoscopy or intraoperative exploration of tumor located in sigmoid colon, and (3) adenocarcinoma confirmed by postoperative pathology.
The exclusion criteria included the following: (1) preoperative radiotherapy or chemotherapy, (2) tumor invading surrounding tissues and organs or distant metastasis, (3) intraoperative conversion to open surgery, (4) severe organ diseases, such as those of heart, lung, liver, and kidney, or hematopoietic and endocrine diseases that may affect survival, (5) complication with severe infection, intestinal obstruction, or other malignant tumors; (6) history of previous abdominal surgery.
Based on SRA preservation, the patients were divided into the SRA preservation (n = 84) and control (n = 123) groups. The control group underwent conventional high ligation of the IMA root. All patient data, including age, gender, body mass index (BMI), operation time, intraoperative blood loss, tumor stage, the number of lymph nodes dissected, tumor differentiation, postoperative adjuvant chemotherapy, and postoperative exhaust and defecation times, were recorded in the database. Postoperative complications, mortality (30 days after surgery or before hospital discharge), and survival were also recorded (Figure 1).
Surgical procedures
Patients were laid in head-down lithotomy position. The peritoneal cavity was routinely explored. The peritoneum was opened on the right side of the IMA root, and the mesenteric membrane was separated upward along the space between Gerota's and Toldt's fascias. In the preservation group, the lymphoid adipose tissue at the root of IMA was dissected, and the IMA root was exposed. Then, the lymphatic adipose tissue around IMA was dissected along the direction of IMA, and the IMA vascular sheath was not opened routinely. The left colic artery and first or second sigmoid artery were divided and cut off along the IMA vascular sheath, and the SRA was preserved (Figures 2A,B). In addition to SRA, the left colic artery or inferior mesenteric vein (IMV) was selectively preserved in several patients, especially those with long sigmoid colon (Figures 2C,D). In the control conventional operation group, high ligation was performed at the roots of IMA and IMV roots (Figures 2E,F, respectively). Both groups followed the principle of complete mesocolic excision and 10 cm proximal and distal margin, and the inferior mesenteric plexus and hypogastric plexus were carefully protected. Before anastomosis, the bowels were fully freed to ensure that the anastomosis was free of tension.
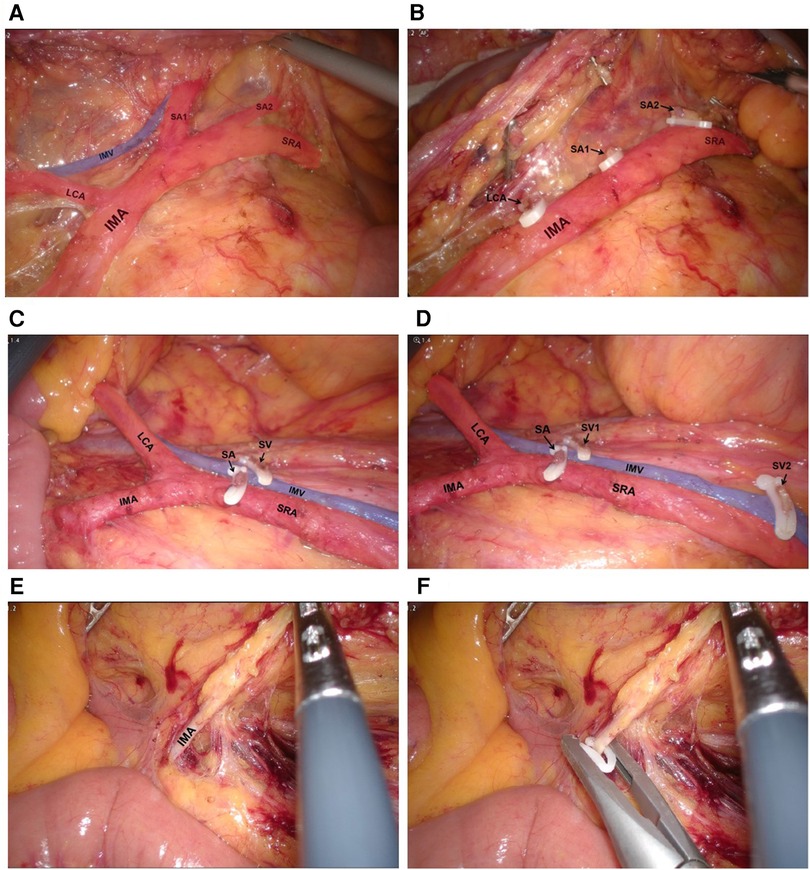
Figure 2. (A,B) Dissect the lymphatic adipose tissue around and at the root of IMA, divide and cut off LCA, SA1 and SA2. (C,D) Operative view of preserving LCA, IMV and SRA. (E,F) Operative view of high ligation of IMA.
Postoperative complications
AL after colorectal resection is defined as the defect of intestinal wall integrity at the colorectal or colo-anal anastomosis site (including suture and staple lines of neorectal reservoirs), leading to a communication between intra- and extraluminal compartments. A pelvic abscess close to the anastomosis is also considered AL. The severity grading of AL is as follows: Grade A: AL requiring no active therapeutic intervention; Grade B: AL requiring active therapeutic intervention but manageable without re-laparotomy; Grade C: AL requiring re-laparotomy (8).
Postoperative ileus (POI) was diagnosed if two or more of the following criteria were met on or after postoperative day 4: absence of flatus for over 24 h, absence of stool for over 72 h, abdominal distension, nausea or vomiting, incapability to tolerate diet in the past 24 h; clinical and imaging examinations (including x-ray radiograph, sonography, and computed tomography) indicative of POI (9).
Statistical analysis
Survival was measured from the time of surgery until death or last-known follow-up (censor date was January 1, 2022). The data were presented as mean ± standard deviation, and Chi-squared test or Fisher's exact test, analysis of variance, or group t test was conducted to test for differences between groups. Kaplan–Meier method was used for survival analysis, and log-rank test was applied for comparison of differences in survival curves. p < 0.05 was considered statistically significant. All statistical analyses were performed using the software SPSS Version 25.0.
Ethical statement
The procedures of this study were in accordance with the Declaration of Helsinki and approved by the Ethics Committee of People's Hospital of Deyang (No.2022-04-051-K01).
Results
Comparison of clinicopathological factors and complications between patient groups
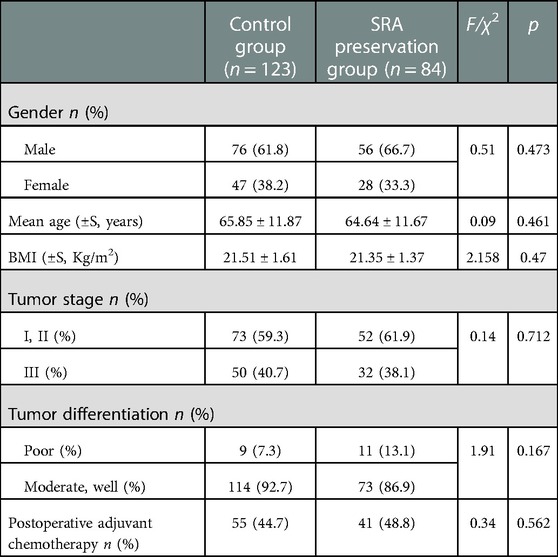
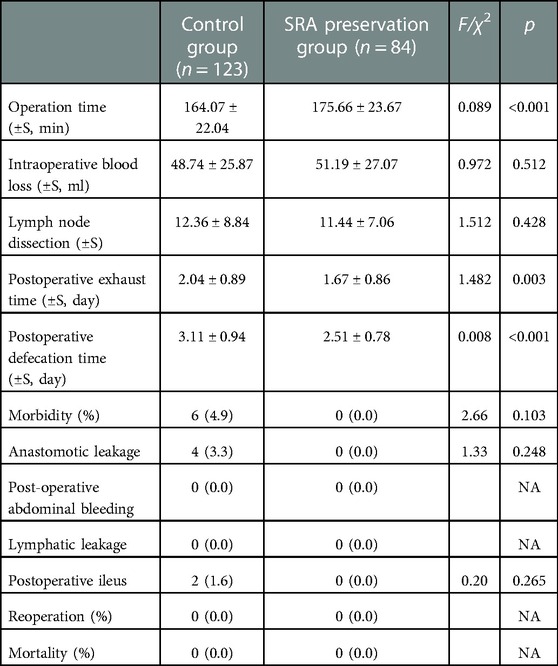
A total of 207 patients with SCC were enrolled in this study. Exactly 84 patients underwent D3 lymph node dissection with preservation of SRA (SRA preservation group), and 123 patients underwent high ligation of the IMA (control group). Table 1 shows the comparison of clinicopathological factors between SRA preservation and control groups. No significant differences were observed in gender, age, BMI, intraoperative blood loss, tumor stage, tumor differentiation, the number of lymph nodes dissected, and postoperative adjuvant chemotherapy (p > 0.05). However, given the anatomy of IMA branches and SRA preservation, the operation time was significantly longer in the preservation group (p < 0.001) (Tables 1, 2).
The postoperative exhaust and defecation times of the SRA preservation group were significantly shorter than those of the control group, and the differences were statistically significant (p = 0.003; p < 0.001) (Table 2). Two cases of POI were observed in the control group, but the difference was not statistically significant compared with that in the preservation group (p = 0.652). Four cases of AL were detected in the control group and none in the preservation group, but the difference was not statistically significant (p = 0.248). No postoperative abdominal bleeding, lymph leakage, death, nor reoperation occurred in the two groups after surgery (Table 2).
Survival
The cumulative 1-, 2-, 3-, and 4-year overall survival rates were 88.7%, 80.4%, 71.7%, and 67.3%, respectively. No significant difference was observed in the overall survival and disease-free survival between the two groups (p = 0.436; p = 0.414) (Figures 3, 4, respectively).

Figure 3. Comparison of overall survival between SRA preservation group and control group (p = 0.436).
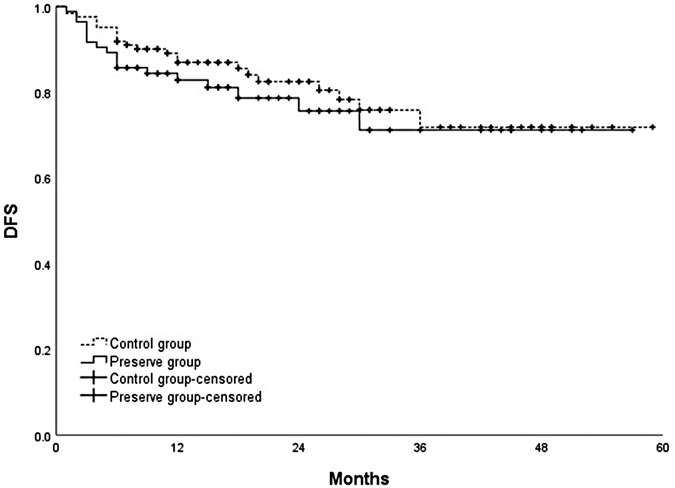
Figure 4. Comparison of disease-free survival between SRA preservation group and control group (p = 0.414).
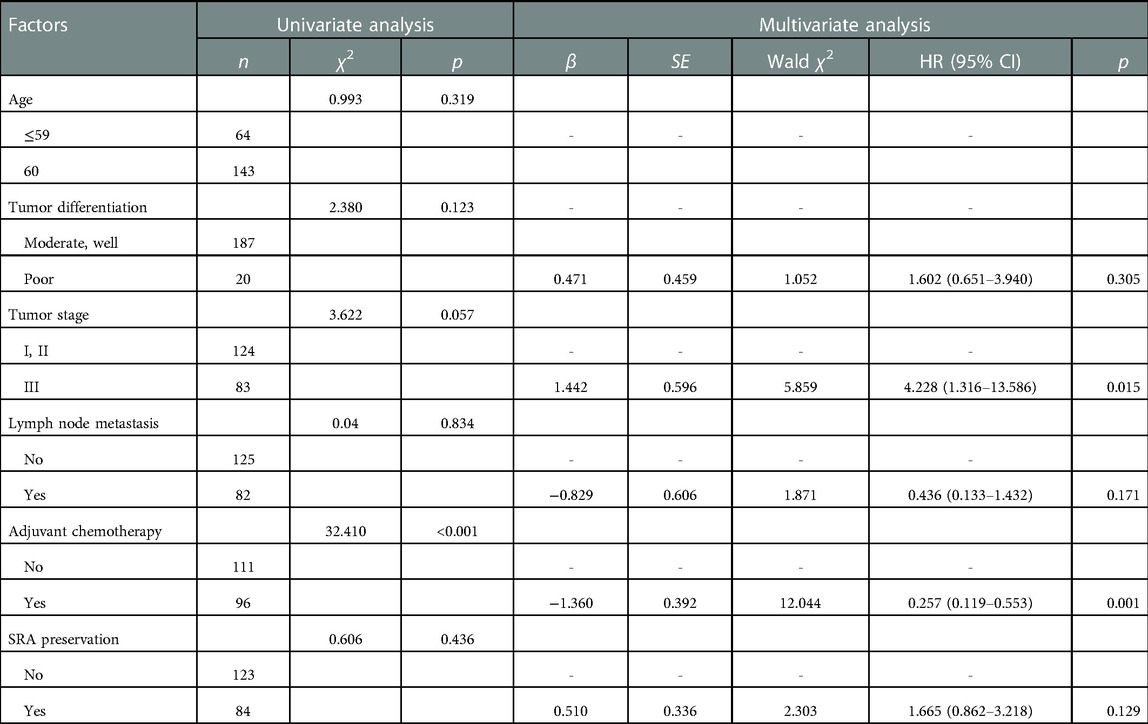
Univariate analysis revealed that adjuvant chemotherapy (p < 0.001) was significantly associated with overall survival. Factors that may affect patient survival were further included in multivariate analysis through the Cox proportional risk model. Multivariate analysis demonstrated that tumor stage (p = 0.015) and adjuvant chemotherapy (p = 0.001) remained independently associated with overall survival. Adjuvant chemotherapy was a protective factor affecting the survival of patients [hazard ratio (HR) = 0.257, 95% confidence interval (CI) (0.119, 0.553, p = 0.001], and tumor stage was an adverse factor [HR = 4.2278, 95% CI (1.316, 13.586), p = 0.015]. However, SRA preservation was not an independent prognostic factor in this cohort (Table 3).
Discussion
High ligation of the IMA is usually recommended during sigmoid colon and rectal cancer surgery to achieve complete D3 lymph node dissection. Several surgeons indicated that high ligation of the IMA for colorectal cancers can improve lymph node harvest, which facilitates accurate tumor staging and thus better disease prognostication, and may improve the survival rate of patients (10, 11). However, an increasing number of studies or meta-analyses showed no significant difference in the number of lymph nodes dissected and overall survival between IMA high ligation versus IMA low ligation (12–15). Similarly, our study compared IMA high ligation with SRA preservation during SCC surgery. No difference was observed in the number of lymph nodes removed or overall survival between the two procedures. The results indicated that SRA preservation during SCC surgery does not increase postoperative morbidity and mortality, does not affect prognosis, and can achieve D3 lymph node dissection, which is technically completely feasible.
However, most previous studies showed that IMA high ligation was associated with a high incidence of AL (14, 15). Early AL is mostly related to technical failure of the anastomosis due to iatrogenic surgical disruption of the peri-anastomotic microvascular blood supply or tension at the anastomotic site (16), whereas late AL is related to pre-existing conditions, such as local sepsis, poor nutrition, immunosuppression, obesity, vascular disease, and radiation exposure (17). A recent study performed real-time indocyanine green fluorescence angiography to measure fluorescence time as a marker of blood flow in the proximal and distal stumps before anastomosis. The results showed that IMA high ligation significantly prolonged fluorescence time in the sigmoid colon during anterior rectal resection compared with low ligation, which indicates that IMA high ligation induced significant colon stump hypoperfusion (4). This finding possibly explains why IMA high ligation resulted in a high rate of AL. As a result, an increasing number of surgeons are opting to perform low ligation of the IMA with D3 lymph node dissection (18–23), particularly in patients with diabetes, obesity, or old patients with cerebrovascular disease and atherosclerotic vessels. Similarly, for SCC, SRA preservation can theoretically increase the blood supply to the distal stump of sigmoid colon or upper rectum and reduce the incidence of AL. However, reports on the preservation of SRA procedure are limited. Our study showed that AL occurred in four cases in the control group but not in the preservation group. Although no statistical difference was observed between the two groups, no AL occurred when SRA was preserved. Whether the differences will become more pronounced if the sample size is large enough should be determined. These results indicate that SRA preservation may have a certain positive significance in reducing the incidence of AL.
In addition, our study revealed that the postoperative exhaust and defecation times were significantly shortened after SRA preservation, and no POI was observed. In preservation of SRA, increased bowel blood supply has significant positive effects on postoperative intestinal function recovery, which is conducive to enhanced recovery after surgery.
Conclusion
In conclusion, SRA preservation plus dissection of lymph nodes around IMA in laparoscopic radical resection for SCC did not increase postoperative morbidity and mortality and did not affect patient prognosis. However, this process may increase the bowel blood supply, which may have a significant positive effect on the recovery of postoperative intestinal function and reduction of AL. Without compromising oncological outcomes, this alternative surgical technique should be recommended as a better intraoperative treatment option for reducing postoperative complications. However, certain technical difficulties need to be addressed by experienced surgeons under the premise of technical assurance. Large and well-designed multicenter randomized clinical trials are necessary to confirm these findings and shed light on this topic.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of People’s Hospital of Deyang. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sekimoto M, Takemasa I, Mizushima T, Ikeda M, Yamamoto H, Doki Y, et al. Laparoscopic lymph node dissection around the inferior mesenteric artery with preservation of the left colic artery. Surg Endosc. (2011) 25:861–6. doi: 10.1007/s00464-010-1284-7
2. Chin CC, Yeh CY, Tang R, Changchien CR, Huang WS, Wang JY. The oncologic benefit of high ligation of the inferior mesenteric artery in the surgical treatment of rectal or sigmoid colon cancer. Int J Colorectal Dis. (2008) 23:783–8. doi: 10.1007/s00384-008-0465-5
3. Kanemitsu Y, Hirai T, Komori K, Kato T. Survival benefit of high ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery. Br J Surg. (2006) 93:609–15. doi: 10.1002/bjs.5327
4. Higashijima J, Kono T, Shimada M, Sugitani A, Kashihara H, Takasu C, et al. High ligation of the Inferior mesenteric artery induces hypoperfusion of the sigmoid colon stump during anterior resection. Front Surg. (2021) 8:756873. doi: 10.3389/fsurg.2021.756873
5. Komen N, Slieker J, de Kort P, de Wilt JH, van der Harst E, Coene PP, et al. High tie versus low tie in rectal surgery: comparison of anastomotic perfusion. Int J Colorectal Dis. (2011) 26(8):1075–8. doi: 10.1007/s00384-011-1188-6; Epub 2011 Mar 29. Erratum in: Int J Colorectal Dis. 2011;26(6):821. Gosselink, Martijn [corrected to Gosselink, Martijn P].21445553
6. Seike K, Koda K, Saito N, Oda K, Kosugi C, Shimizu K, et al. Laser Doppler assessment of the influence of division at the root of the inferior mesenteric artery on anastomotic blood flow in rectosigmoid cancer surgery. Int J Colorectal Dis. (2007) 22(6):689–97. doi: 10.1007/s00384-006-0221-7
7. Yang XF, Zhang JF, Li JJ, Zhao S, Shi S, Wu JC, et al. The pathological behaviors and prognostic factors of Chinese and Japanese colorectal cancers from general hospitals: a comparative study of the inpatients with surgical operation. Oncotarget. (2016) 7(51):84155–64. doi: 10.18632/oncotarget.12228
8. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study group of rectal cancer. Surgery. (2010) 147(3):339–51. doi: 10.1016/j.surg.2009.10.012
9. Jin Y, Geng R, Liu Y, Liu L, Jin X, Zhao F, et al. Prediction of postoperative ileus in patients with colorectal cancer by preoperative gut microbiota. Front Oncol. (2020) 10:526009. doi: 10.3389/fonc.2020.526009
10. Charan I, Kapoor A, Singhal MK, Jagawat N, Bhavsar D, Jain V, et al. High ligation of inferior mesenteric artery in left colonic and rectal cancers: lymph node yield and survival benefit. Indian J Surg. (2015) 77(Suppl 3):1103–8. doi: 10.1007/s12262-014-1179-2
11. Hida J, Okuno K. High ligation of the inferior mesenteric artery in rectal cancer surgery. Surg Today. (2013) 43(1):8–19. doi: 10.1007/s00595-012-0359-6
12. Yang Y, Wang G, He J, Zhang J, Xi J, Wang F. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: a meta-analysis. Int J Surg. (2018) 52:20–4. doi: 10.1016/j.ijsu.2017.12.030
13. Cirocchi R, Trastulli S, Farinella E, Desiderio J, Vettoretto N, Parisis A, et al. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: a RCT is needed. Surg Oncol. (2012) 21(3):e111–23. doi: 10.1016/j.suronc.2012.04.004
14. Yin TC, Chen YC, Su WC, Chen PJ, Chang TK, Huang CW, et al. Low ligation plus high dissection versus high ligation of the inferior mesenteric artery in sigmoid colon and rectal cancer surgery: a meta-analysis. Front Oncol. (2021) 11:774782. doi: 10.3389/fonc.2021.774782
15. Zeng J, Su G. High ligation of the inferior mesenteric artery during sigmoid colon and rectal cancer surgery increases the risk of anastomotic leakage: a meta-analysis. World J Surg Oncol. (2018) 16(1):157. doi: 10.1186/s12957-018-1458-7
16. Chiarello MM, Fransvea P, Cariati M, Adams NJ, Bianchi V, Brisinda G. Anastomotic leakage in colorectal cancer surgery. Surg Oncol. (2022) 40:101708. doi: 10.1016/j.suronc.2022.101708
17. Sparreboom CL, van Groningen JT, Lingsma HF, Wouters MWJM, Menon AG, Kleinrensink GJ, et al. Different risk factors for early and late colorectal anastomotic leakage in a nationwide audit. Dis Colon Rectum. (2018) 61(11):1258–66. doi: 10.1097/DCR.0000000000001202
18. Matsuda K, Hotta T, Takifuji K, Yokoyama S, Oku Y, Watanabe T, et al. Randomized clinical trial of defaecatory function after anterior resection for rectal cancer with high versus low ligation of the Inferior mesenteric artery. Br J Surg. (2015) 102(5):501–8. doi: 10.1002/bjs.9739
19. Yasuda K, Kawai K, Ishihara S, Murono K, Otani K, Nishikawa T, et al. Level of arterial ligation in sigmoid colon and rectal cancer surgery. World J Surg Oncol. (2016) 14:99. doi: 10.1186/s12957-016-0819-3
20. Hinoi T, Okajima M, Shimomura M, Egi H, Ohdan H, Konishi F, et al. Effect of left colonic artery preservation on anastomotic leakage in laparoscopic anterior resection for middle and low rectal cancer. World J Surg. (2013) 37(12):2935–43. doi: 10.1007/s00268-013-2194-3
21. Yamamoto M, Okuda J, Tanaka K, Ishii M, Hamamoto H, Uchiyama K. Oncological impact of laparoscopic lymphadenectomy with preservation of the left colic artery for advanced sigmoid and rectosigmoid colon cancer. Dig Surg. (2014) 31(6):452–8. doi: 10.1159/000369938
22. Guo Y, Wang D, He L, Zhang Y, Zhao S, Zhang L, et al. Marginal artery stump pressure in left colic artery-preserving rectal cancer surgery: a clinical trial. ANZ J Surg. (2017) 87(7-8):576–81. doi: 10.1111/ans.13032
Keywords: sigmoid colon cancer, superior rectal artery, inferior mesenteric artery, anastomotic leakage, overall survival
Citation: Ren H, Liu Y, Zhang M and An L (2023) Feasibility of preservation of superior rectal artery plus dissection of lymph nodes around inferior mesenteric artery in laparoscopic resection for sigmoid colon cancer. Front. Surg. 10:1086868. doi: 10.3389/fsurg.2023.1086868
Received: 1 November 2022; Accepted: 26 January 2023;
Published: 14 February 2023.
Edited by:
Bo Zhang, West China Hospital, Sichuan University, ChinaReviewed by:
Ming Wang, Shanghai Jiao Tong University, ChinaZhengrong Li, The First Affiliated Hospital of Nanchang University, China
© 2023 Ren, Liu, Zhang and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haoyuan Ren cmh5MTk4NjA5MThAaG90bWFpbC5jb20= Yong Liu cmh5MTk4NjkxOEAxNjMuY29t
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Haoyuan Ren
Haoyuan Ren Yong Liu
Yong Liu Mingran Zhang
Mingran Zhang